 Found 1168 hits with Last Name = 'ogilvie' and Initial = 'dj'
Found 1168 hits with Last Name = 'ogilvie' and Initial = 'dj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50236369
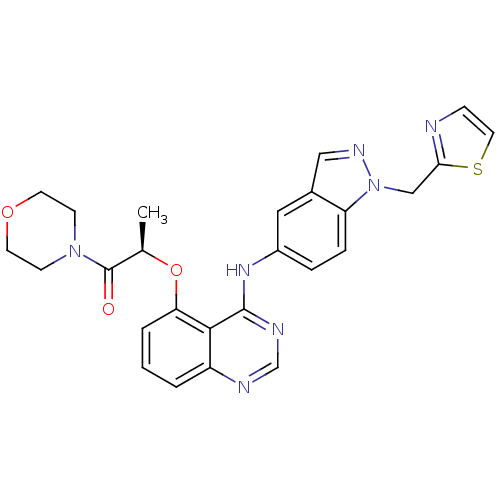
((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50236369

((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153906
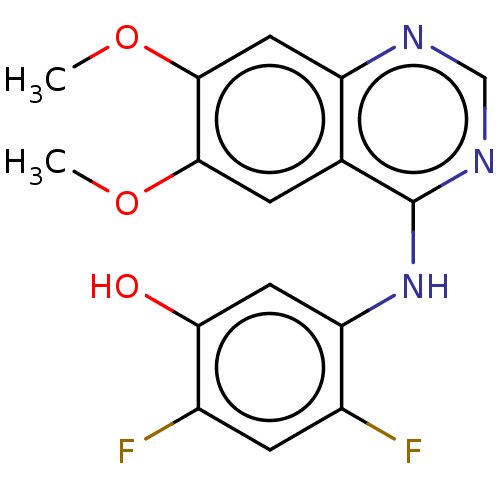
(CHEMBL3775169)Show InChI InChI=1S/C16H13F2N3O3/c1-23-14-3-8-11(6-15(14)24-2)19-7-20-16(8)21-12-5-13(22)10(18)4-9(12)17/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM4627
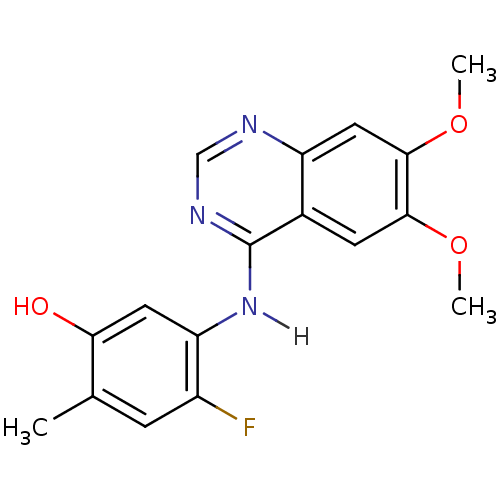
(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...)Show InChI InChI=1S/C17H16FN3O3/c1-9-4-11(18)13(6-14(9)22)21-17-10-5-15(23-2)16(24-3)7-12(10)19-8-20-17/h4-8,22H,1-3H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4683

(4-Anilinoquinazoline 30 | N-(4-bromo-2-fluoropheny...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OC[C@@H]1CCCN(C)C1 |r| Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-3-4-14(11-28)12-30-21-10-19-16(9-20(21)29-2)22(26-13-25-19)27-18-6-5-15(23)8-17(18)24/h5-6,8-10,13-14H,3-4,7,11-12H2,1-2H3,(H,25,26,27)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 45: 1300-12 (2002)
Article DOI: 10.1021/jm011022e
BindingDB Entry DOI: 10.7270/Q29C6VMW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190001
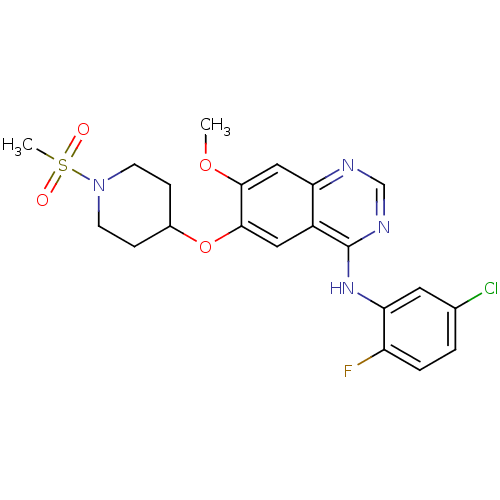
(CHEMBL213007 | N-(5-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cc(Cl)ccc3F)c2cc1OC1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C21H22ClFN4O4S/c1-30-19-11-17-15(10-20(19)31-14-5-7-27(8-6-14)32(2,28)29)21(25-12-24-17)26-18-9-13(22)3-4-16(18)23/h3-4,9-12,14H,5-8H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50373856

(CHEMBL271705)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCOCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H24ClN7O3/c24-18-13-16(4-5-19(18)34-14-17-3-1-2-6-25-17)28-21-20-22(27-15-26-21)29-30-23(20)33-12-9-31-7-10-32-11-8-31/h1-6,13,15H,7-12,14H2,(H2,26,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373853
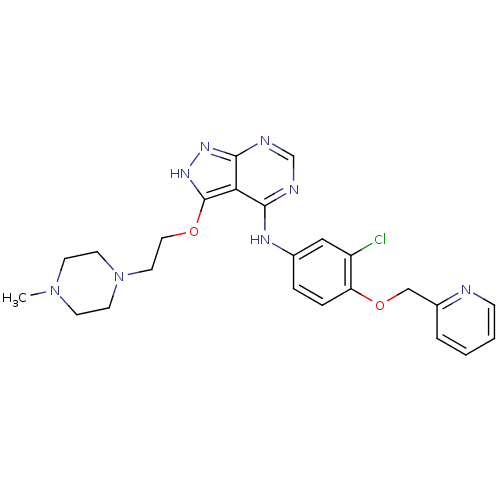
(CHEMBL258282)Show SMILES CN1CCN(CCOc2[nH]nc3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C24H27ClN8O2/c1-32-8-10-33(11-9-32)12-13-34-24-21-22(27-16-28-23(21)30-31-24)29-17-5-6-20(19(25)14-17)35-15-18-4-2-3-7-26-18/h2-7,14,16H,8-13,15H2,1H3,(H2,27,28,29,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153979
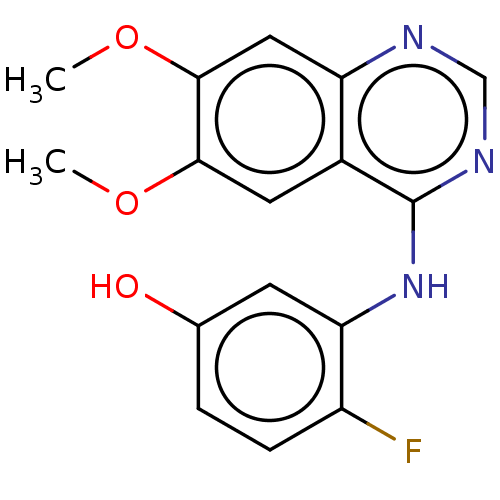
(CHEMBL3774904)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-13-5-9(21)3-4-11(13)17/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153902
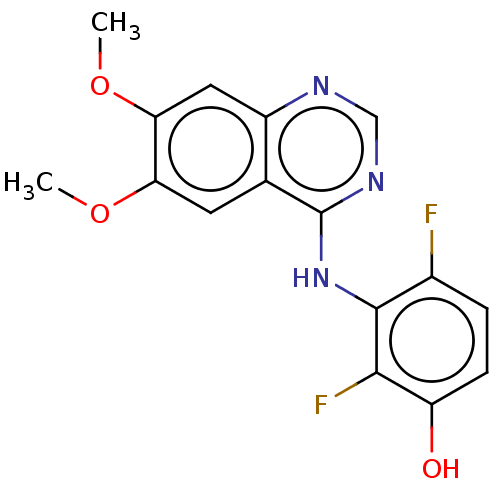
(CHEMBL3775557)Show InChI InChI=1S/C16H13F2N3O3/c1-23-12-5-8-10(6-13(12)24-2)19-7-20-16(8)21-15-9(17)3-4-11(22)14(15)18/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189995

(CHEMBL213874 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24ClFN4O2/c1-28-8-6-14(7-9-28)12-30-20-10-15-18(11-19(20)29-2)25-13-26-22(15)27-17-5-3-4-16(23)21(17)24/h3-5,10-11,13-14H,6-9,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373862

(CHEMBL402553)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H24ClN7O2/c24-18-13-16(6-7-19(18)33-14-17-5-1-2-8-25-17)28-21-20-22(27-15-26-21)29-30-23(20)32-12-11-31-9-3-4-10-31/h1-2,5-8,13,15H,3-4,9-12,14H2,(H2,26,27,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190002

(CHEMBL387265 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C21H22ClFN4O4S/c1-30-18-11-17-14(10-19(18)31-13-6-8-27(9-7-13)32(2,28)29)21(25-12-24-17)26-16-5-3-4-15(22)20(16)23/h3-5,10-13H,6-9H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189993

(2-(4-(4-(3-chloro-2-fluorophenylamino)-7-methoxyqu...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(CC(N)=O)CC1 Show InChI InChI=1S/C22H23ClFN5O3/c1-31-18-10-17-14(9-19(18)32-13-5-7-29(8-6-13)11-20(25)30)22(27-12-26-17)28-16-4-2-3-15(23)21(16)24/h2-4,9-10,12-13H,5-8,11H2,1H3,(H2,25,30)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222489

((R)-N-(2-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](CN(C)C(=O)CO)Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C26H26ClN5O4/c1-17(13-32(2)24(34)14-33)36-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)35-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222495

((S)-1-(2-((4-(3-chloro-4-(pyridin-2-ylmethoxy)phen...)Show SMILES OCC(=O)N1CCC[C@H]1COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C27H26ClN5O4/c28-21-13-18(9-10-23(21)36-15-19-5-1-2-11-29-19)32-27-26-22(30-17-31-27)7-3-8-24(26)37-16-20-6-4-12-33(20)25(35)14-34/h1-3,5,7-11,13,17,20,34H,4,6,12,14-16H2,(H,30,31,32)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM4630
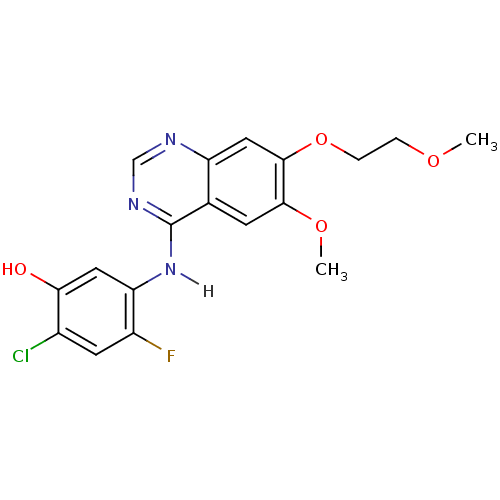
(2-chloro-4-fluoro-5-{[6-methoxy-7-(2-methoxyethoxy...)Show InChI InChI=1S/C18H17ClFN3O4/c1-25-3-4-27-17-8-13-10(5-16(17)26-2)18(22-9-21-13)23-14-7-15(24)11(19)6-12(14)20/h5-9,24H,3-4H2,1-2H3,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 42: 5369-89 (1999)
Article DOI: 10.1021/jm990345w
BindingDB Entry DOI: 10.7270/Q2F47MBM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4630

(2-chloro-4-fluoro-5-{[6-methoxy-7-(2-methoxyethoxy...)Show InChI InChI=1S/C18H17ClFN3O4/c1-25-3-4-27-17-8-13-10(5-16(17)26-2)18(22-9-21-13)23-14-7-15(24)11(19)6-12(14)20/h5-9,24H,3-4H2,1-2H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 42: 5369-89 (1999)
Article DOI: 10.1021/jm990345w
BindingDB Entry DOI: 10.7270/Q2F47MBM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4633
(2-chloro-4-fluoro-5-{[6-methoxy-7-(2-methoxyethoxy...)Show InChI InChI=1S/C19H18ClFN2O4/c1-25-5-6-27-19-10-15-11(7-18(19)26-2)14(3-4-22-15)23-16-9-17(24)12(20)8-13(16)21/h3-4,7-10,24H,5-6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 42: 5369-89 (1999)
Article DOI: 10.1021/jm990345w
BindingDB Entry DOI: 10.7270/Q2F47MBM |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371358
(CHEMBL257478)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N(C)CCO Show InChI InChI=1S/C27H29N5O4/c1-17-14-20(9-11-23(17)36-21-10-8-18(2)28-15-21)31-26-25-22(29-16-30-26)6-5-7-24(25)35-19(3)27(34)32(4)12-13-33/h5-11,14-16,19,33H,12-13H2,1-4H3,(H,29,30,31)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153903
(CHEMBL3775336)Show InChI InChI=1S/C16H13F2N3O3/c1-23-12-5-8-11(6-13(12)24-2)19-7-20-16(8)21-10-4-3-9(17)15(22)14(10)18/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190000

(CHEMBL214798 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(C)CC1 Show InChI InChI=1S/C21H22ClFN4O2/c1-27-8-6-13(7-9-27)29-19-10-14-17(11-18(19)28-2)24-12-25-21(14)26-16-5-3-4-15(22)20(16)23/h3-5,10-13H,6-9H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4627

(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...)Show InChI InChI=1S/C17H16FN3O3/c1-9-4-11(18)13(6-14(9)22)21-17-10-5-15(23-2)16(24-3)7-12(10)19-8-20-17/h4-8,22H,1-3H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154001
(CHEMBL3775934)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-12-13(7-15(14)23-2)18-8-19-16(12)20-10-3-9(17)4-11(21)5-10/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153908
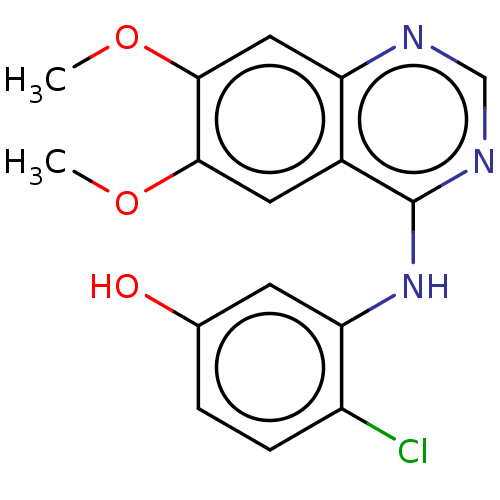
(CHEMBL3774489)Show InChI InChI=1S/C16H14ClN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-13-5-9(21)3-4-11(13)17/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189990

(CHEMBL215786 | N-(3-chloro-4-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24ClFN4O2/c1-28-7-5-14(6-8-28)12-30-21-10-16-19(11-20(21)29-2)25-13-26-22(16)27-15-3-4-18(24)17(23)9-15/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189991

(CHEMBL214857 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COCCN1CCC(CC1)Oc1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC Show InChI InChI=1S/C23H26ClFN4O3/c1-30-11-10-29-8-6-15(7-9-29)32-21-12-16-19(13-20(21)31-2)26-14-27-23(16)28-18-5-3-4-17(24)22(18)25/h3-5,12-15H,6-11H2,1-2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222493

((R)-N-(1-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)N(C)C(=O)CO Show InChI InChI=1S/C26H26ClN5O4/c1-17(32(2)24(34)13-33)14-35-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)36-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM4633

(2-chloro-4-fluoro-5-{[6-methoxy-7-(2-methoxyethoxy...)Show InChI InChI=1S/C19H18ClFN2O4/c1-25-5-6-27-19-10-15-11(7-18(19)26-2)14(3-4-22-15)23-16-9-17(24)12(20)8-13(16)21/h3-4,7-10,24H,5-6H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 42: 5369-89 (1999)
Article DOI: 10.1021/jm990345w
BindingDB Entry DOI: 10.7270/Q2F47MBM |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373852

(CHEMBL257430)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCNCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H25ClN8O2/c24-18-13-16(4-5-19(18)34-14-17-3-1-2-6-26-17)29-21-20-22(28-15-27-21)30-31-23(20)33-12-11-32-9-7-25-8-10-32/h1-6,13,15,25H,7-12,14H2,(H2,27,28,29,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373864

(CHEMBL402339)Show SMILES COc1cc(Nc2ncnc3n[nH]c(OCCN4CCC(O)CC4)c23)ccc1OCc1cccc(F)c1 Show InChI InChI=1S/C26H29FN6O4/c1-35-22-14-19(5-6-21(22)37-15-17-3-2-4-18(27)13-17)30-24-23-25(29-16-28-24)31-32-26(23)36-12-11-33-9-7-20(34)8-10-33/h2-6,13-14,16,20,34H,7-12,15H2,1H3,(H2,28,29,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50373869

(CHEMBL258270)Show SMILES CC(=O)N1CCN(CCOc2[nH]nc3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C25H27ClN8O3/c1-17(35)34-10-8-33(9-11-34)12-13-36-25-22-23(28-16-29-24(22)31-32-25)30-18-5-6-21(20(26)14-18)37-15-19-4-2-3-7-27-19/h2-7,14,16H,8-13,15H2,1H3,(H2,28,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373857
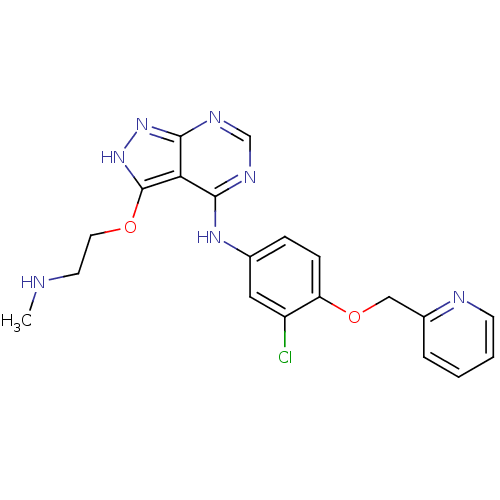
(CHEMBL255135)Show SMILES CNCCOc1[nH]nc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C20H20ClN7O2/c1-22-8-9-29-20-17-18(24-12-25-19(17)27-28-20)26-13-5-6-16(15(21)10-13)30-11-14-4-2-3-7-23-14/h2-7,10,12,22H,8-9,11H2,1H3,(H2,24,25,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50179758
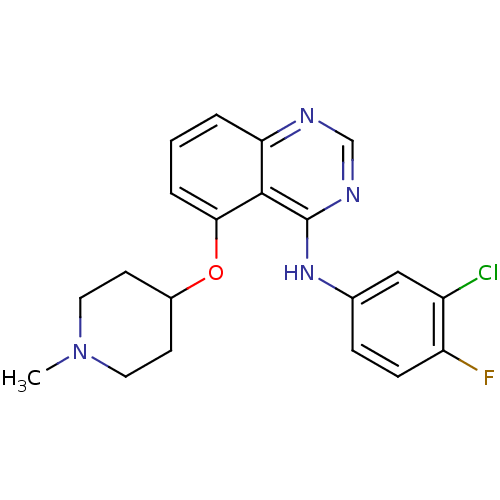
(CHEMBL203725 | N-(3-chloro-4-fluorophenyl)-5-(1-me...)Show SMILES CN1CCC(CC1)Oc1cccc2ncnc(Nc3ccc(F)c(Cl)c3)c12 Show InChI InChI=1S/C20H20ClFN4O/c1-26-9-7-14(8-10-26)27-18-4-2-3-17-19(18)20(24-12-23-17)25-13-5-6-16(22)15(21)11-13/h2-6,11-12,14H,7-10H2,1H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against EGFR |
Bioorg Med Chem Lett 16: 1633-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.028
BindingDB Entry DOI: 10.7270/Q2P84BGH |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4627

(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...)Show InChI InChI=1S/C17H16FN3O3/c1-9-4-11(18)13(6-14(9)22)21-17-10-5-15(23-2)16(24-3)7-12(10)19-8-20-17/h4-8,22H,1-3H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 42: 5369-89 (1999)
Article DOI: 10.1021/jm990345w
BindingDB Entry DOI: 10.7270/Q2F47MBM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154249
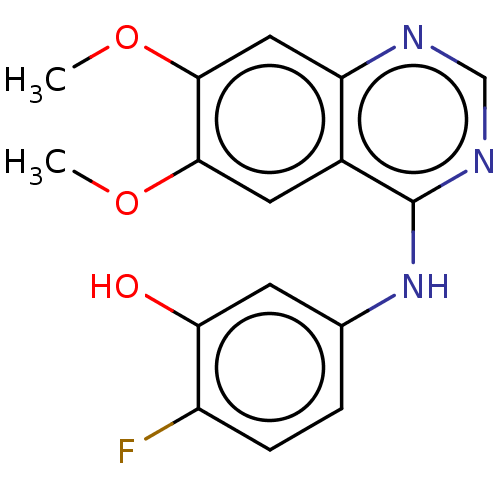
(CHEMBL3775879)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-9-3-4-11(17)13(21)5-9/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153895
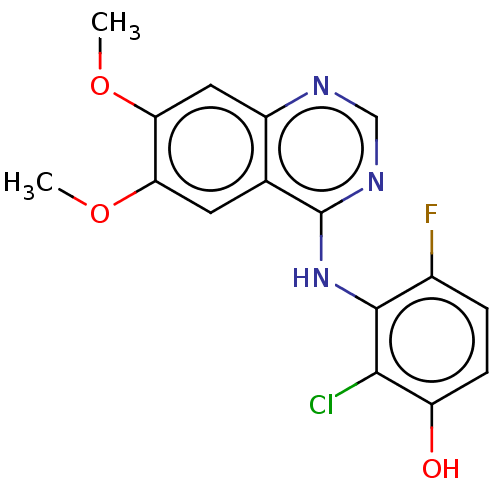
(CHEMBL3774953)Show InChI InChI=1S/C16H13ClFN3O3/c1-23-12-5-8-10(6-13(12)24-2)19-7-20-16(8)21-15-9(18)3-4-11(22)14(15)17/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154246
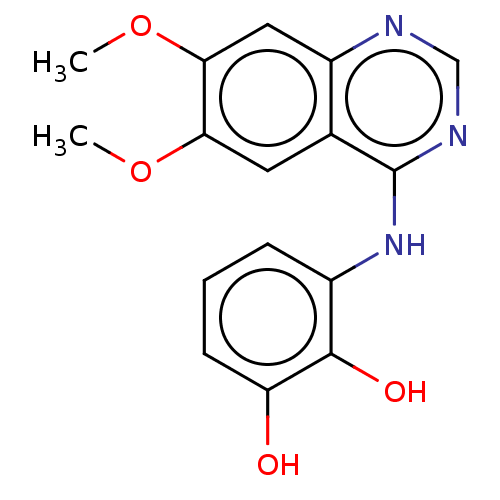
(CHEMBL3774580)Show InChI InChI=1S/C16H15N3O4/c1-22-13-6-9-11(7-14(13)23-2)17-8-18-16(9)19-10-4-3-5-12(20)15(10)21/h3-8,20-21H,1-2H3,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222491

(CHEMBL249928 | N-(2-(4-(3-chloro-4-(pyridin-2-ylme...)Show SMILES CN(CCOc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)C(=O)CO Show InChI InChI=1S/C25H24ClN5O4/c1-31(23(33)14-32)11-12-34-22-7-4-6-20-24(22)25(29-16-28-20)30-17-8-9-21(19(26)13-17)35-15-18-5-2-3-10-27-18/h2-10,13,16,32H,11-12,14-15H2,1H3,(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4635

(2-chloro-4-fluoro-5-{[6-methoxy-7-(2-methoxyethoxy...)Show InChI InChI=1S/C18H17ClFN3O4/c1-25-3-4-27-18-8-13-10(5-17(18)26-2)15(9-21-23-13)22-14-7-16(24)11(19)6-12(14)20/h5-9,24H,3-4H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 42: 5369-89 (1999)
Article DOI: 10.1021/jm990345w
BindingDB Entry DOI: 10.7270/Q2F47MBM |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373855
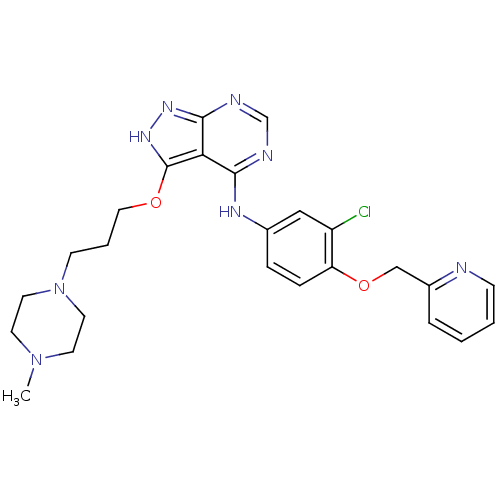
(CHEMBL272935)Show SMILES CN1CCN(CCCOc2[nH]nc3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C25H29ClN8O2/c1-33-10-12-34(13-11-33)9-4-14-35-25-22-23(28-17-29-24(22)31-32-25)30-18-6-7-21(20(26)15-18)36-16-19-5-2-3-8-27-19/h2-3,5-8,15,17H,4,9-14,16H2,1H3,(H2,28,29,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373861

(CHEMBL411897)Show SMILES COC1CCN(CCOc2[nH]nc3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C25H28ClN7O3/c1-34-19-7-10-33(11-8-19)12-13-35-25-22-23(28-16-29-24(22)31-32-25)30-17-5-6-21(20(26)14-17)36-15-18-4-2-3-9-27-18/h2-6,9,14,16,19H,7-8,10-13,15H2,1H3,(H2,28,29,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373859

(CHEMBL257861)Show SMILES OCCOc1[nH]nc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C19H17ClN6O3/c20-14-9-12(4-5-15(14)29-10-13-3-1-2-6-21-13)24-17-16-18(23-11-22-17)25-26-19(16)28-8-7-27/h1-6,9,11,27H,7-8,10H2,(H2,22,23,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM4622
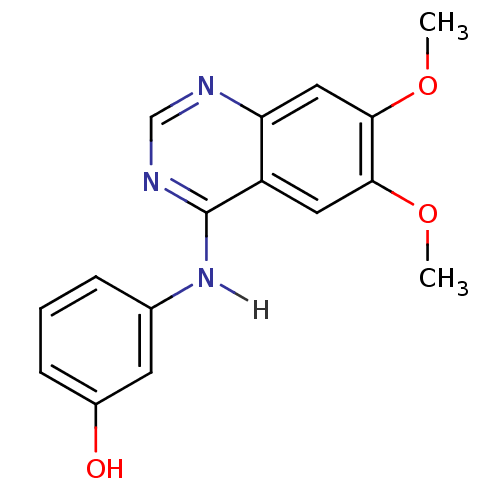
(3-[(6,7-dimethoxyquinazolin-4-yl)amino]phenol | An...)Show InChI InChI=1S/C16H15N3O3/c1-21-14-7-12-13(8-15(14)22-2)17-9-18-16(12)19-10-4-3-5-11(20)6-10/h3-9,20H,1-2H3,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM26477
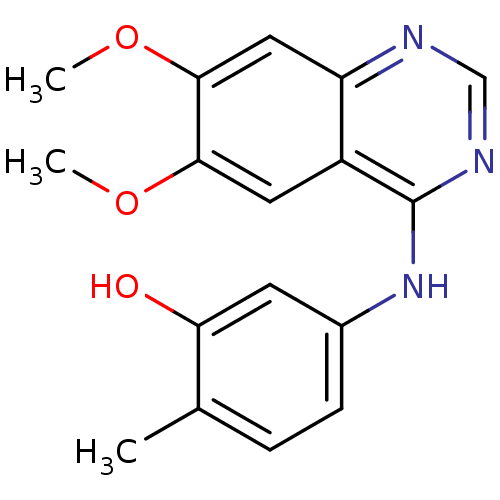
(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-2-methylph...)Show InChI InChI=1S/C17H17N3O3/c1-10-4-5-11(6-14(10)21)20-17-12-7-15(22-2)16(23-3)8-13(12)18-9-19-17/h4-9,21H,1-3H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153901
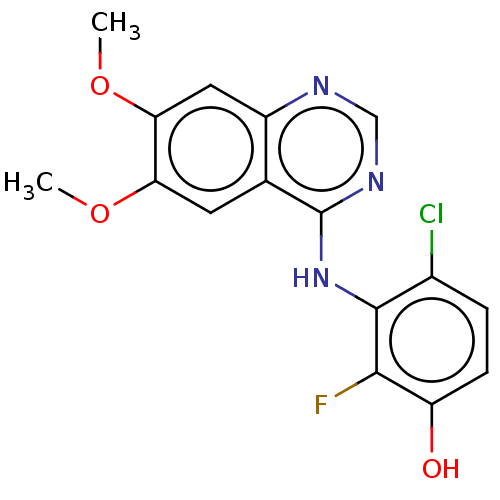
(CHEMBL3775511)Show InChI InChI=1S/C16H13ClFN3O3/c1-23-12-5-8-10(6-13(12)24-2)19-7-20-16(8)21-15-9(17)3-4-11(22)14(15)18/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4682
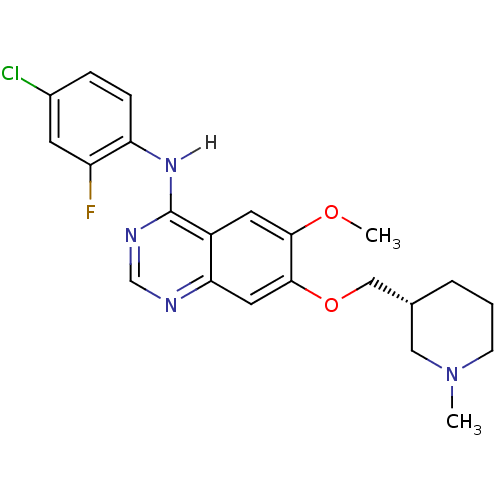
(4-Anilinoquinazoline 29 | N-(4-chloro-2-fluorophen...)Show SMILES COc1cc2c(Nc3ccc(Cl)cc3F)ncnc2cc1OC[C@@H]1CCCN(C)C1 |r| Show InChI InChI=1S/C22H24ClFN4O2/c1-28-7-3-4-14(11-28)12-30-21-10-19-16(9-20(21)29-2)22(26-13-25-19)27-18-6-5-15(23)8-17(18)24/h5-6,8-10,13-14H,3-4,7,11-12H2,1-2H3,(H,25,26,27)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 45: 1300-12 (2002)
Article DOI: 10.1021/jm011022e
BindingDB Entry DOI: 10.7270/Q29C6VMW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4629

(3-{[6-methoxy-7-(2-methoxyethoxy)quinazolin-4-yl]a...)Show InChI InChI=1S/C18H19N3O4/c1-23-6-7-25-17-10-15-14(9-16(17)24-2)18(20-11-19-15)21-12-4-3-5-13(22)8-12/h3-5,8-11,22H,6-7H2,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca
| Assay Description
An ELISA assay was used to determine the ability of inhibitor to inhibit VEGF-R RTK activity. The compounds were incubated with enzyme 20 min at room... |
J Med Chem 42: 5369-89 (1999)
Article DOI: 10.1021/jm990345w
BindingDB Entry DOI: 10.7270/Q2F47MBM |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50171256

(CHEMBL444619 | [3-Chloro-4-(pyridin-2-ylmethoxy)-p...)Show SMILES COc1cc(ON2CCC(C)CC2)c2c(Nc3ccc(OCc4ccccn4)c(Cl)c3)ncnc2c1 Show InChI InChI=1S/C27H28ClN5O3/c1-18-8-11-33(12-9-18)36-25-15-21(34-2)14-23-26(25)27(31-17-30-23)32-19-6-7-24(22(28)13-19)35-16-20-5-3-4-10-29-20/h3-7,10,13-15,17-18H,8-9,11-12,16H2,1-2H3,(H,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory concentration against erbB2 kinase phosphorylation using synthetic peptide as a substrate |
Bioorg Med Chem Lett 15: 4226-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.068
BindingDB Entry DOI: 10.7270/Q2TQ612W |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50171256

(CHEMBL444619 | [3-Chloro-4-(pyridin-2-ylmethoxy)-p...)Show SMILES COc1cc(ON2CCC(C)CC2)c2c(Nc3ccc(OCc4ccccn4)c(Cl)c3)ncnc2c1 Show InChI InChI=1S/C27H28ClN5O3/c1-18-8-11-33(12-9-18)36-25-15-21(34-2)14-23-26(25)27(31-17-30-23)32-19-6-7-24(22(28)13-19)35-16-20-5-3-4-10-29-20/h3-7,10,13-15,17-18H,8-9,11-12,16H2,1-2H3,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory concentration against EGFR kinase phosphorylation using synthetic peptide as a substrate |
Bioorg Med Chem Lett 15: 4226-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.068
BindingDB Entry DOI: 10.7270/Q2TQ612W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data