 Found 426 hits with Last Name = 'o''neil' and Initial = 'sv'
Found 426 hits with Last Name = 'o''neil' and Initial = 'sv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50240692
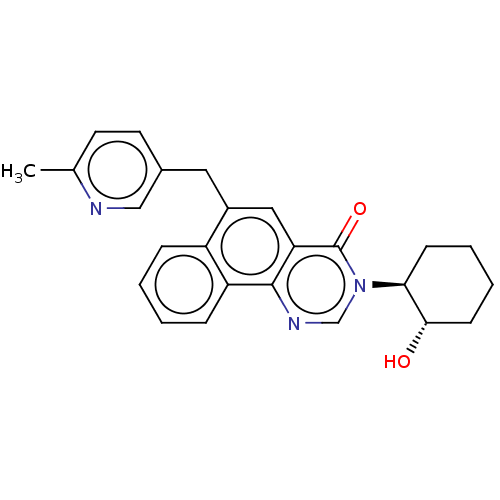
(CHEMBL4078588)Show SMILES Cc1ccc(Cc2cc3c(ncn([C@H]4CCCC[C@@H]4O)c3=O)c3ccccc23)cn1 |r| Show InChI InChI=1S/C25H25N3O2/c1-16-10-11-17(14-26-16)12-18-13-21-24(20-7-3-2-6-19(18)20)27-15-28(25(21)30)22-8-4-5-9-23(22)29/h2-3,6-7,10-11,13-15,22-23,29H,4-5,8-9,12H2,1H3/t22-,23-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PT-1284 human muscarinic acetylcholine receptor M1 expressed in CHO cell membranes after 90 mins scintillation counting method |
J Med Chem 60: 6649-6663 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00597
BindingDB Entry DOI: 10.7270/Q2ZK5JS0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50240694
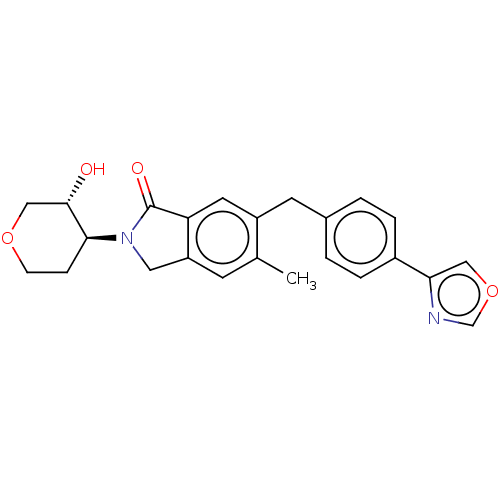
(CHEMBL4067722)Show SMILES Cc1cc2CN([C@H]3CCOC[C@@H]3O)C(=O)c2cc1Cc1ccc(cc1)-c1cocn1 |r| Show InChI InChI=1S/C24H24N2O4/c1-15-8-19-11-26(22-6-7-29-13-23(22)27)24(28)20(19)10-18(15)9-16-2-4-17(5-3-16)21-12-30-14-25-21/h2-5,8,10,12,14,22-23,27H,6-7,9,11,13H2,1H3/t22-,23-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PT-1284 human muscarinic acetylcholine receptor M1 expressed in CHO cell membranes after 90 mins scintillation counting method |
J Med Chem 60: 6649-6663 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00597
BindingDB Entry DOI: 10.7270/Q2ZK5JS0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50240693
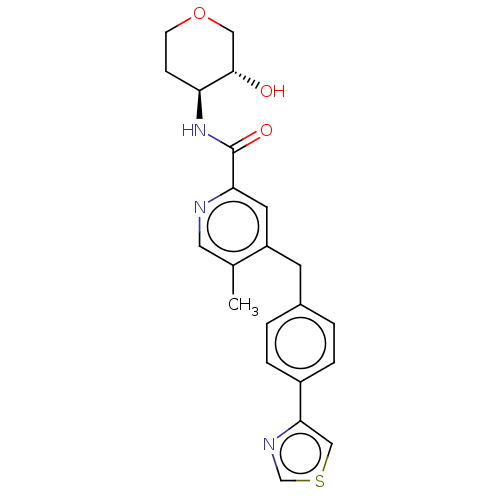
(CHEMBL4097569)Show SMILES Cc1cnc(cc1Cc1ccc(cc1)-c1cscn1)C(=O)N[C@H]1CCOC[C@@H]1O |r| Show InChI InChI=1S/C22H23N3O3S/c1-14-10-23-19(22(27)25-18-6-7-28-11-21(18)26)9-17(14)8-15-2-4-16(5-3-15)20-12-29-13-24-20/h2-5,9-10,12-13,18,21,26H,6-8,11H2,1H3,(H,25,27)/t18-,21-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PT-1284 human muscarinic acetylcholine receptor M1 expressed in CHO cell membranes after 90 mins scintillation counting method |
J Med Chem 60: 6649-6663 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00597
BindingDB Entry DOI: 10.7270/Q2ZK5JS0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50135213
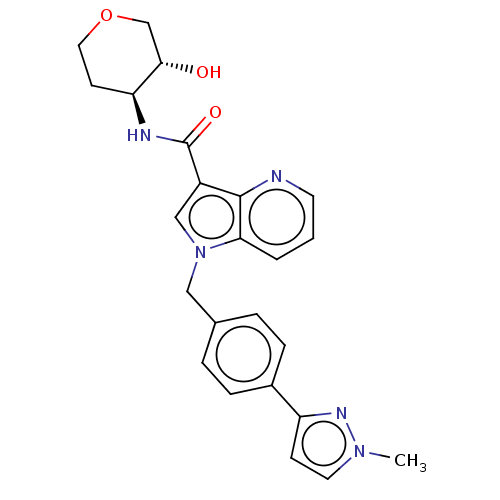
(CHEMBL3747016)Show SMILES Cn1ccc(n1)-c1ccc(Cn2cc(C(=O)N[C@H]3CCOC[C@@H]3O)c3ncccc23)cc1 |r| Show InChI InChI=1S/C24H25N5O3/c1-28-11-8-19(27-28)17-6-4-16(5-7-17)13-29-14-18(23-21(29)3-2-10-25-23)24(31)26-20-9-12-32-15-22(20)30/h2-8,10-11,14,20,22,30H,9,12-13,15H2,1H3,(H,26,31)/t20-,22-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against [3H]- (+)-3-PPP binding to Sigma opioid receptor in guinea pig brain membrane homogenates |
J Med Chem 60: 6649-6663 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00597
BindingDB Entry DOI: 10.7270/Q2ZK5JS0 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175328

((4S,7S,11aS)-benzyl 4-(((3S)-2-hydroxy-5-oxo-tetra...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2C\C=C/C[C@H](NC(=O)c3ccccc3)C(=O)N12)C(=O)OCc1ccccc1 |c:17| Show InChI InChI=1S/C30H32N4O8/c35-25-15-23(29(39)42-25)32-27(37)24-17-33(30(40)41-18-19-9-3-1-4-10-19)16-21-13-7-8-14-22(28(38)34(21)24)31-26(36)20-11-5-2-6-12-20/h1-12,21-24,29,39H,13-18H2,(H,31,36)(H,32,37)/b8-7-/t21-,22-,23-,24-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193734
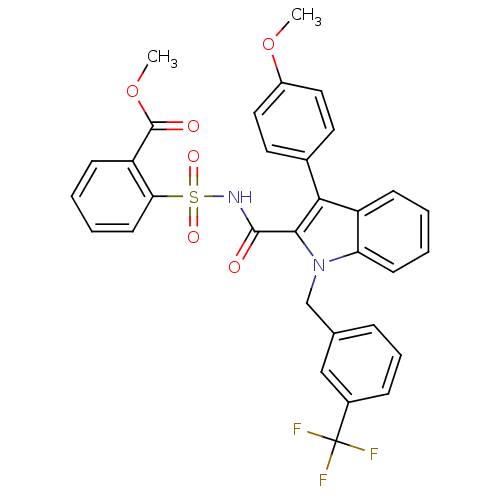
(2-{[3-(4-methoxy-phenyl)-1-(3-trifluoromethyl-benz...)Show SMILES COC(=O)c1ccccc1S(=O)(=O)NC(=O)c1c(-c2ccc(OC)cc2)c2ccccc2n1Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C32H25F3N2O6S/c1-42-23-16-14-21(15-17-23)28-24-10-3-5-12-26(24)37(19-20-8-7-9-22(18-20)32(33,34)35)29(28)30(38)36-44(40,41)27-13-6-4-11-25(27)31(39)43-2/h3-18H,19H2,1-2H3,(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175338

((4S,7S,11aS)-7-(benzo[b]thiophene-2-carboxamido)-N...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2CCCC[C@H](NC(=O)c3cc4ccccc4s3)C(=O)N12 Show InChI InChI=1S/C24H27N3O7S/c28-20-10-16(24(32)34-20)26-21(29)17-12-33-11-14-6-2-3-7-15(23(31)27(14)17)25-22(30)19-9-13-5-1-4-8-18(13)35-19/h1,4-5,8-9,14-17,24,32H,2-3,6-7,10-12H2,(H,25,30)(H,26,29)/t14-,15-,16-,17-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175337

((4S,7S,11aS)-benzyl 4-(((3S)-2-hydroxy-5-oxo-tetra...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2C\C=C/C[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12)C(=O)OCc1ccccc1 |c:17| Show InChI InChI=1S/C33H33N5O8/c39-27-16-25(32(43)46-27)36-29(40)26-18-37(33(44)45-19-20-8-2-1-3-9-20)17-22-11-5-7-13-24(31(42)38(22)26)35-30(41)28-23-12-6-4-10-21(23)14-15-34-28/h1-10,12,14-15,22,24-26,32,43H,11,13,16-19H2,(H,35,41)(H,36,40)/b7-5-/t22-,24-,25-,26-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175335

((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CNC[C@@H]2CCCC[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12 Show InChI InChI=1S/C26H30N4O6/c31-22-12-20(26(35)36-22)29-24(33)21-14-27-13-18-7-3-4-8-19(25(34)30(18)21)28-23(32)17-10-9-15-5-1-2-6-16(15)11-17/h1-2,5-6,9-11,18-21,26-27,35H,3-4,7-8,12-14H2,(H,28,32)(H,29,33)/t18-,19-,20-,21-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175347
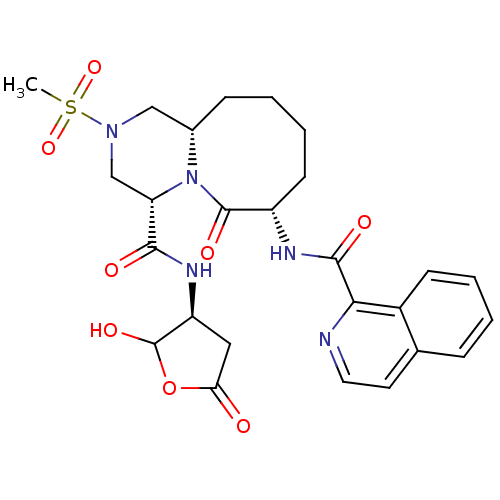
((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES CS(=O)(=O)N1C[C@@H]2CCCC[C@H](NC(=O)c3nccc4ccccc34)C(=O)N2[C@@H](C1)C(=O)N[C@H]1CC(=O)OC1O Show InChI InChI=1S/C26H31N5O8S/c1-40(37,38)30-13-16-7-3-5-9-18(28-24(34)22-17-8-4-2-6-15(17)10-11-27-22)25(35)31(16)20(14-30)23(33)29-19-12-21(32)39-26(19)36/h2,4,6,8,10-11,16,18-20,26,36H,3,5,7,9,12-14H2,1H3,(H,28,34)(H,29,33)/t16-,18-,19-,20-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175346
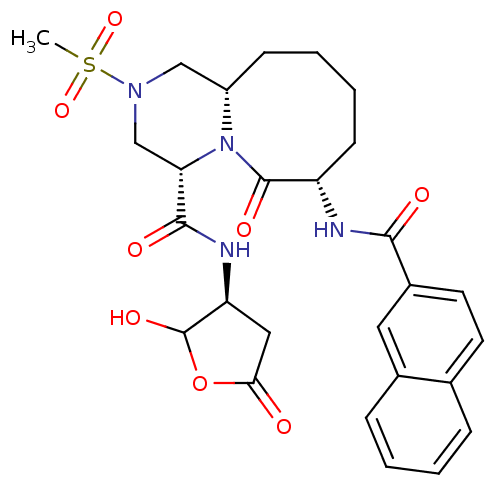
((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES CS(=O)(=O)N1C[C@@H]2CCCC[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N2[C@@H](C1)C(=O)N[C@H]1CC(=O)OC1O Show InChI InChI=1S/C27H32N4O8S/c1-40(37,38)30-14-19-8-4-5-9-20(28-24(33)18-11-10-16-6-2-3-7-17(16)12-18)26(35)31(19)22(15-30)25(34)29-21-13-23(32)39-27(21)36/h2-3,6-7,10-12,19-22,27,36H,4-5,8-9,13-15H2,1H3,(H,28,33)(H,29,34)/t19-,20-,21-,22-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175323
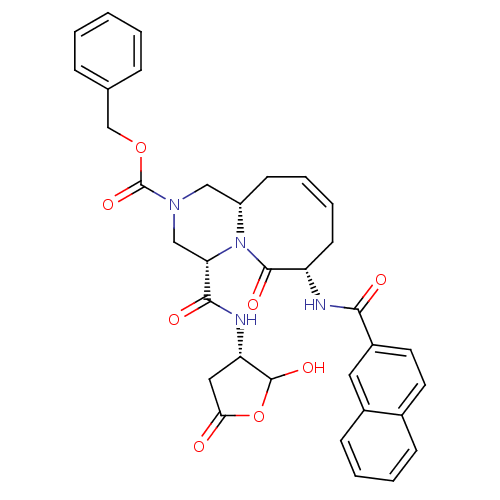
((4S,7S,11aS)-benzyl 4-(((3S)-2-hydroxy-5-oxo-tetra...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2C\C=C/C[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12)C(=O)OCc1ccccc1 |c:17| Show InChI InChI=1S/C34H34N4O8/c39-29-17-27(33(43)46-29)36-31(41)28-19-37(34(44)45-20-21-8-2-1-3-9-21)18-25-12-6-7-13-26(32(42)38(25)28)35-30(40)24-15-14-22-10-4-5-11-23(22)16-24/h1-11,14-16,25-28,33,43H,12-13,17-20H2,(H,35,40)(H,36,41)/b7-6-/t25-,26-,27-,28-,33?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175340
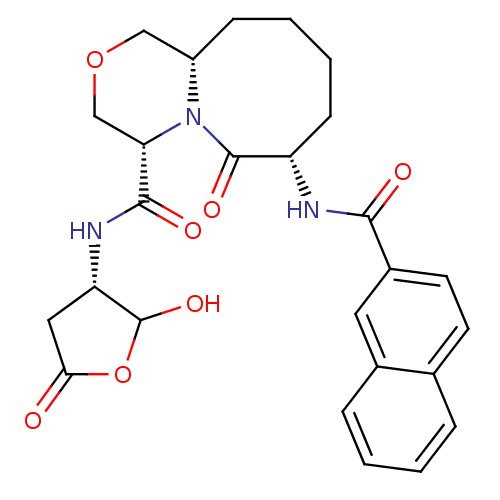
((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2CCCC[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12 Show InChI InChI=1S/C26H29N3O7/c30-22-12-20(26(34)36-22)28-24(32)21-14-35-13-18-7-3-4-8-19(25(33)29(18)21)27-23(31)17-10-9-15-5-1-2-6-16(15)11-17/h1-2,5-6,9-11,18-21,26,34H,3-4,7-8,12-14H2,(H,27,31)(H,28,32)/t18-,19-,20-,21-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175327
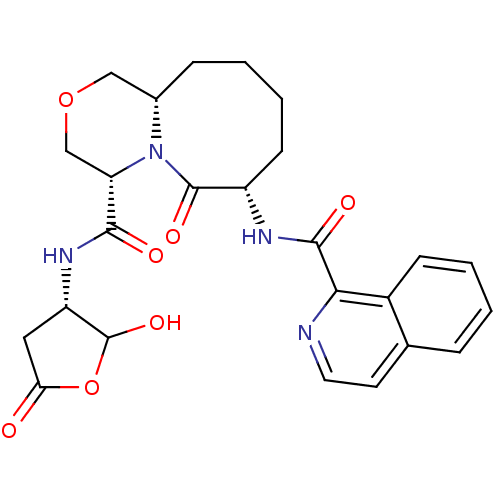
((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2CCCC[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12 Show InChI InChI=1S/C25H28N4O7/c30-20-11-18(25(34)36-20)28-22(31)19-13-35-12-15-6-2-4-8-17(24(33)29(15)19)27-23(32)21-16-7-3-1-5-14(16)9-10-26-21/h1,3,5,7,9-10,15,17-19,25,34H,2,4,6,8,11-13H2,(H,27,32)(H,28,31)/t15-,17-,18-,19-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175326
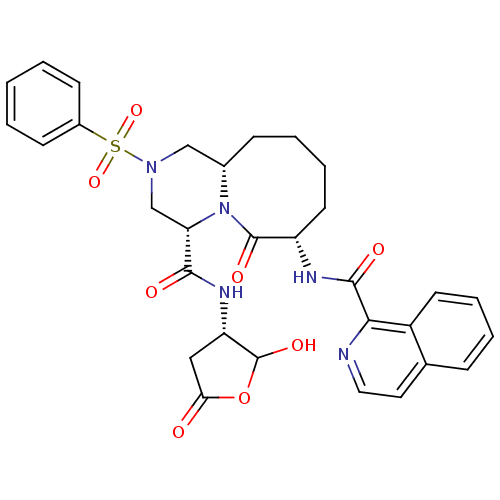
((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2CCCC[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C31H33N5O8S/c37-26-16-24(31(41)44-26)34-28(38)25-18-35(45(42,43)21-10-2-1-3-11-21)17-20-9-5-7-13-23(30(40)36(20)25)33-29(39)27-22-12-6-4-8-19(22)14-15-32-27/h1-4,6,8,10-12,14-15,20,23-25,31,41H,5,7,9,13,16-18H2,(H,33,39)(H,34,38)/t20-,23-,24-,25-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175348

((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2CCCC[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C32H34N4O8S/c37-28-17-26(32(41)44-28)34-30(39)27-19-35(45(42,43)24-11-2-1-3-12-24)18-23-10-6-7-13-25(31(40)36(23)27)33-29(38)22-15-14-20-8-4-5-9-21(20)16-22/h1-5,8-9,11-12,14-16,23,25-27,32,41H,6-7,10,13,17-19H2,(H,33,38)(H,34,39)/t23-,25-,26-,27-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50255776
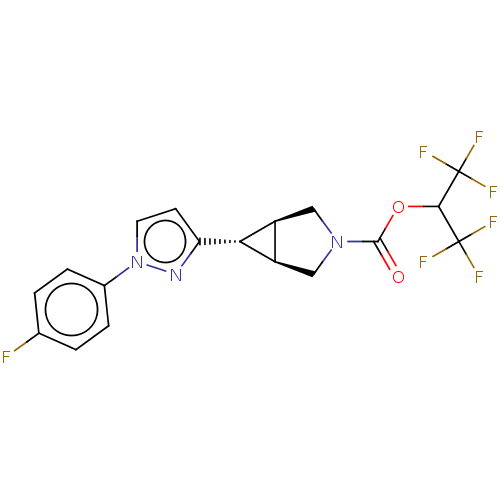
(CHEMBL4075310)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1ccn(n1)-c1ccc(F)cc1)C(=O)OC(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C18H14F7N3O2/c19-9-1-3-10(4-2-9)28-6-5-13(26-28)14-11-7-27(8-12(11)14)16(29)30-15(17(20,21)22)18(23,24)25/h1-6,11-12,14-15H,7-8H2/t11-,12+,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 61: 3008-3026 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00070
BindingDB Entry DOI: 10.7270/Q2S46VD4 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199762
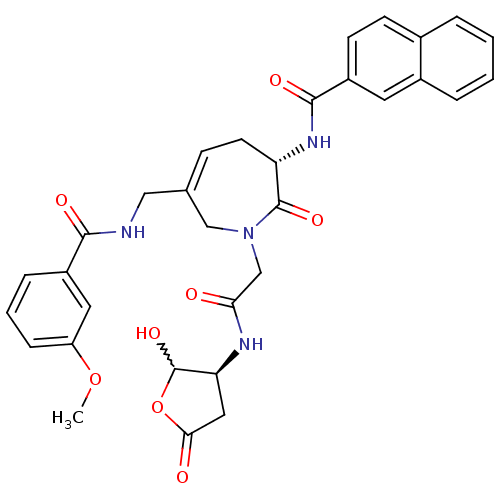
(CHEMBL411178 | N-((S,Z)-1-(2-((3S)-2-hydroxy-5-oxo...)Show SMILES COc1cccc(c1)C(=O)NCC1=CC[C@H](NC(=O)c2ccc3ccccc3c2)C(=O)N(CC(=O)N[C@H]2CC(=O)OC2O)C1 |w:41.45,t:13| Show InChI InChI=1S/C32H32N4O8/c1-43-24-8-4-7-22(14-24)29(39)33-16-19-9-12-25(35-30(40)23-11-10-20-5-2-3-6-21(20)13-23)31(41)36(17-19)18-27(37)34-26-15-28(38)44-32(26)42/h2-11,13-14,25-26,32,42H,12,15-18H2,1H3,(H,33,39)(H,34,37)(H,35,40)/t25-,26-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199749
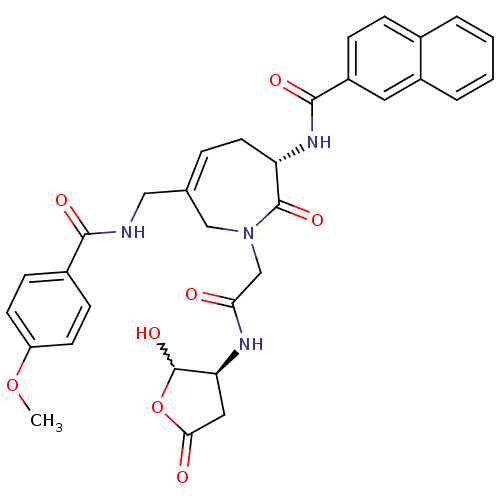
(CHEMBL268772 | N-((S,Z)-1-(2-((3S)-2-hydroxy-5-oxo...)Show SMILES COc1ccc(cc1)C(=O)NCC1=CC[C@H](NC(=O)c2ccc3ccccc3c2)C(=O)N(CC(=O)N[C@H]2CC(=O)OC2O)C1 |w:41.45,t:13| Show InChI InChI=1S/C32H32N4O8/c1-43-24-11-9-21(10-12-24)29(39)33-16-19-6-13-25(35-30(40)23-8-7-20-4-2-3-5-22(20)14-23)31(41)36(17-19)18-27(37)34-26-15-28(38)44-32(26)42/h2-12,14,25-26,32,42H,13,15-18H2,1H3,(H,33,39)(H,34,37)(H,35,40)/t25-,26-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199766
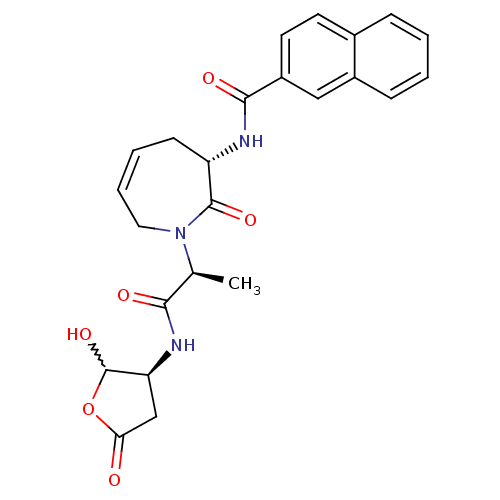
(CHEMBL394351 | N-((S,Z)-1-((S)-1-((3S)-2-hydroxy-5...)Show SMILES C[C@H](N1CC=CC[C@H](NC(=O)c2ccc3ccccc3c2)C1=O)C(=O)N[C@H]1CC(=O)OC1O |w:31.35,c:4| Show InChI InChI=1S/C24H25N3O6/c1-14(21(29)26-19-13-20(28)33-24(19)32)27-11-5-4-8-18(23(27)31)25-22(30)17-10-9-15-6-2-3-7-16(15)12-17/h2-7,9-10,12,14,18-19,24,32H,8,11,13H2,1H3,(H,25,30)(H,26,29)/t14-,18-,19-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175329
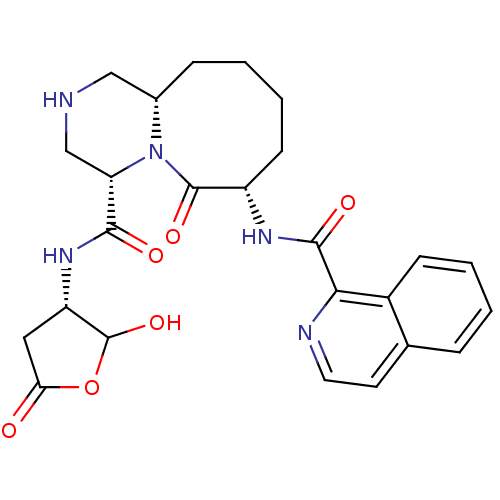
((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CNC[C@@H]2CCCC[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12 Show InChI InChI=1S/C25H29N5O6/c31-20-11-18(25(35)36-20)29-22(32)19-13-26-12-15-6-2-4-8-17(24(34)30(15)19)28-23(33)21-16-7-3-1-5-14(16)9-10-27-21/h1,3,5,7,9-10,15,17-19,25-26,35H,2,4,6,8,11-13H2,(H,28,33)(H,29,32)/t15-,17-,18-,19-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175345

((4S,7S,11aS)-7-benzamido-N-((3S)-2-hydroxy-5-oxo-t...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2CCCC[C@H](NC(=O)c3ccccc3)C(=O)N12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H32N4O8S/c33-24-15-22(28(37)40-24)30-26(35)23-17-31(41(38,39)20-12-5-2-6-13-20)16-19-11-7-8-14-21(27(36)32(19)23)29-25(34)18-9-3-1-4-10-18/h1-6,9-10,12-13,19,21-23,28,37H,7-8,11,14-17H2,(H,29,34)(H,30,35)/t19-,21-,22-,23-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50189360

((1S,10S)-9-[(Isoquinoline-1-carbonyl)-amino]-6,10-...)Show SMILES CCO[C@@H]1OC(=O)C[C@@H]1NC(=O)[C@@H]1CCCN2N1C(=O)[C@H](CCC2=O)NC(=O)c1nccc2ccccc12 Show InChI InChI=1S/C26H29N5O7/c1-2-37-26-18(14-21(33)38-26)29-23(34)19-8-5-13-30-20(32)10-9-17(25(36)31(19)30)28-24(35)22-16-7-4-3-6-15(16)11-12-27-22/h3-4,6-7,11-12,17-19,26H,2,5,8-10,13-14H2,1H3,(H,28,35)(H,29,34)/t17-,18-,19-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter& Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of caspase-1 using fluorogenic substrate and BMG Fluostar plate reader for 30 min at 37 degree C |
Bioorg Med Chem Lett 15: 4291-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.050
BindingDB Entry DOI: 10.7270/Q2J103ZQ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199750

(CHEMBL243823 | N-((S,Z)-1-(2-((3S)-2-hydroxy-5-oxo...)Show SMILES COc1ccccc1C(=O)NCC1=CC[C@H](NC(=O)c2ccc3ccccc3c2)C(=O)N(CC(=O)N[C@H]2CC(=O)OC2O)C1 |w:41.45,t:13| Show InChI InChI=1S/C32H32N4O8/c1-43-26-9-5-4-8-23(26)30(40)33-16-19-10-13-24(35-29(39)22-12-11-20-6-2-3-7-21(20)14-22)31(41)36(17-19)18-27(37)34-25-15-28(38)44-32(25)42/h2-12,14,24-25,32,42H,13,15-18H2,1H3,(H,33,40)(H,34,37)(H,35,39)/t24-,25-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199763

(CHEMBL397184 | N-((S,Z)-6-(benzamidomethyl)-1-(2-(...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)CN1CC(CNC(=O)c2ccccc2)=CC[C@H](NC(=O)c2ccc3ccccc3c2)C1=O |w:1.0,c:25| Show InChI InChI=1S/C31H30N4O7/c36-26(33-25-15-27(37)42-31(25)41)18-35-17-19(16-32-28(38)21-7-2-1-3-8-21)10-13-24(30(35)40)34-29(39)23-12-11-20-6-4-5-9-22(20)14-23/h1-12,14,24-25,31,41H,13,15-18H2,(H,32,38)(H,33,36)(H,34,39)/t24-,25-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193736
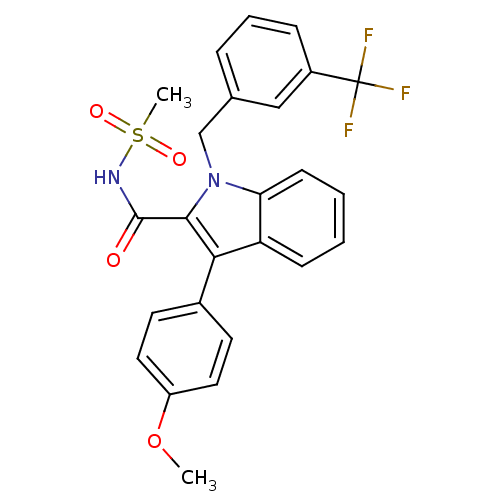
(1-(3-(trifluoromethyl)benzyl)-3-(4-methoxyphenyl)-...)Show SMILES COc1ccc(cc1)-c1c(C(=O)NS(C)(=O)=O)n(Cc2cccc(c2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C25H21F3N2O4S/c1-34-19-12-10-17(11-13-19)22-20-8-3-4-9-21(20)30(23(22)24(31)29-35(2,32)33)15-16-6-5-7-18(14-16)25(26,27)28/h3-14H,15H2,1-2H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199768
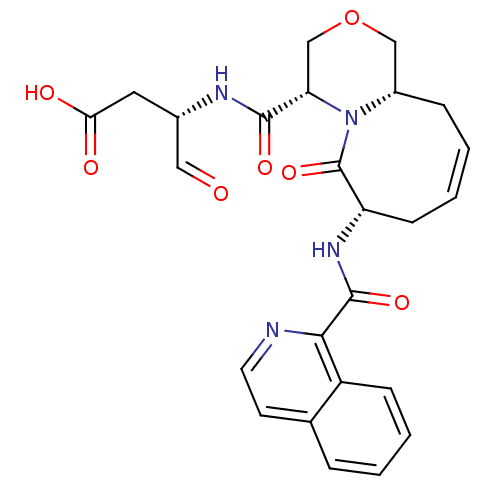
((S)-3-((4S,7S,11aS,Z)-7-(isoquinoline-1-carboxamid...)Show SMILES OC(=O)C[C@H](NC(=O)[C@@H]1COC[C@@H]2C\C=C/C[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12)C=O |c:14| Show InChI InChI=1S/C25H26N4O7/c30-12-16(11-21(31)32)27-23(33)20-14-36-13-17-6-2-4-8-19(25(35)29(17)20)28-24(34)22-18-7-3-1-5-15(18)9-10-26-22/h1-5,7,9-10,12,16-17,19-20H,6,8,11,13-14H2,(H,27,33)(H,28,34)(H,31,32)/b4-2-/t16-,17-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175333

((4S,7S,11aS)-7-(benzo[b]thiophene-2-carboxamido)-N...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2C\C=C/C[C@H](NC(=O)c3cc4ccccc4s3)C(=O)N12 |c:17| Show InChI InChI=1S/C24H25N3O7S/c28-20-10-16(24(32)34-20)26-21(29)17-12-33-11-14-6-2-3-7-15(23(31)27(14)17)25-22(30)19-9-13-5-1-4-8-18(13)35-19/h1-5,8-9,14-17,24,32H,6-7,10-12H2,(H,25,30)(H,26,29)/b3-2-/t14-,15-,16-,17-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175324
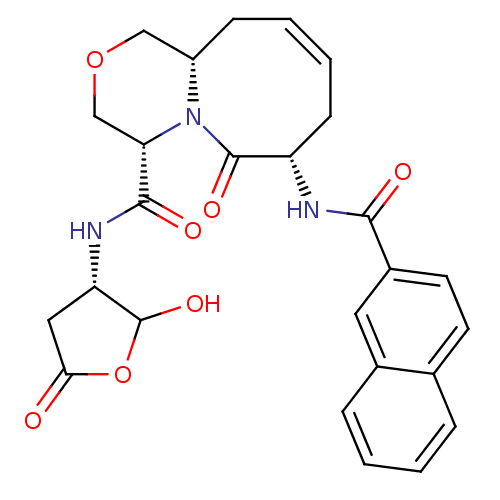
((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2C\C=C/C[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12 |c:17| Show InChI InChI=1S/C26H27N3O7/c30-22-12-20(26(34)36-22)28-24(32)21-14-35-13-18-7-3-4-8-19(25(33)29(18)21)27-23(31)17-10-9-15-5-1-2-6-16(15)11-17/h1-6,9-11,18-21,26,34H,7-8,12-14H2,(H,27,31)(H,28,32)/b4-3-/t18-,19-,20-,21-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175342

((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2C\C=C/C[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12 |c:17| Show InChI InChI=1S/C25H26N4O7/c30-20-11-18(25(34)36-20)28-22(31)19-13-35-12-15-6-2-4-8-17(24(33)29(15)19)27-23(32)21-16-7-3-1-5-14(16)9-10-26-21/h1-5,7,9-10,15,17-19,25,34H,6,8,11-13H2,(H,27,32)(H,28,31)/b4-2-/t15-,17-,18-,19-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193748

(1-(3-(trifluoromethyl)benzyl)-3-(4-methoxyphenyl)-...)Show SMILES COc1ccc(cc1)-c1c(C(=O)NS(=O)(=O)c2ccccc2)n(Cc2cccc(c2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C30H23F3N2O4S/c1-39-23-16-14-21(15-17-23)27-25-12-5-6-13-26(25)35(19-20-8-7-9-22(18-20)30(31,32)33)28(27)29(36)34-40(37,38)24-10-3-2-4-11-24/h2-18H,19H2,1H3,(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50255775

(CHEMBL4079080)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1ccn(n1)-c1ccc(F)cc1)C(=O)O[C@H](CO)C(F)(F)F |r| Show InChI InChI=1S/C18H17F4N3O3/c19-10-1-3-11(4-2-10)25-6-5-14(23-25)16-12-7-24(8-13(12)16)17(27)28-15(9-26)18(20,21)22/h1-6,12-13,15-16,26H,7-9H2/t12-,13+,15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 61: 3008-3026 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00070
BindingDB Entry DOI: 10.7270/Q2S46VD4 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199759
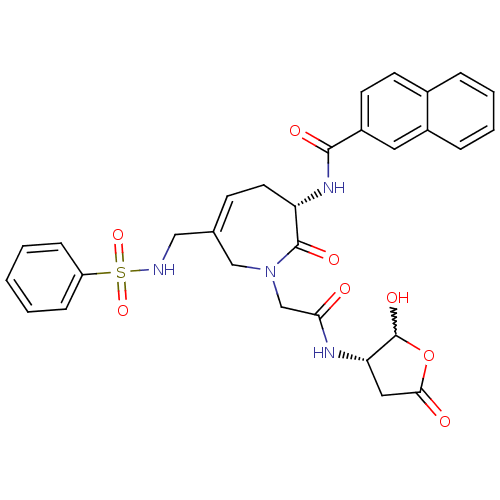
(CHEMBL411759 | N-((S,E)-1-(2-((3S)-2-hydroxy-5-oxo...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)CN1CC(CNS(=O)(=O)c2ccccc2)=CC[C@H](NC(=O)c2ccc3ccccc3c2)C1=O |w:1.0,c:26| Show InChI InChI=1S/C30H30N4O8S/c35-26(32-25-15-27(36)42-30(25)39)18-34-17-19(16-31-43(40,41)23-8-2-1-3-9-23)10-13-24(29(34)38)33-28(37)22-12-11-20-6-4-5-7-21(20)14-22/h1-12,14,24-25,30-31,39H,13,15-18H2,(H,32,35)(H,33,37)/t24-,25-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50189360

((1S,10S)-9-[(Isoquinoline-1-carbonyl)-amino]-6,10-...)Show SMILES CCO[C@@H]1OC(=O)C[C@@H]1NC(=O)[C@@H]1CCCN2N1C(=O)[C@H](CCC2=O)NC(=O)c1nccc2ccccc12 Show InChI InChI=1S/C26H29N5O7/c1-2-37-26-18(14-21(33)38-26)29-23(34)19-8-5-13-30-20(32)10-9-17(25(36)31(19)30)28-24(35)22-16-7-4-3-6-15(16)11-12-27-22/h3-4,6-7,11-12,17-19,26H,2,5,8-10,13-14H2,1H3,(H,28,35)(H,29,34)/t17-,18-,19-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 16: 4233-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.076
BindingDB Entry DOI: 10.7270/Q20001RW |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50189360

((1S,10S)-9-[(Isoquinoline-1-carbonyl)-amino]-6,10-...)Show SMILES CCO[C@@H]1OC(=O)C[C@@H]1NC(=O)[C@@H]1CCCN2N1C(=O)[C@H](CCC2=O)NC(=O)c1nccc2ccccc12 Show InChI InChI=1S/C26H29N5O7/c1-2-37-26-18(14-21(33)38-26)29-23(34)19-8-5-13-30-20(32)10-9-17(25(36)31(19)30)28-24(35)22-16-7-4-3-6-15(16)11-12-27-22/h3-4,6-7,11-12,17-19,26H,2,5,8-10,13-14H2,1H3,(H,28,35)(H,29,34)/t17-,18-,19-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem Lett 16: 4728-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.016
BindingDB Entry DOI: 10.7270/Q26T0NFF |
More data for this
Ligand-Target Pair | |
Caspase-8
(Homo sapiens (Human)) | BDBM50175337

((4S,7S,11aS)-benzyl 4-(((3S)-2-hydroxy-5-oxo-tetra...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2C\C=C/C[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12)C(=O)OCc1ccccc1 |c:17| Show InChI InChI=1S/C33H33N5O8/c39-27-16-25(32(43)46-27)36-29(40)26-18-37(33(44)45-19-20-8-2-1-3-9-20)17-22-11-5-7-13-24(31(42)38(22)26)35-30(41)28-23-12-6-4-10-21(23)14-15-34-28/h1-10,12,14-15,22,24-26,32,43H,11,13,16-19H2,(H,35,41)(H,36,40)/b7-5-/t22-,24-,25-,26-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 8 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199769
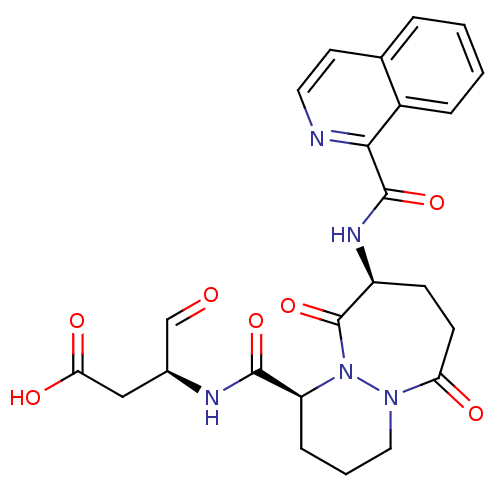
((S)-3-((1S,9S)-9-(isoquinoline-1-carboxamido)-6,10...)Show SMILES OC(=O)C[C@H](NC(=O)[C@@H]1CCCN2N1C(=O)[C@H](CCC2=O)NC(=O)c1nccc2ccccc12)C=O Show InChI InChI=1S/C24H25N5O7/c30-13-15(12-20(32)33)26-22(34)18-6-3-11-28-19(31)8-7-17(24(36)29(18)28)27-23(35)21-16-5-2-1-4-14(16)9-10-25-21/h1-2,4-5,9-10,13,15,17-18H,3,6-8,11-12H2,(H,26,34)(H,27,35)(H,32,33)/t15-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199755
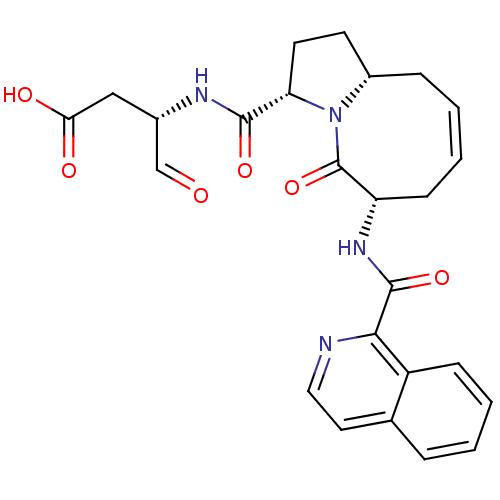
((S)-3-((3S,6S,10aR,Z)-6-(isoquinoline-1-carboxamid...)Show SMILES OC(=O)C[C@H](NC(=O)[C@@H]1CC[C@@H]2C\C=C/C[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12)C=O |c:13| Show InChI InChI=1S/C25H26N4O6/c30-14-16(13-21(31)32)27-23(33)20-10-9-17-6-2-4-8-19(25(35)29(17)20)28-24(34)22-18-7-3-1-5-15(18)11-12-26-22/h1-5,7,11-12,14,16-17,19-20H,6,8-10,13H2,(H,27,33)(H,28,34)(H,31,32)/b4-2-/t16-,17-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50191852

(2-(5-(4-fluoro-3-methylphenyl)-4-(4-(4-methyl-1H-i...)Show SMILES Cc1nc[nH]c1C1CCN(CC1)c1nc(CC#N)ncc1-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H23FN6/c1-14-11-17(3-4-19(14)23)18-12-25-20(5-8-24)28-22(18)29-9-6-16(7-10-29)21-15(2)26-13-27-21/h3-4,11-13,16H,5-7,9-10H2,1-2H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human NHE1 expressed in Ap1 cell line |
Bioorg Med Chem Lett 16: 4796-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.077
BindingDB Entry DOI: 10.7270/Q2DN44P1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175330
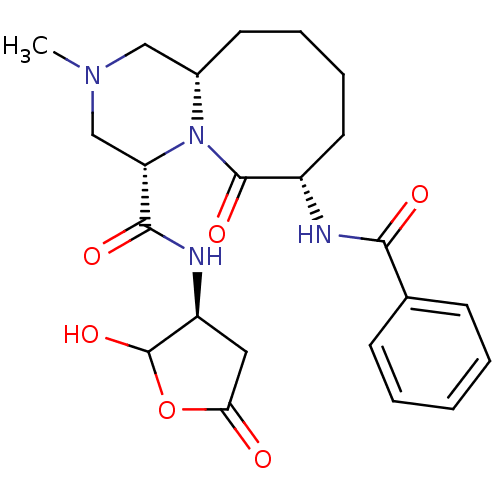
((4S,7S,11aS)-7-benzamido-N-((3S)-2-hydroxy-5-oxo-t...)Show SMILES CN1C[C@@H]2CCCC[C@H](NC(=O)c3ccccc3)C(=O)N2[C@@H](C1)C(=O)N[C@H]1CC(=O)OC1O Show InChI InChI=1S/C23H30N4O6/c1-26-12-15-9-5-6-10-16(24-20(29)14-7-3-2-4-8-14)22(31)27(15)18(13-26)21(30)25-17-11-19(28)33-23(17)32/h2-4,7-8,15-18,23,32H,5-6,9-13H2,1H3,(H,24,29)(H,25,30)/t15-,16-,17-,18-,23?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175341
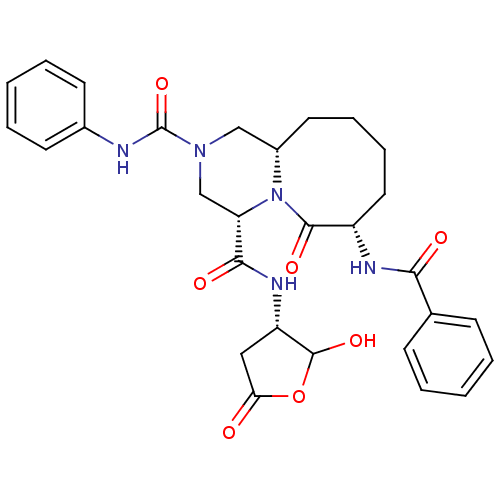
((4S,7S,11aS)-7-benzamido-N4-((3S)-2-hydroxy-5-oxo-...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2CCCC[C@H](NC(=O)c3ccccc3)C(=O)N12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C29H33N5O7/c35-24-15-22(28(39)41-24)32-26(37)23-17-33(29(40)30-19-11-5-2-6-12-19)16-20-13-7-8-14-21(27(38)34(20)23)31-25(36)18-9-3-1-4-10-18/h1-6,9-12,20-23,28,39H,7-8,13-17H2,(H,30,40)(H,31,36)(H,32,37)/t20-,21-,22-,23-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-8
(Homo sapiens (Human)) | BDBM50175323

((4S,7S,11aS)-benzyl 4-(((3S)-2-hydroxy-5-oxo-tetra...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2C\C=C/C[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12)C(=O)OCc1ccccc1 |c:17| Show InChI InChI=1S/C34H34N4O8/c39-29-17-27(33(43)46-29)36-31(41)28-19-37(34(44)45-20-21-8-2-1-3-9-21)18-25-12-6-7-13-26(32(42)38(25)28)35-30(40)24-15-14-22-10-4-5-11-23(22)16-24/h1-11,14-16,25-28,33,43H,12-13,17-20H2,(H,35,40)(H,36,41)/b7-6-/t25-,26-,27-,28-,33?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 8 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50191839
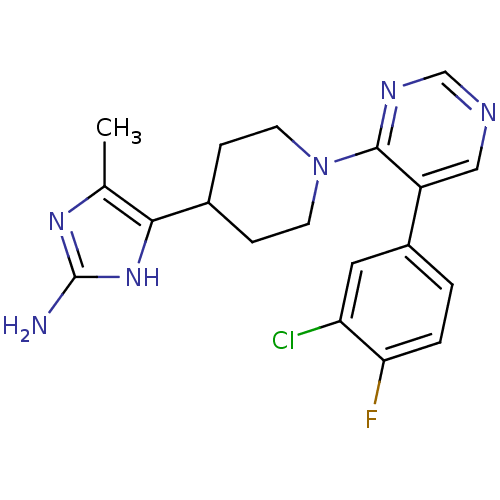
(5-(1-(5-(3-chloro-4-fluorophenyl)pyrimidin-4-yl)pi...)Show SMILES Cc1nc(N)[nH]c1C1CCN(CC1)c1ncncc1-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C19H20ClFN6/c1-11-17(26-19(22)25-11)12-4-6-27(7-5-12)18-14(9-23-10-24-18)13-2-3-16(21)15(20)8-13/h2-3,8-10,12H,4-7H2,1H3,(H3,22,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human NHE1 expressed in Ap1 cell line |
Bioorg Med Chem Lett 16: 4796-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.077
BindingDB Entry DOI: 10.7270/Q2DN44P1 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
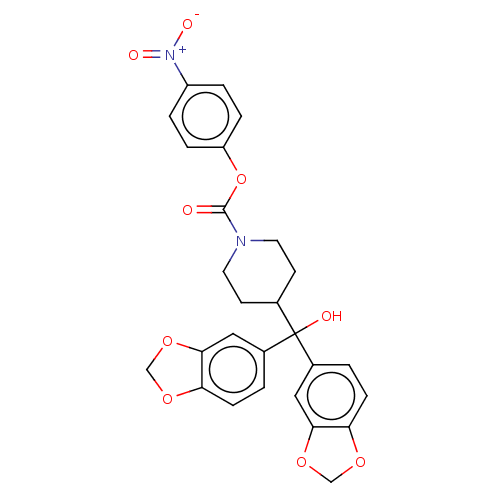
(Homo sapiens (Human)) | BDBM60622
(BDBM50300355 | US11753371, Compound JZL-184 | US91...)Show SMILES OC(C1CCN(CC1)C(=O)Oc1ccc(cc1)[N+]([O-])=O)(c1ccc2OCOc2c1)c1ccc2OCOc2c1 Show InChI InChI=1S/C27H24N2O9/c30-26(38-21-5-3-20(4-6-21)29(32)33)28-11-9-17(10-12-28)27(31,18-1-7-22-24(13-18)36-15-34-22)19-2-8-23-25(14-19)37-16-35-23/h1-8,13-14,17,31H,9-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 61: 3008-3026 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00070
BindingDB Entry DOI: 10.7270/Q2S46VD4 |
More data for this
Ligand-Target Pair | |
Caspase-1
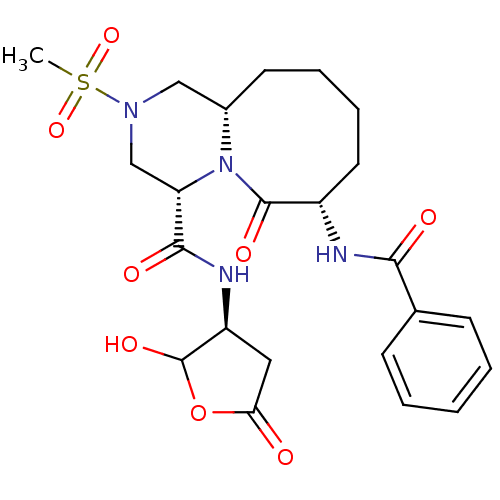
(Homo sapiens (Human)) | BDBM50175339
((4S,7S,11aS)-7-benzamido-N-((3S)-2-hydroxy-5-oxo-t...)Show SMILES CS(=O)(=O)N1C[C@@H]2CCCC[C@H](NC(=O)c3ccccc3)C(=O)N2[C@@H](C1)C(=O)N[C@H]1CC(=O)OC1O Show InChI InChI=1S/C23H30N4O8S/c1-36(33,34)26-12-15-9-5-6-10-16(24-20(29)14-7-3-2-4-8-14)22(31)27(15)18(13-26)21(30)25-17-11-19(28)35-23(17)32/h2-4,7-8,15-18,23,32H,5-6,9-13H2,1H3,(H,24,29)(H,25,30)/t15-,16-,17-,18-,23?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
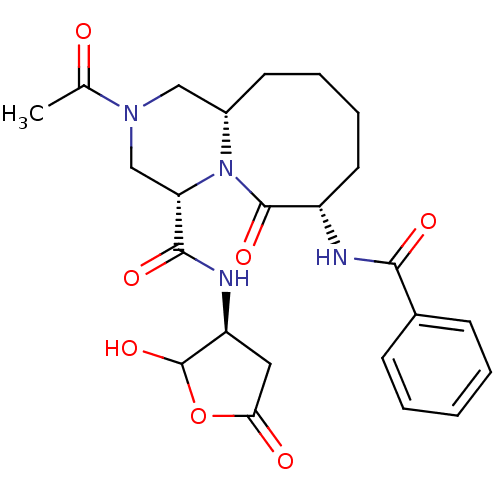
(Homo sapiens (Human)) | BDBM50175332
((4S,7S,11aS)-2-acetyl-7-benzamido-N-((3S)-2-hydrox...)Show SMILES CC(=O)N1C[C@@H]2CCCC[C@H](NC(=O)c3ccccc3)C(=O)N2[C@@H](C1)C(=O)N[C@H]1CC(=O)OC1O Show InChI InChI=1S/C24H30N4O7/c1-14(29)27-12-16-9-5-6-10-17(25-21(31)15-7-3-2-4-8-15)23(33)28(16)19(13-27)22(32)26-18-11-20(30)35-24(18)34/h2-4,7-8,16-19,24,34H,5-6,9-13H2,1H3,(H,25,31)(H,26,32)/t16-,17-,18-,19-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50255806
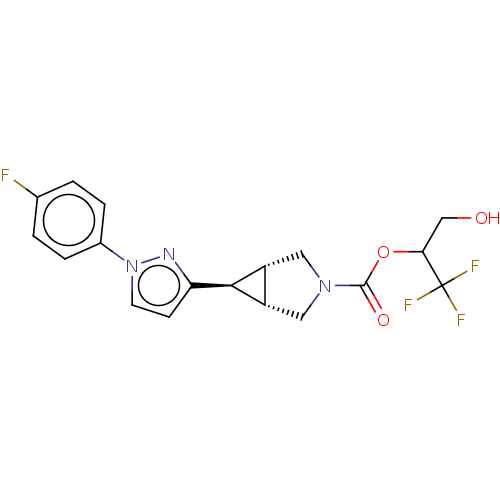
(CHEMBL4097100)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1ccn(n1)-c1ccc(F)cc1)C(=O)OC(CO)C(F)(F)F |r| Show InChI InChI=1S/C18H17F4N3O3/c19-10-1-3-11(4-2-10)25-6-5-14(23-25)16-12-7-24(8-13(12)16)17(27)28-15(9-26)18(20,21)22/h1-6,12-13,15-16,26H,7-9H2/t12-,13+,15?,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 61: 3008-3026 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00070
BindingDB Entry DOI: 10.7270/Q2S46VD4 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 1
(Homo sapiens (Human)) | BDBM50191831
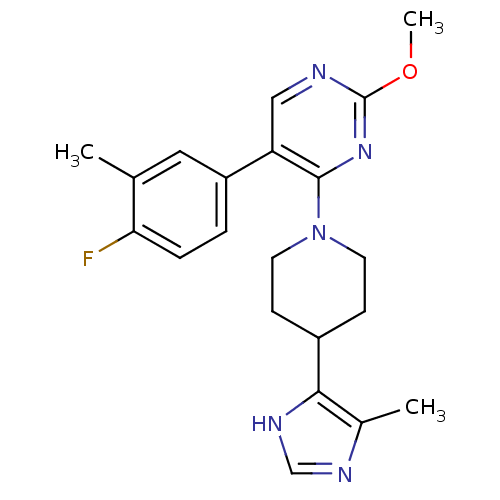
(5-(4-fluoro-3-methylphenyl)-2-methoxy-4-(4-(4-meth...)Show SMILES COc1ncc(-c2ccc(F)c(C)c2)c(n1)N1CCC(CC1)c1[nH]cnc1C Show InChI InChI=1S/C21H24FN5O/c1-13-10-16(4-5-18(13)22)17-11-23-21(28-3)26-20(17)27-8-6-15(7-9-27)19-14(2)24-12-25-19/h4-5,10-12,15H,6-9H2,1-3H3,(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human NHE1 expressed in Ap1 cell line |
Bioorg Med Chem Lett 16: 4796-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.077
BindingDB Entry DOI: 10.7270/Q2DN44P1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193744

(1-(3-(trifluoromethyl)benzyl)-N-(4-fluorophenylsul...)Show SMILES COc1ccc(cc1)-c1c(C(=O)NS(=O)(=O)c2ccc(F)cc2)n(Cc2cccc(c2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C30H22F4N2O4S/c1-40-23-13-9-20(10-14-23)27-25-7-2-3-8-26(25)36(18-19-5-4-6-21(17-19)30(32,33)34)28(27)29(37)35-41(38,39)24-15-11-22(31)12-16-24/h2-17H,18H2,1H3,(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193751
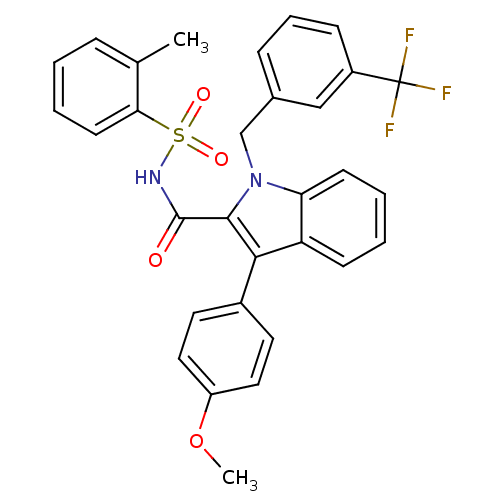
(1-(3-(trifluoromethyl)benzyl)-3-(4-methoxyphenyl)-...)Show SMILES COc1ccc(cc1)-c1c(C(=O)NS(=O)(=O)c2ccccc2C)n(Cc2cccc(c2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C31H25F3N2O4S/c1-20-8-3-6-13-27(20)41(38,39)35-30(37)29-28(22-14-16-24(40-2)17-15-22)25-11-4-5-12-26(25)36(29)19-21-9-7-10-23(18-21)31(32,33)34/h3-18H,19H2,1-2H3,(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data