

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122294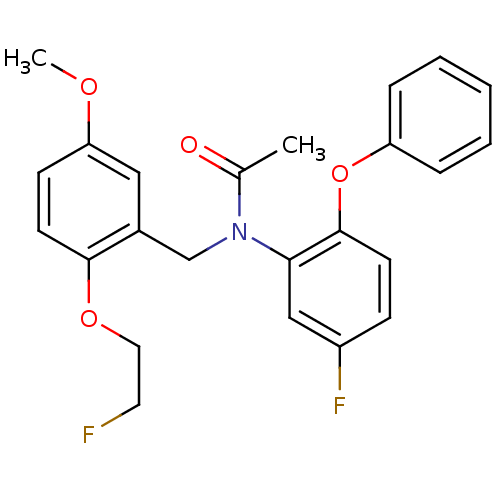 (CHEMBL292092 | N-(5-fluoro-2-phenoxyphenyl)-N-(2-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122293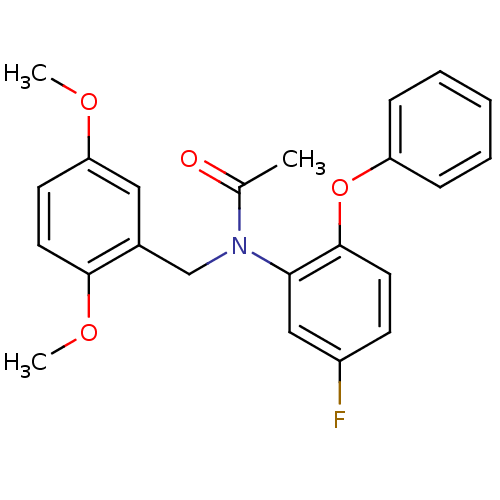 (CHEMBL401000 | CHEMBL63064 | N-(2,5-Dimethoxy-benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122295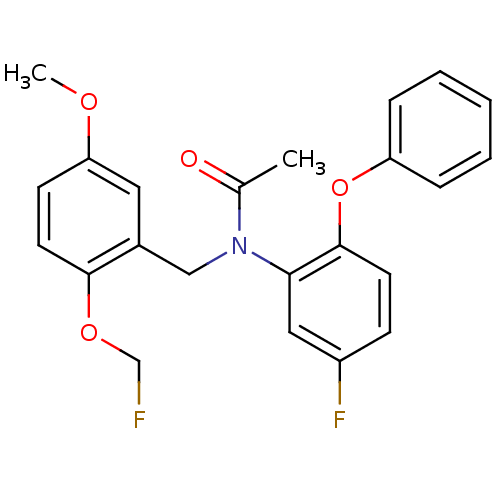 (CHEMBL63065 | N-(2-Fluoromethoxy-5-methoxy-benzyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22032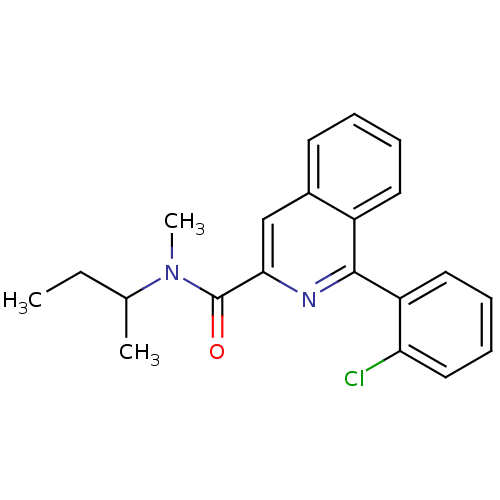 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50144889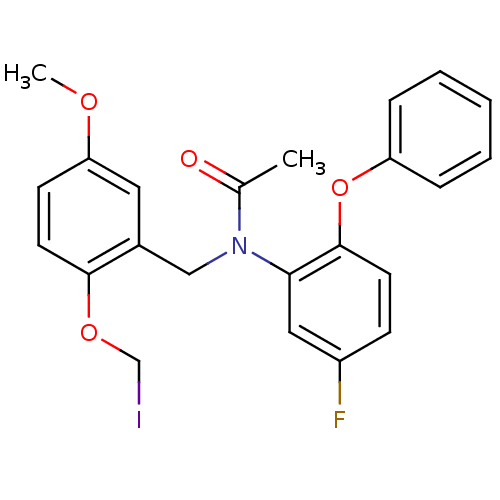 (CHEMBL72521 | N-(5-Fluoro-2-phenoxy-phenyl)-N-(2-i...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Rattus norvegicus (rat)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095778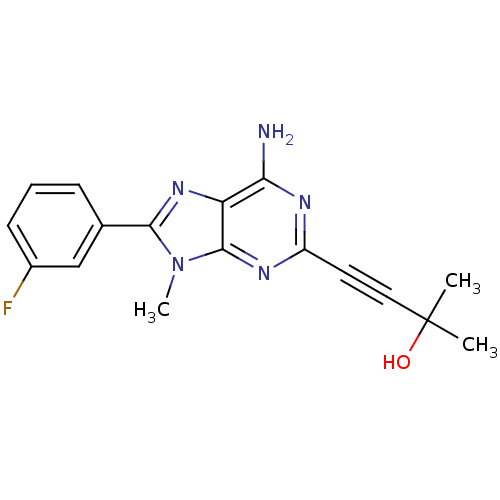 (4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095790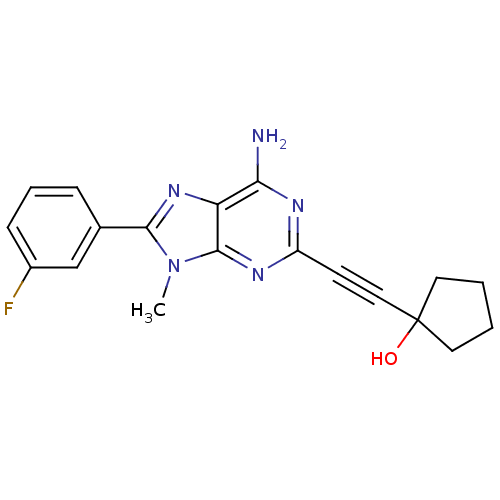 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095786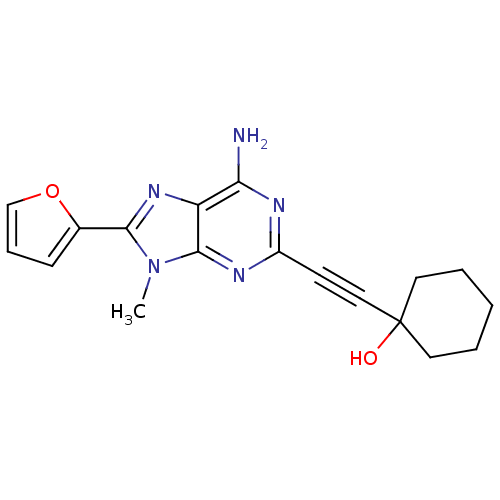 (1-(6-Amino-8-furan-2-yl-9-methyl-9H-purin-2-ylethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095784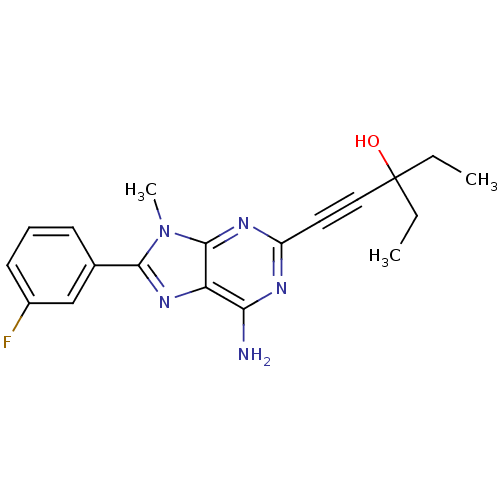 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50059376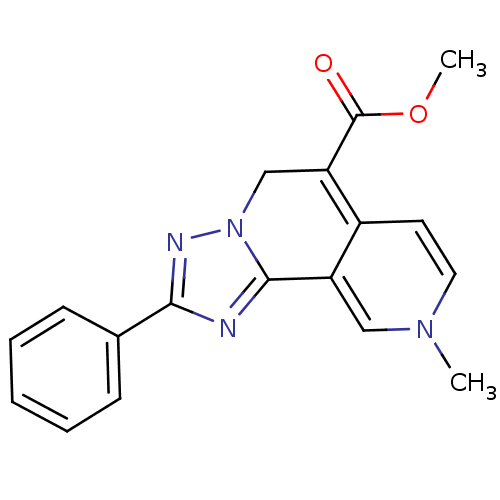 (9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095787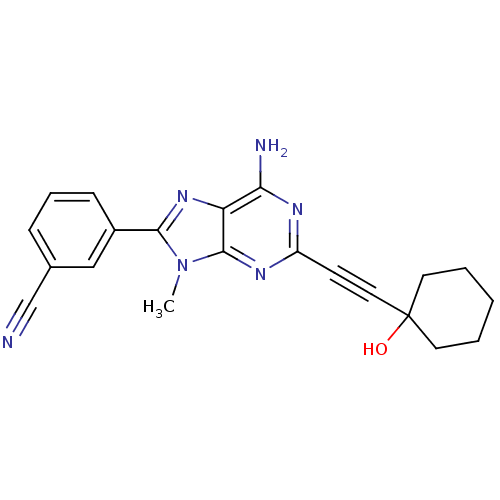 (3-[6-Amino-2-(1-hydroxy-cyclohexylethynyl)-9-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095793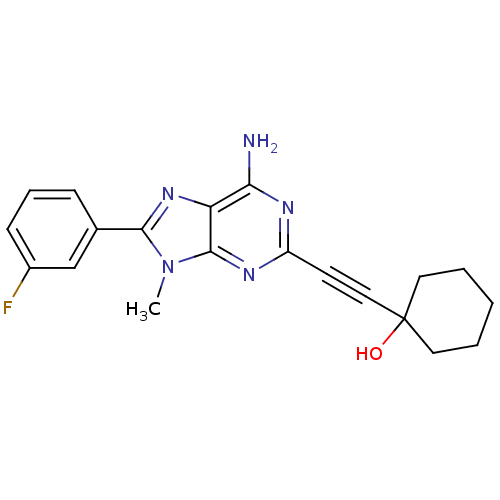 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095788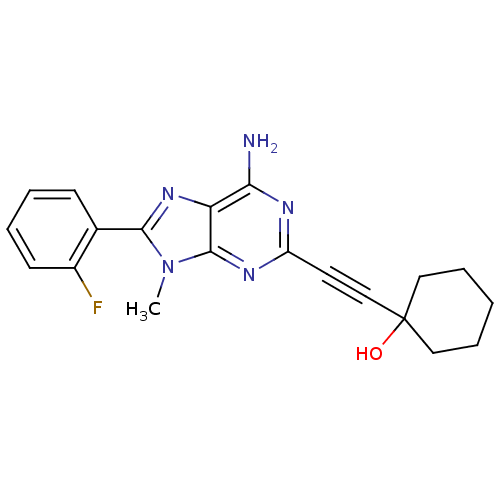 (1-[6-Amino-8-(2-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095793 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50079652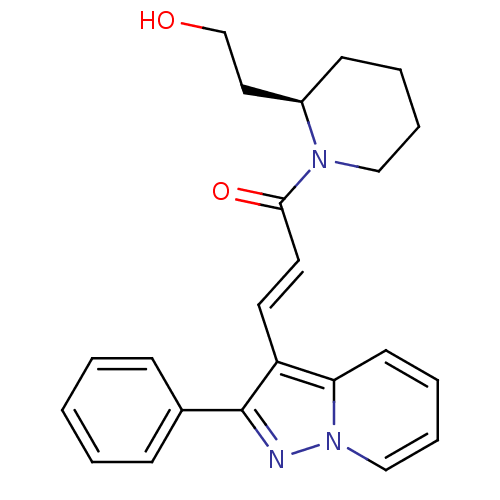 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50144887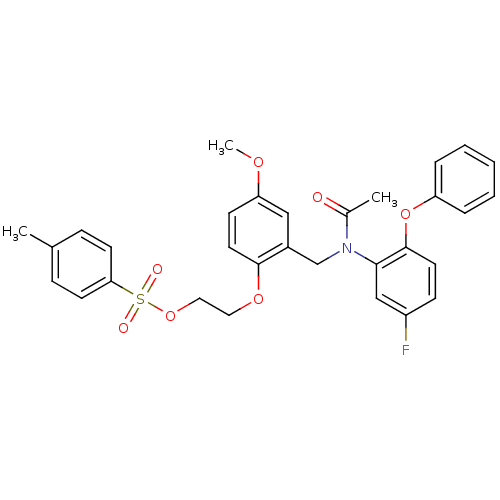 (CHEMBL443526 | Toluene-4-sulfonic acid 2-(2-{[acet...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description In vitro inhibition of [11C]2 binding to Peripheral benzodiazepine receptor (PBR) in rat brain | J Med Chem 47: 2228-35 (2004) Article DOI: 10.1021/jm0304919 BindingDB Entry DOI: 10.7270/Q20G3KXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095781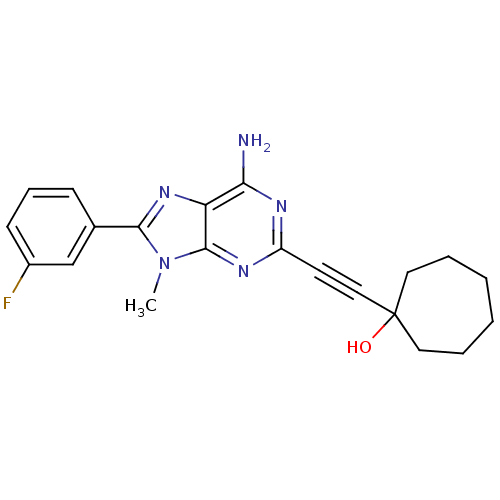 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095788 (1-[6-Amino-8-(2-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095790 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095786 (1-(6-Amino-8-furan-2-yl-9-methyl-9H-purin-2-ylethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095781 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095784 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095778 (4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17283 ((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50006710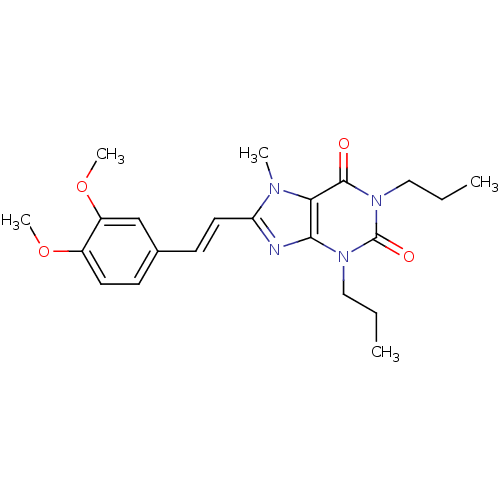 (8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095787 (3-[6-Amino-2-(1-hydroxy-cyclohexylethynyl)-9-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50095784 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50095793 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332461 ((R)-3-carba cyclic-phosphatidic acid | CHEMBL16300...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibition of recombinant ATX mediated hydrolysis of FS-3 | Bioorg Med Chem Lett 20: 7525-8 (2010) Article DOI: 10.1016/j.bmcl.2010.09.115 BindingDB Entry DOI: 10.7270/Q2XD11X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50095790 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50079652 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332460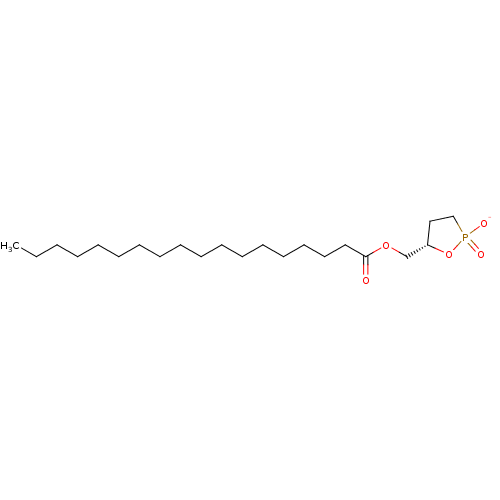 ((S)-carba cyclic-phosphatidic acid | CHEMBL1630084) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibition of recombinant ATX mediated hydrolysis of FS-3 | Bioorg Med Chem Lett 20: 7525-8 (2010) Article DOI: 10.1016/j.bmcl.2010.09.115 BindingDB Entry DOI: 10.7270/Q2XD11X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50095778 (4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50006710 (8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human thrombin by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50079652 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153106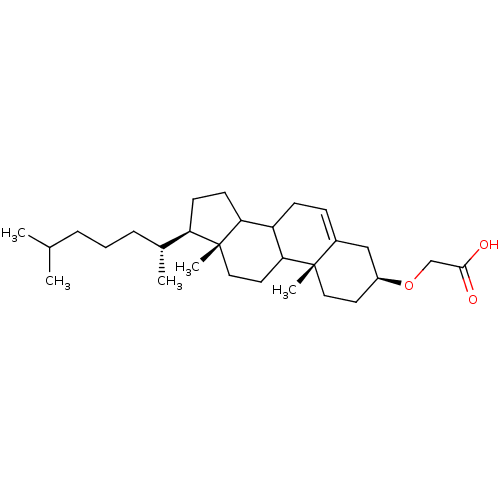 (CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50395262 (CHEMBL2163999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Teijin Pharma Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of MAPKAP-K2 using KKLNRTLSVA as substrate and [33P]-gamma-ATP by Lineweaver-Burke plot analysis | J Med Chem 55: 6700-15 (2012) Article DOI: 10.1021/jm300411k BindingDB Entry DOI: 10.7270/Q20K29PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153111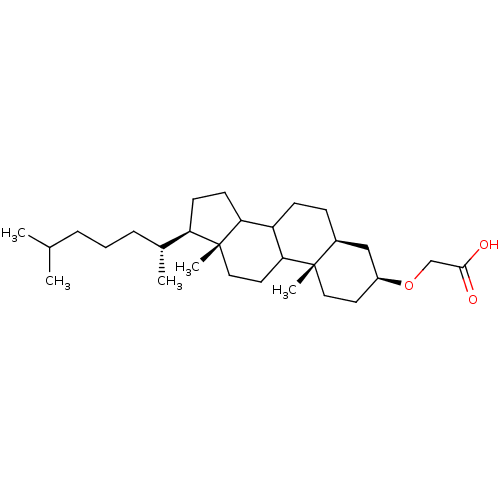 (CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50059376 (9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50006710 (8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50059376 (9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153113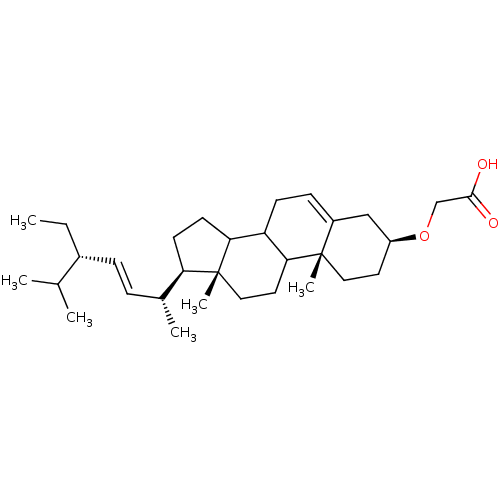 (CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153111 (CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha competitively on DNA template | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153106 (CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha competitively on DNA template | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17283 ((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant tissue plasminogen activator by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17283 ((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human plasmin by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1538 total ) | Next | Last >> |