 Found 175 hits with Last Name = 'oblin' and Initial = 'a'
Found 175 hits with Last Name = 'oblin' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(BOVINE) | BDBM81790
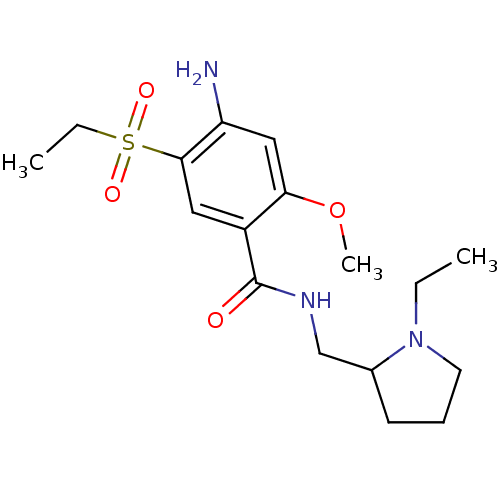
(Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...)Show InChI InChI=1S/C17H27N3O4S/c1-4-20-8-6-7-12(20)11-19-17(21)13-9-16(25(22,23)5-2)14(18)10-15(13)24-3/h9-10,12H,4-8,11,18H2,1-3H3,(H,19,21) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108
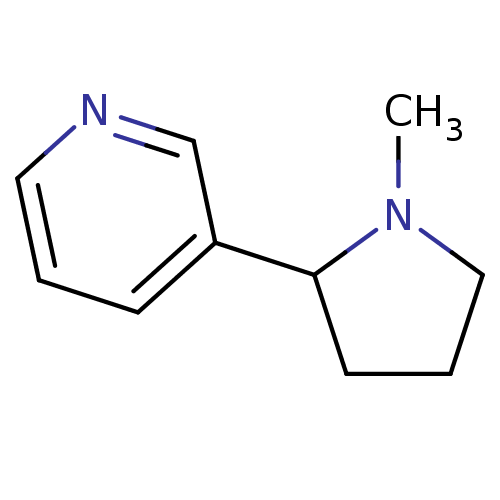
((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50000766
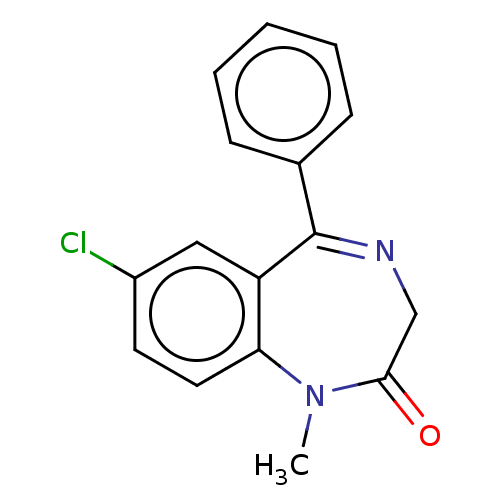
(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(BOVINE) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(PIG) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3
(RAT) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM81790

(Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...)Show InChI InChI=1S/C17H27N3O4S/c1-4-20-8-6-7-12(20)11-19-17(21)13-9-16(25(22,23)5-2)14(18)10-15(13)24-3/h9-10,12H,4-8,11,18H2,1-3H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Mus musculus (Mouse)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50170601
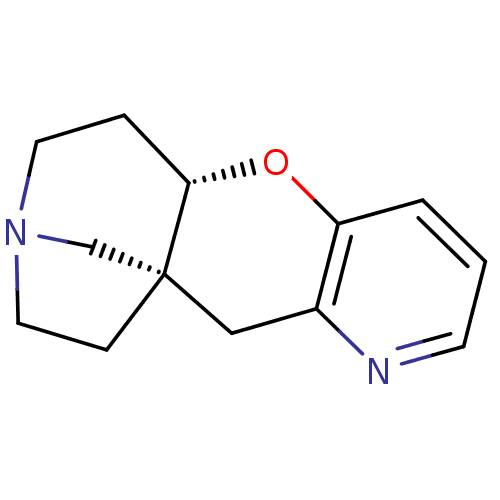
((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(RAT) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 80.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50170601

((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(BOVINE) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM81790

(Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...)Show InChI InChI=1S/C17H27N3O4S/c1-4-20-8-6-7-12(20)11-19-17(21)13-9-16(25(22,23)5-2)14(18)10-15(13)24-3/h9-10,12H,4-8,11,18H2,1-3H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine 1D receptor
(Bos taurus (Bovine)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50170601

((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM81790

(Amisulpride | CAS_71675-85-9 | NSC_2159 | US101672...)Show InChI InChI=1S/C17H27N3O4S/c1-4-20-8-6-7-12(20)11-19-17(21)13-9-16(25(22,23)5-2)14(18)10-15(13)24-3/h9-10,12H,4-8,11,18H2,1-3H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 83-97 (1997)
BindingDB Entry DOI: 10.7270/Q2NZ865X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data