

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499811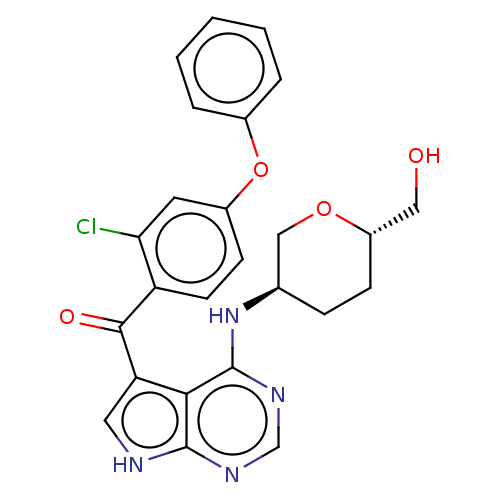 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/67/2005(H1N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501949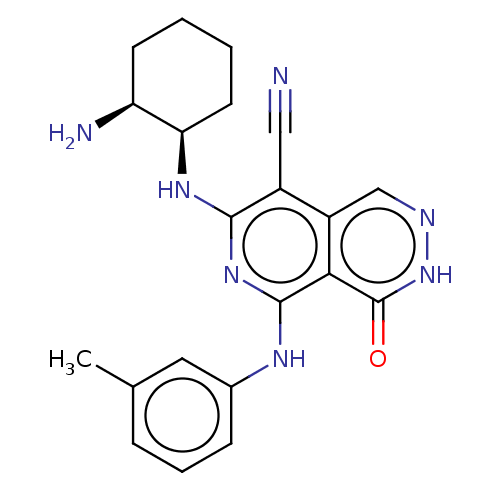 (CHEMBL4461851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499810 (N-((S)-1-((2S,5R)-5-((5-(2-chloro-4- phenoxybenzoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499814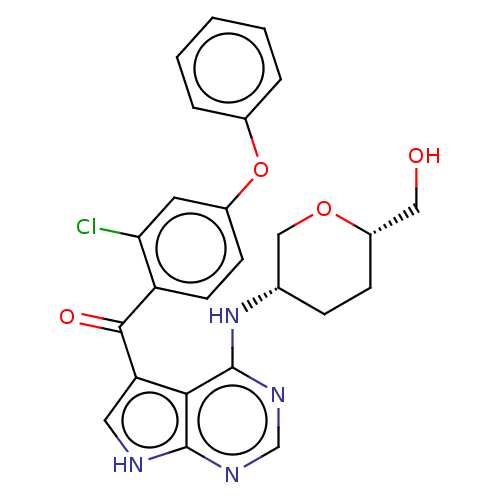 ((2-chloro-4-phenoxyphenyl)(4- (((3S,6S)-6-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/700/2007(H7N7)) neuraminidase N7 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499838 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6S)-6-((R)-1- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499976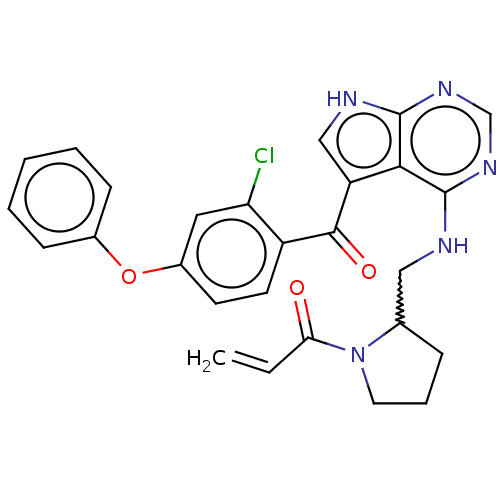 ((racemic)-1-(2-(((5-(2-chloro-4- phenoxybenzoyl)-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499924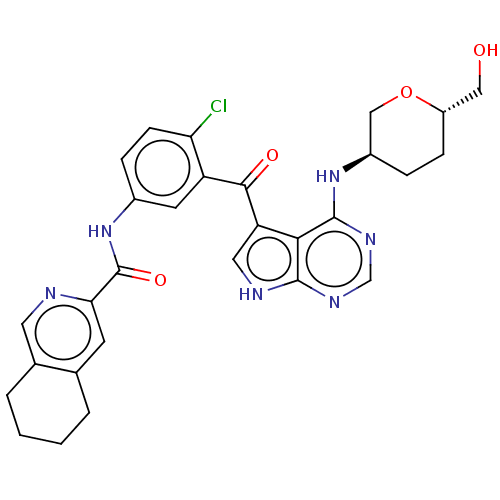 (N-(4-chloro-3-(4-(((3R,6S)-6- (hydroxymethyl)tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499869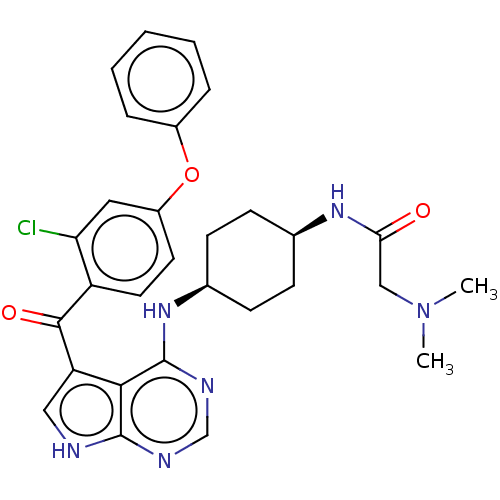 (N-(cis-4-((5-(2-chloro-4- phenoxybenzoyl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499974 ((racemic)-1-(2-(((5-(2-chloro-4- phenoxybenzoyl)-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Shiga/8/2004(H4N6)) neuraminidase N6 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499890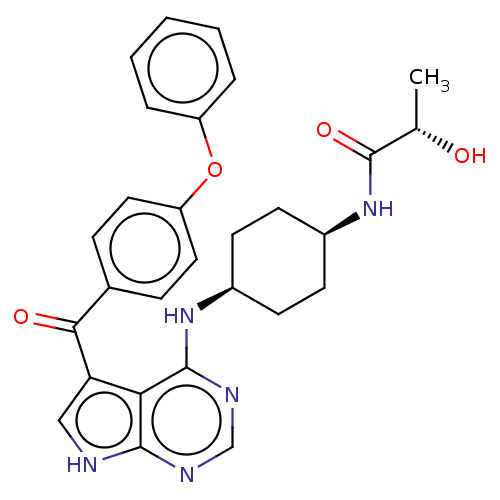 ((S)-2-hydroxy-N-(cis-4-((5-(4- phenoxybenzoyl)-7H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499991 (US11020398, Compound I-360e | racemic-cis-(2-chlor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499854 ((4-(((3R,6S)-6-((S)-1- aminoethyl)tetrahydro-2H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499892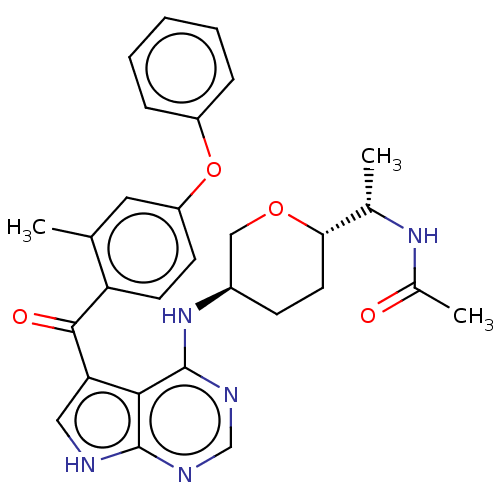 (N-((S)-1-((2S,5R)-5-((5-(2-methyl-4- phenoxybenzoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Yamaguchi/20/06(H1N1) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by ... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499881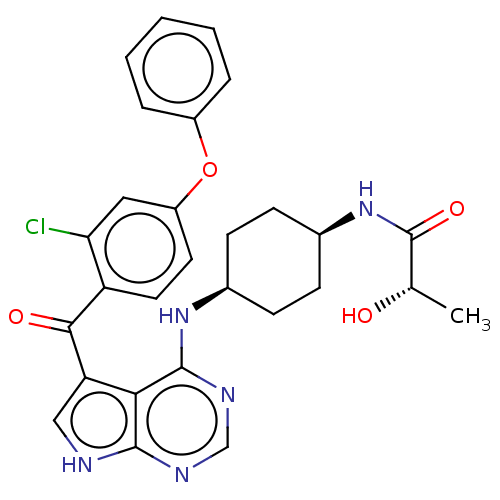 ((S)-N-(cis-4-((5-(2-chloro-4- phenoxybenzoyl)-7H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/duck/Tsukuba/441/05(H11N9) neuraminidase N9 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followe... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499839 (N-(cis-4-((5-(2-chloro-4- phenoxybenzoyl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499855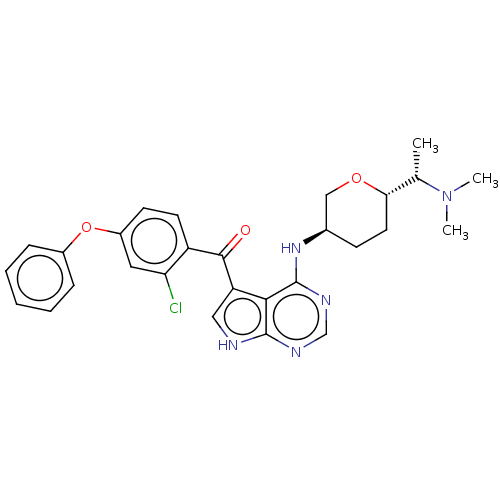 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6S)-6-((S)-1- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499888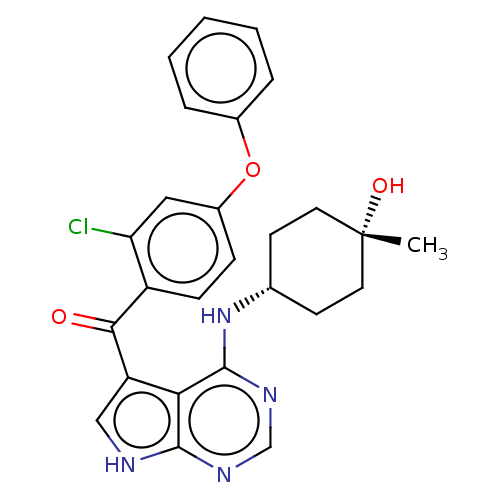 ((2-chloro-4-phenoxyphenyl)(4-((cis-4- hydroxy-4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499891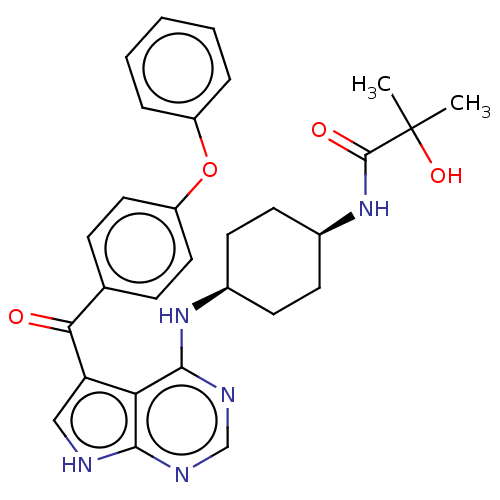 (2-hydroxy-2-methyl-N-(cis-4-((5-(4- phenoxybenzoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499882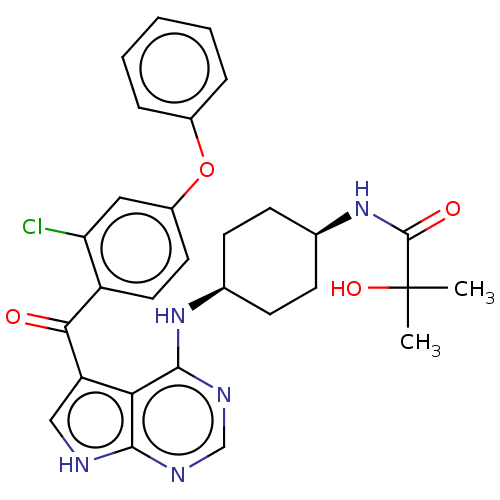 (N-(cis-4-((5-(2-chloro-4- phenoxybenzoyl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499870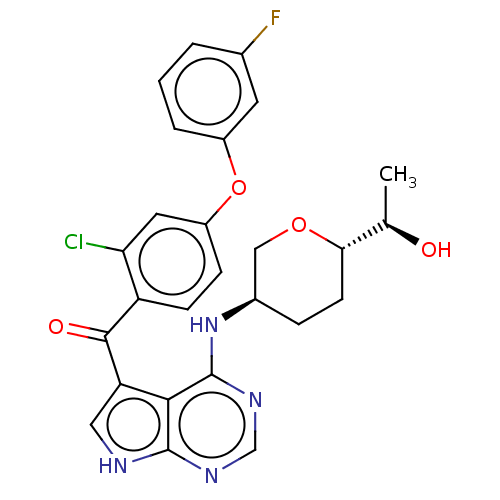 ((2-chloro-4-(3- fluorophenoxy)phenyl)(4-(((3R,6S)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499889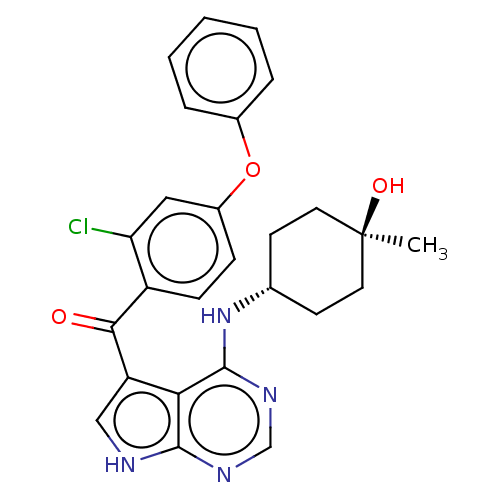 ((2-chloro-4-phenoxyphenyl)(4-((trans- 4-hydroxy-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499893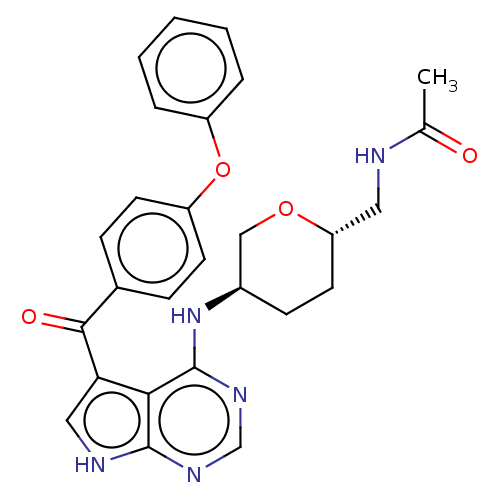 (N-(((2S,5R)-5-((5-(4-phenoxybenzoyl)- 7H-pyrrolo[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499885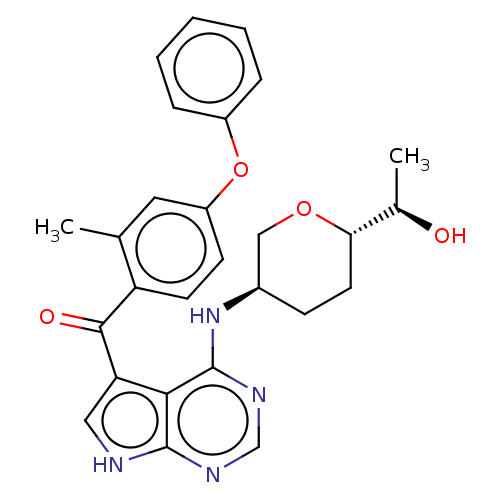 ((4-(((3R,6S)-6-((R)-1- hydroxyethyl)tetrahydro-2H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499815 (N-(((2S,5R)-5-((5-(2-chloro-4- phenoxybenzoyl)-7H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499857 ((2-chloro-4-phenoxyphenyl)(4-((trans- 4-hydroxycyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499876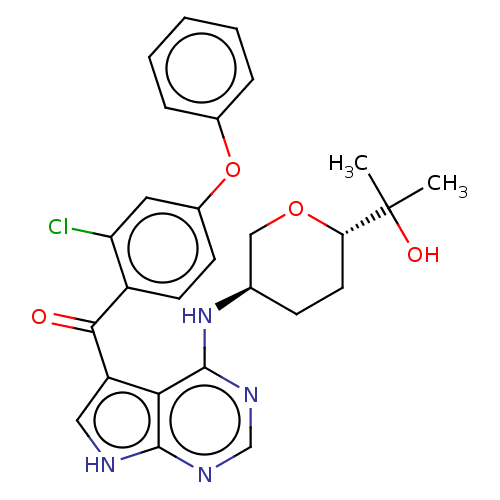 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6S)-6-(2-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/mallard/Hokkaido/24/2009(H5N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins f... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499865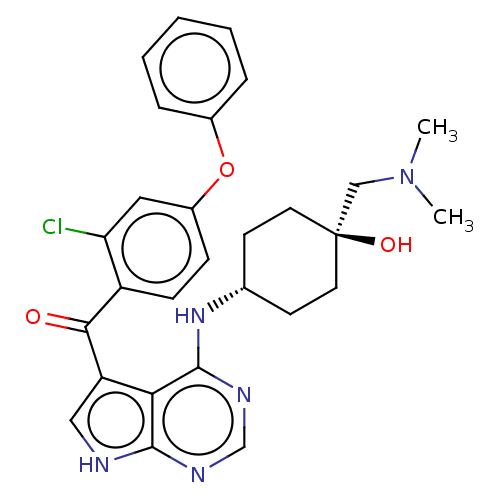 ((2-chloro-4-phenoxyphenyl)(4-((trans- 4-((dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Narita/1/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15 mins... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Aichi/102/2008(H3N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499867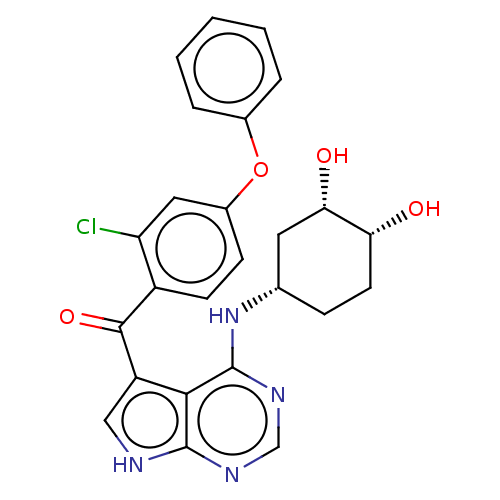 (US11020398, Compound I-179 | rac-(2-chloro-4-pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499868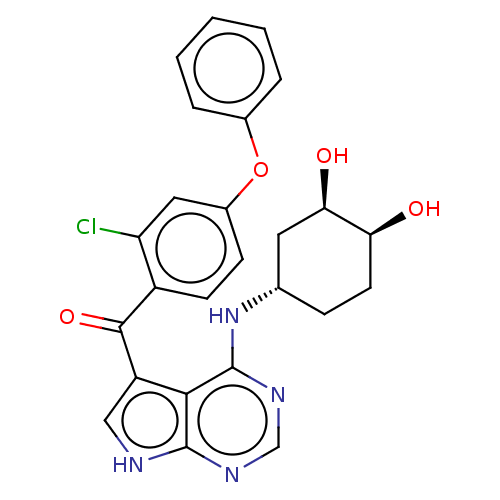 (US11020398, Compound I-180 | rac-(2-chloro-4-pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499894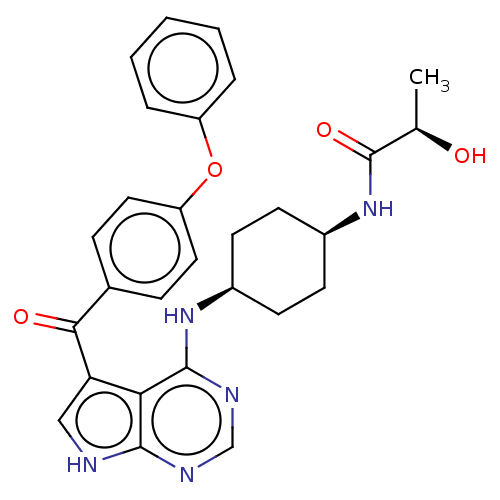 ((R)-2-hydroxy-N-(cis-4-((5-(4- phenoxybenzoyl)-7H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499739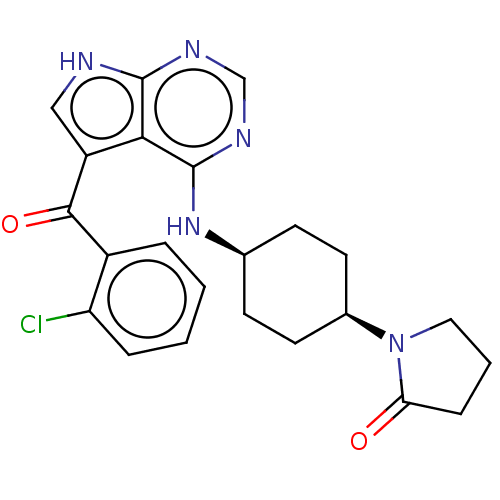 (1-(cis-4-((5-(2-chlorobenzoyl)-7H- pyrrolo[2,3-d]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499790 (N-(cis-4-((5-(2-chlorobenzoyl)-7H- pyrrolo[2,3-d]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499898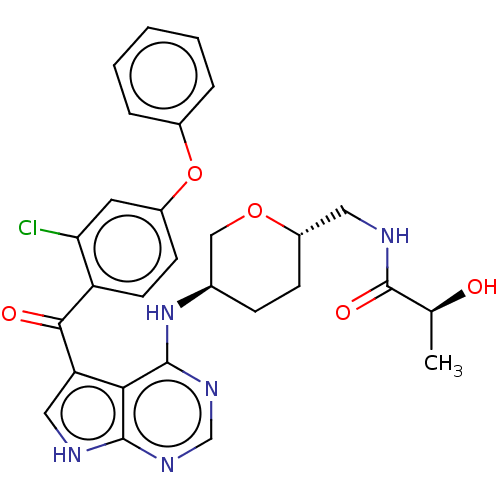 ((S)-N-(((2S,5R)-5-((5-(2-chloro-4- phenoxybenzoyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 488 total ) | Next | Last >> |