

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50095493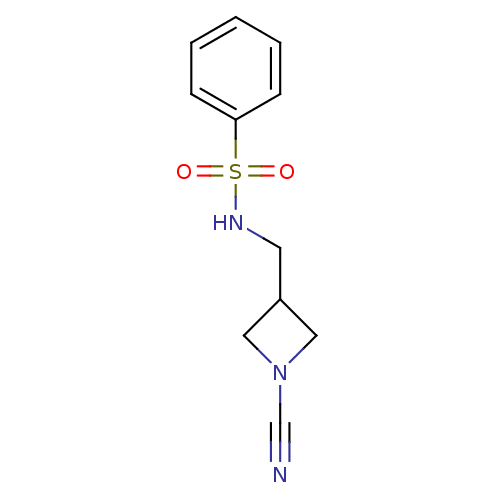 (CHEMBL276169 | N-(1-Cyano-azetidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095489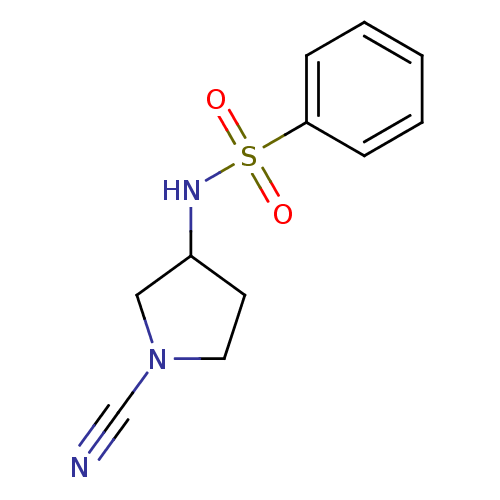 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491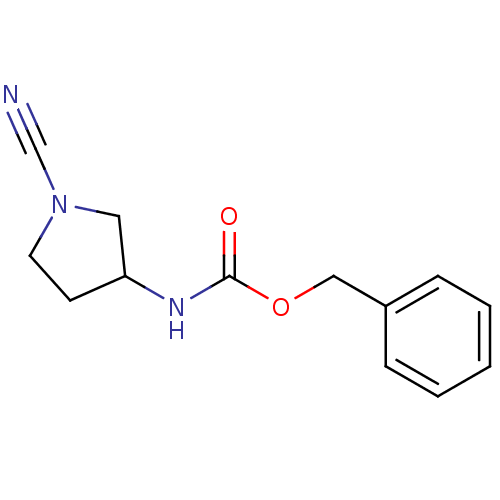 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488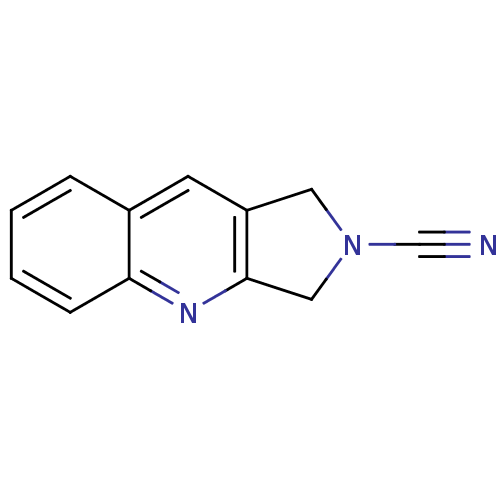 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19908 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-methyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | 17 | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 3146-51 (2007) Article DOI: 10.1016/j.bmcl.2007.03.028 BindingDB Entry DOI: 10.7270/Q21834SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50255925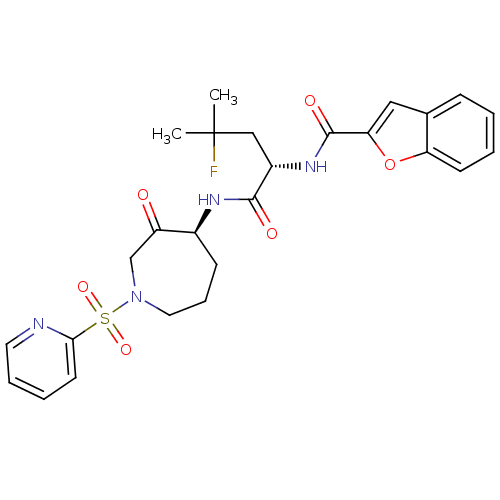 (CHEMBL474438 | N-((S)-4-fluoro-4-methyl-1-oxo-1-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 19: 675-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.053 BindingDB Entry DOI: 10.7270/Q21V5DVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306304 ((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50255753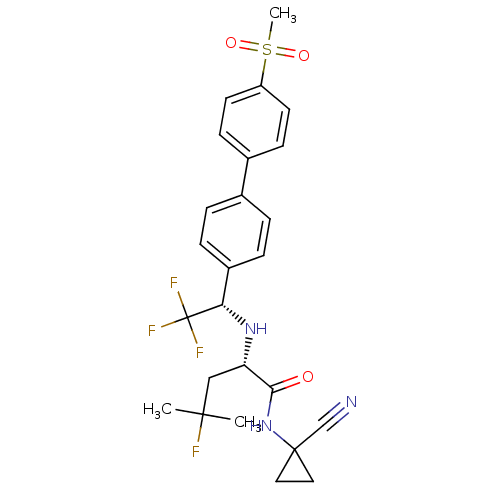 (CHEMBL481611 | MK-0822 | Odanacatib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM50118514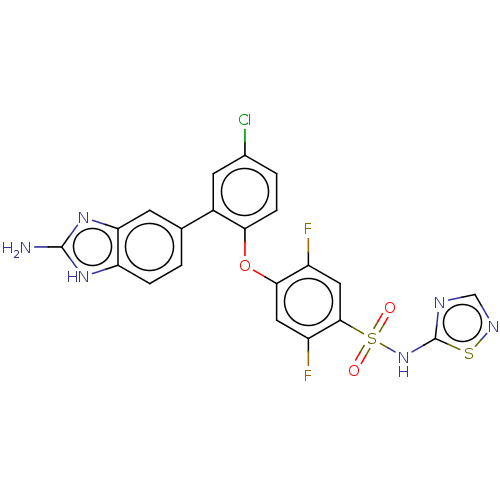 (CHEMBL3617063 | US9481677, 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human Nav1.2 channel expressed in HEK cells co-expressing human sodium channel subunit beta1 at holding potential -35 mV by... | ACS Med Chem Lett 7: 277-82 (2016) Article DOI: 10.1021/acsmedchemlett.5b00447 BindingDB Entry DOI: 10.7270/Q2J1052W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306306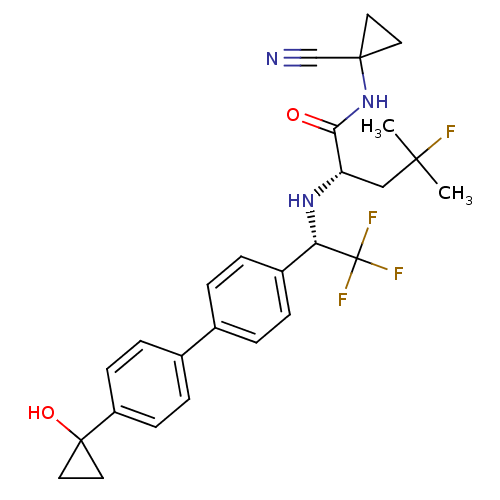 ((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306307 ((S)-2-((S)-1-(4'-((S)-1-amino-1-oxopropan-2-yl)bip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50255753 (CHEMBL481611 | MK-0822 | Odanacatib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 19: 675-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.053 BindingDB Entry DOI: 10.7270/Q21V5DVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50255753 (CHEMBL481611 | MK-0822 | Odanacatib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50255753 (CHEMBL481611 | MK-0822 | Odanacatib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19489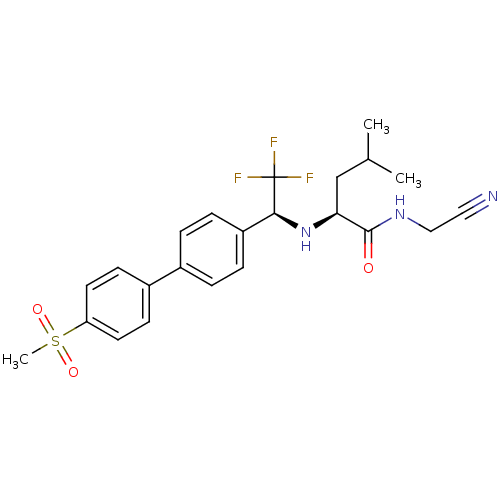 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306309 (1-(4'-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM254126 (US9481677, 98) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
XENON PHARMACEUTICALS INC. US Patent | Assay Description Patch voltage clamp electrophysiology allows for the direct measurement and quantification of block of voltage-gated sodium channels (Nav's), and... | US Patent US9481677 (2016) BindingDB Entry DOI: 10.7270/Q20V8BQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50233032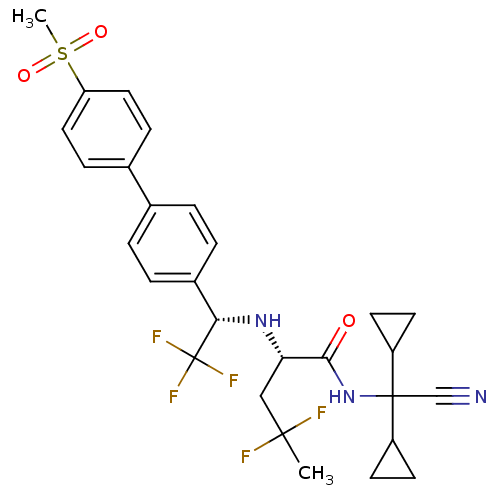 ((S)-4,4-difluoro-2-[(S)-2,2,2-trifluoro-1-(4'-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19908 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-methyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | 17 | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 3146-51 (2007) Article DOI: 10.1016/j.bmcl.2007.03.028 BindingDB Entry DOI: 10.7270/Q21834SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Mus musculus) | BDBM50169425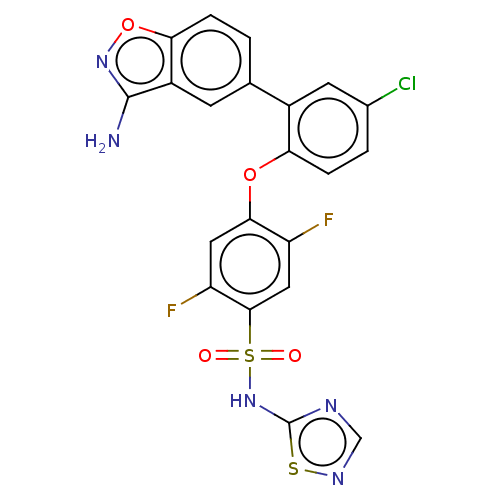 (CHEMBL3341983 | US9481677, 44) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 channel in mouse CAD cells by whole-cell patch clamp assay | ACS Med Chem Lett 7: 277-82 (2016) Article DOI: 10.1021/acsmedchemlett.5b00447 BindingDB Entry DOI: 10.7270/Q2J1052W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19847 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-[4-(meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 49: 1066-79 (2006) Article DOI: 10.1021/jm051059p BindingDB Entry DOI: 10.7270/Q2DF6PH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50233036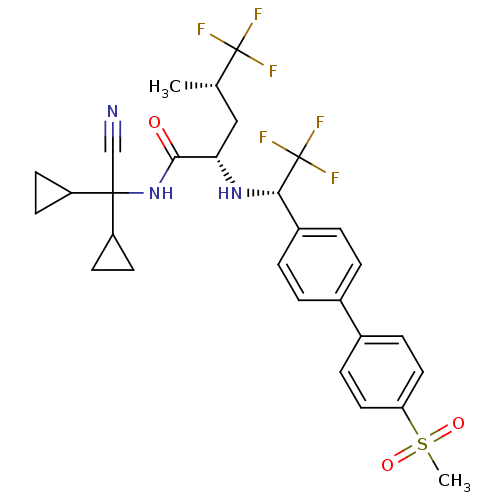 ((2S,4S)-5,5,5-trifluoro-4-methyl-2-[(S)-2,2,2-trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50118514 (CHEMBL3617063 | US9481677, 38) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human Nav1.7 channel expressed in HEK cells co-expressing human sodium channel subunit beta3 at -150 mV by manual voltage c... | ACS Med Chem Lett 7: 277-82 (2016) Article DOI: 10.1021/acsmedchemlett.5b00447 BindingDB Entry DOI: 10.7270/Q2J1052W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50233039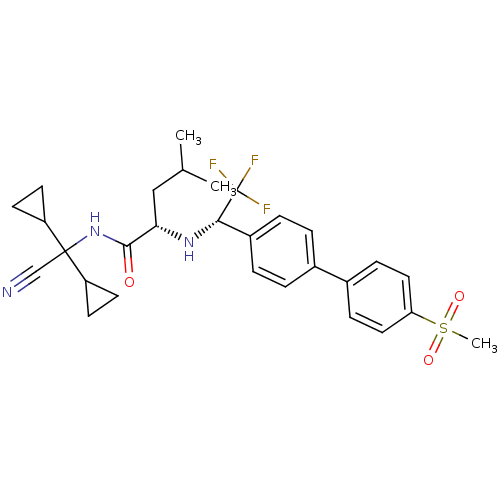 ((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306310 ((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19847 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-[4-(meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | 16 | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 3146-51 (2007) Article DOI: 10.1016/j.bmcl.2007.03.028 BindingDB Entry DOI: 10.7270/Q21834SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306305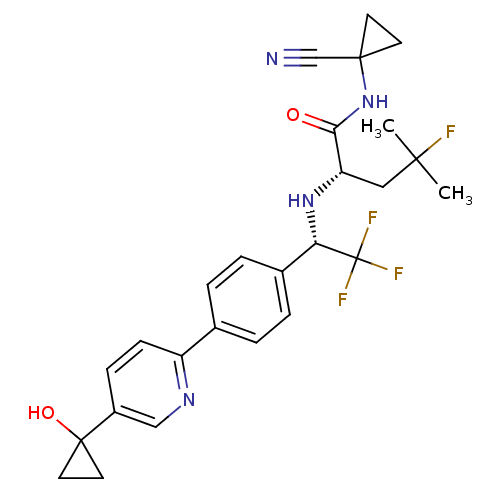 ((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19847 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-[4-(meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | 16 | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 3146-51 (2007) Article DOI: 10.1016/j.bmcl.2007.03.028 BindingDB Entry DOI: 10.7270/Q21834SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50118514 (CHEMBL3617063 | US9481677, 38) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human Nav1.7 channel expressed in HEK cells co-expressing human sodium channel subunit beta1 at holding potential -60 mV by... | ACS Med Chem Lett 7: 277-82 (2016) Article DOI: 10.1021/acsmedchemlett.5b00447 BindingDB Entry DOI: 10.7270/Q2J1052W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50118514 (CHEMBL3617063 | US9481677, 38) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
XENON PHARMACEUTICALS INC. US Patent | Assay Description Patch voltage clamp electrophysiology allows for the direct measurement and quantification of block of voltage-gated sodium channels (Nav's), and... | US Patent US9481677 (2016) BindingDB Entry DOI: 10.7270/Q20V8BQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM254114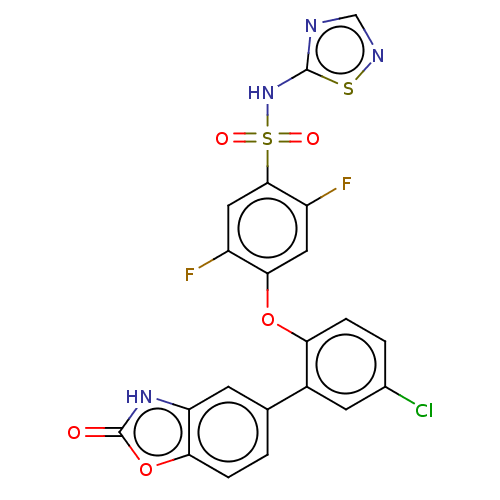 (US9481677, 52) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
XENON PHARMACEUTICALS INC. US Patent | Assay Description Patch voltage clamp electrophysiology allows for the direct measurement and quantification of block of voltage-gated sodium channels (Nav's), and... | US Patent US9481677 (2016) BindingDB Entry DOI: 10.7270/Q20V8BQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19847 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-[4-(meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rabbit cathepsin K | J Med Chem 51: 6410-20 (2008) Article DOI: 10.1021/jm800610j BindingDB Entry DOI: 10.7270/Q261105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19847 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-[4-(meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 49: 1066-79 (2006) Article DOI: 10.1021/jm051059p BindingDB Entry DOI: 10.7270/Q2DF6PH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306303 ((S)-N-(1-cyanocyclopropyl)-2-((S)-1-(4'-((S)-2,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306302 ((S)-N-(1-cyanocyclopropyl)-2-((S)-1-(4'-((R)-2,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50169425 (CHEMBL3341983 | US9481677, 44) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human Nav1.7 channel expressed in HEK cells co-expressing human sodium channel subunit beta3 at -150 mV by manual voltage c... | ACS Med Chem Lett 7: 277-82 (2016) Article DOI: 10.1021/acsmedchemlett.5b00447 BindingDB Entry DOI: 10.7270/Q2J1052W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19909 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{1-methyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | 41 | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 3146-51 (2007) Article DOI: 10.1016/j.bmcl.2007.03.028 BindingDB Entry DOI: 10.7270/Q21834SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306311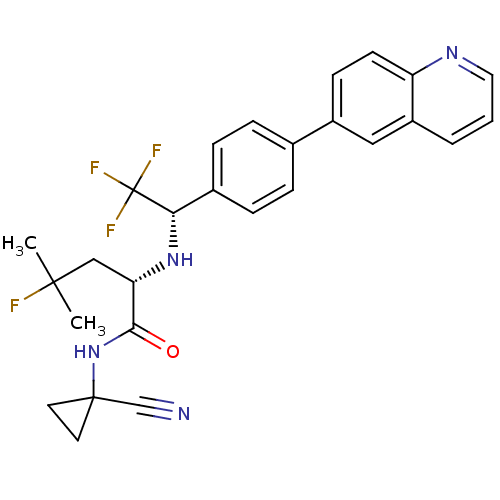 ((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50233034 ((2S,4R)-5,5,5-trifluoro-4-methyl-2-[(S)-2,2,2-trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50169425 (CHEMBL3341983 | US9481677, 44) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human Nav1.7 channel expressed in HEK cells co-expressing human sodium channel subunit beta1 at holding potential -60 mV by... | ACS Med Chem Lett 7: 277-82 (2016) Article DOI: 10.1021/acsmedchemlett.5b00447 BindingDB Entry DOI: 10.7270/Q2J1052W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19909 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{1-methyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | 41 | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 3146-51 (2007) Article DOI: 10.1016/j.bmcl.2007.03.028 BindingDB Entry DOI: 10.7270/Q21834SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19909 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{1-methyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rabbit cathepsin K | J Med Chem 51: 6410-20 (2008) Article DOI: 10.1021/jm800610j BindingDB Entry DOI: 10.7270/Q261105F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19847 ((1R,2R)-N-(cyanomethyl)-5,5-difluoro-2-{2-[4-(meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 49: 1066-79 (2006) Article DOI: 10.1021/jm051059p BindingDB Entry DOI: 10.7270/Q2DF6PH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50169426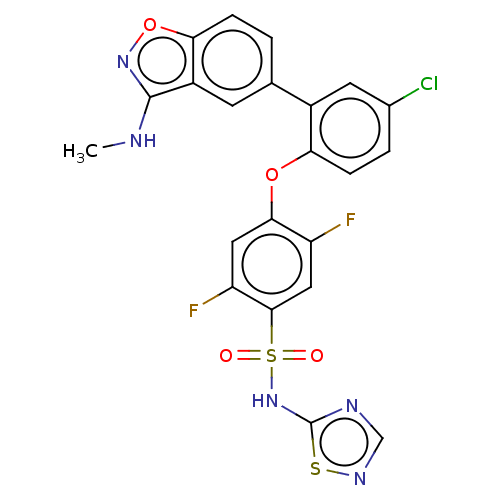 (CHEMBL3806065 | US9481677, 69) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
XENON PHARMACEUTICALS INC. US Patent | Assay Description Patch voltage clamp electrophysiology allows for the direct measurement and quantification of block of voltage-gated sodium channels (Nav's), and... | US Patent US9481677 (2016) BindingDB Entry DOI: 10.7270/Q20V8BQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50306308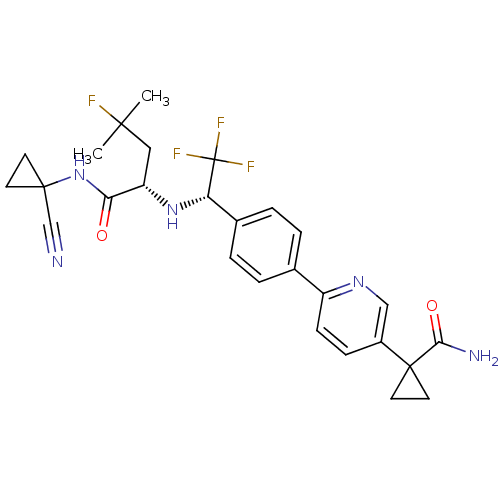 (1-(6-(4-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of humanized rabbit cathepsin K | Bioorg Med Chem Lett 20: 887-92 (2010) Article DOI: 10.1016/j.bmcl.2009.12.083 BindingDB Entry DOI: 10.7270/Q27D2V75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50169426 (CHEMBL3806065 | US9481677, 69) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human Nav1.7 channel expressed in HEK cells co-expressing human sodium channel subunit beta1 at holding potential -60 mV by... | ACS Med Chem Lett 7: 277-82 (2016) Article DOI: 10.1021/acsmedchemlett.5b00447 BindingDB Entry DOI: 10.7270/Q2J1052W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19845 ((1R,2S)-N-(cyanomethyl)-2-[(Z)-2-[4-(methylsulfany...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 49: 1066-79 (2006) Article DOI: 10.1021/jm051059p BindingDB Entry DOI: 10.7270/Q2DF6PH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rabbit cathepsin K | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1062 total ) | Next | Last >> |