

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194658 ((2R)-N-(1-cyclopropylmethyl-4-piperidinylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194659 ((2R)-N-(1-cyclopentylmethyl-4-piperidinylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194640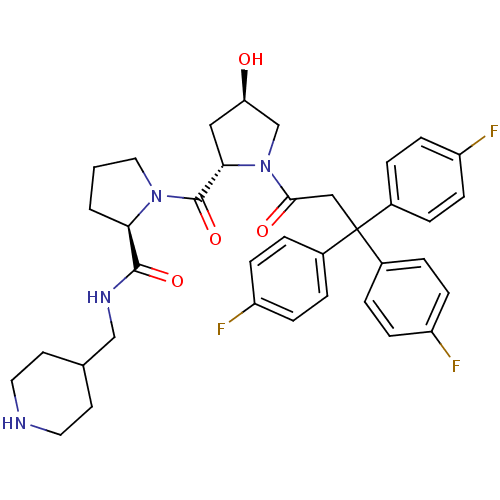 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194654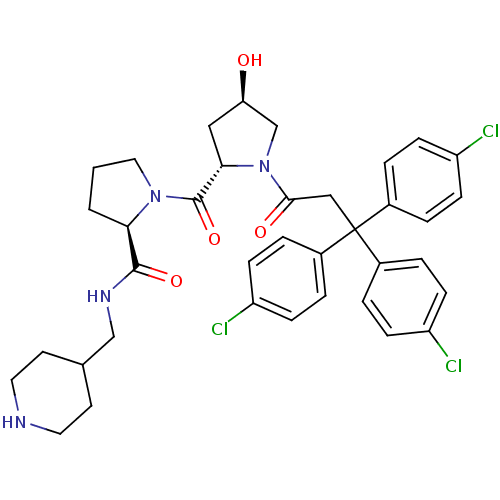 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194641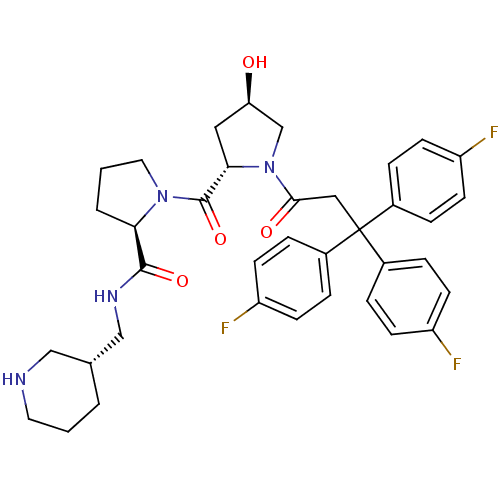 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194645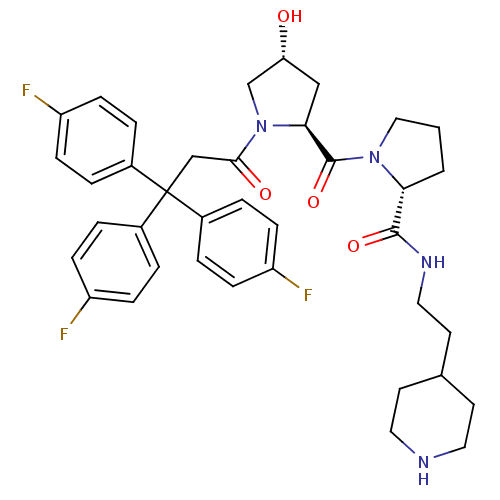 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM82372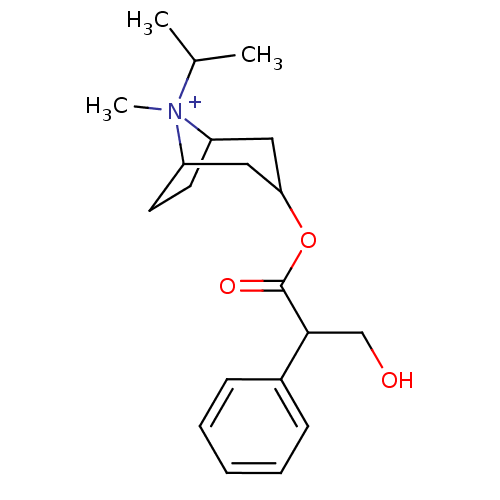 (CAS_22254-24-6 | Ipratropium | NSC_3746) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM82372 (CAS_22254-24-6 | Ipratropium | NSC_3746) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194655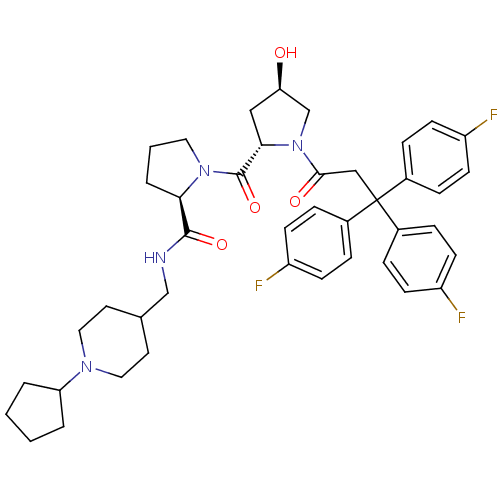 ((2R)-N-(1-cyclopentyl-4-piperidinylmethyl)-1-((2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194642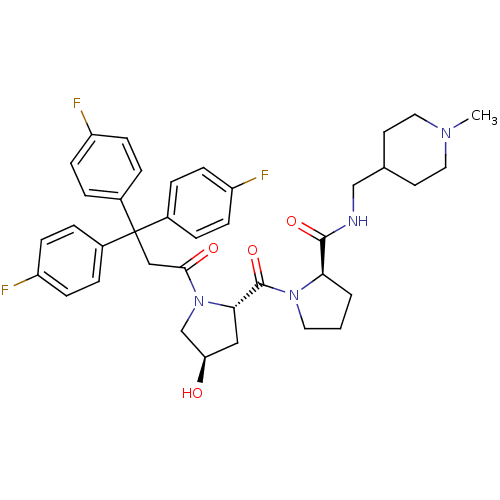 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-fluoroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194639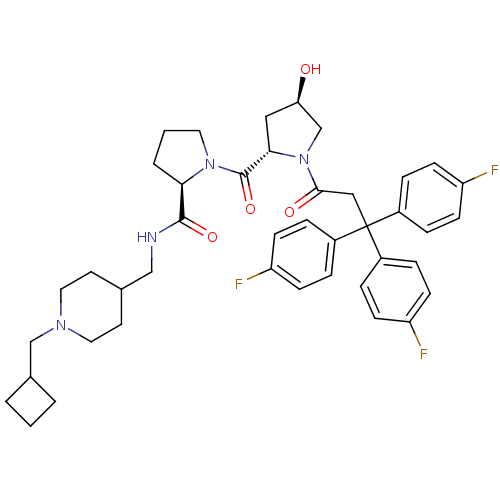 ((2R)-N-(1-cyclobutylmethyl-4-piperidinylmethyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM82372 (CAS_22254-24-6 | Ipratropium | NSC_3746) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50109647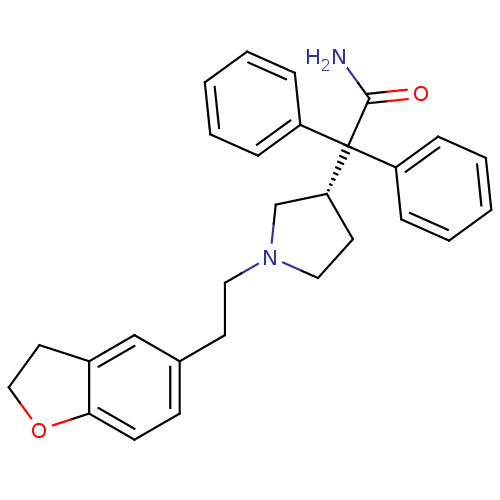 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50109647 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity (Ki) against binding of [3H]NMS using membranes from CHO cells expressing cloned human Muscarinic acetylcholine receptor M3 | J Med Chem 45: 984-7 (2002) BindingDB Entry DOI: 10.7270/Q2P26ZVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50095656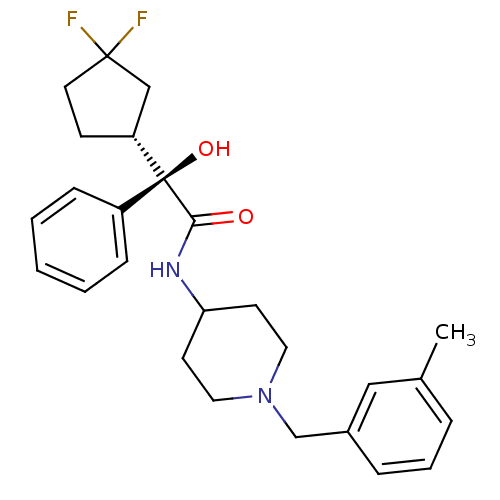 (2-(3,3-Difluoro-cyclopentyl)-2-hydroxy-N-[1-(3-met...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-NMS binding to human muscarinic acetylcholine receptor M1 expressed in CHO cells | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194657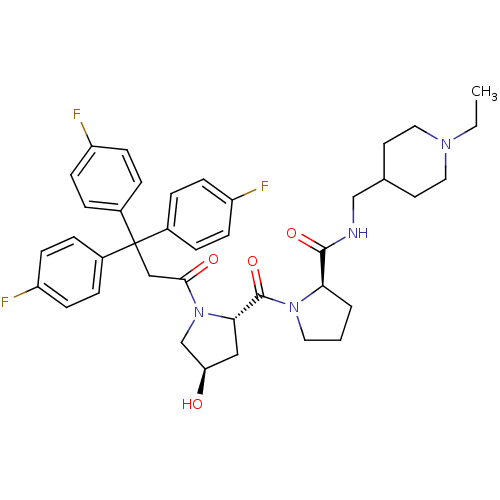 ((2R)-N-(1-ethyl-4-piperidinylmethyl)-1-((2S,4R)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129387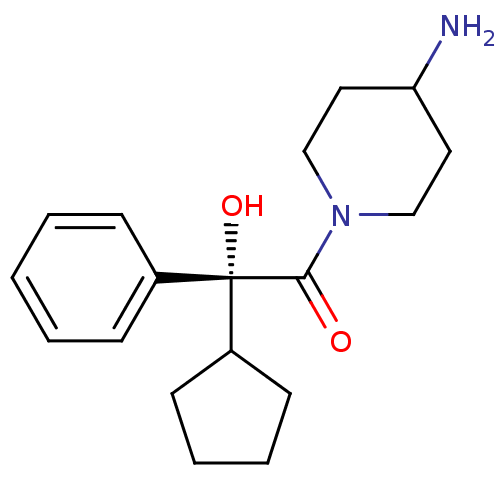 ((R)-1-(4-Amino-piperidin-1-yl)-2-cyclopentyl-2-hyd...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129390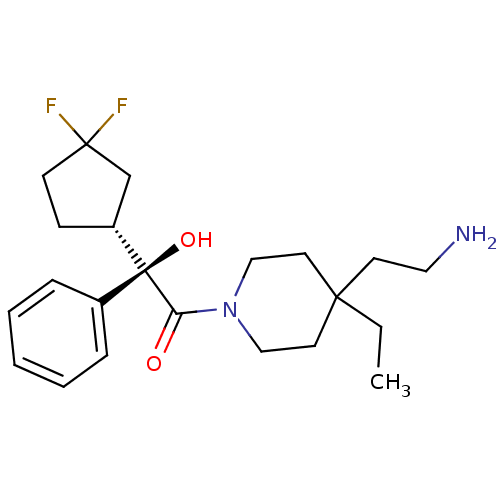 ((R)-1-[4-(2-Amino-ethyl)-4-ethyl-piperidin-1-yl]-2...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50129399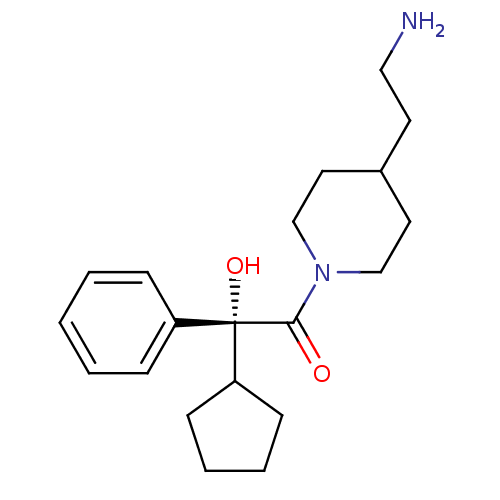 ((R)-1-[4-(2-Amino-ethyl)-piperidin-1-yl]-2-cyclope...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human muscarinic acetylcholine receptor M3 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194649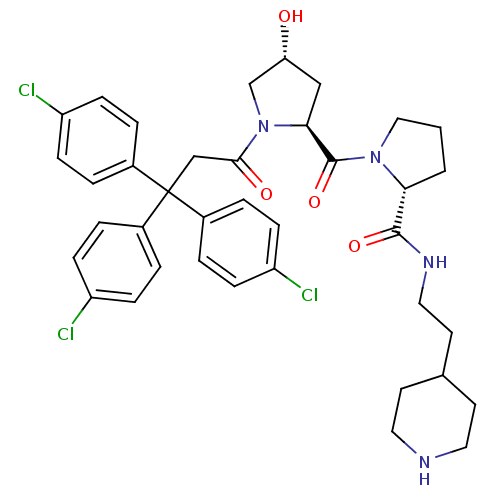 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50095679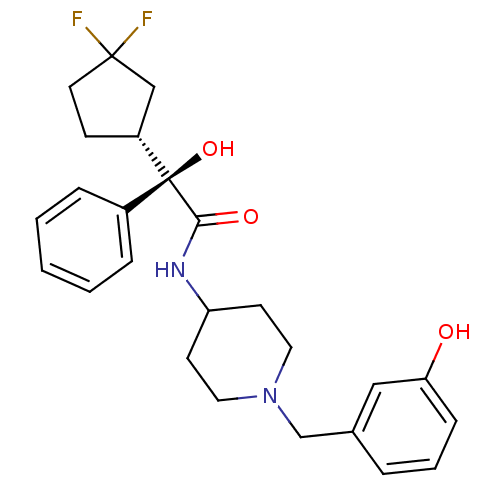 (2-(3,3-Difluoro-cyclopentyl)-2-hydroxy-N-[1-(3-hyd...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-NMS binding to human muscarinic acetylcholine receptor M1 expressed in CHO cells | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50129392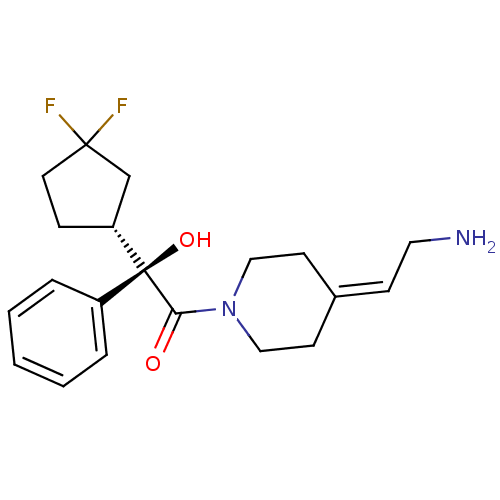 ((R)-1-[4-(2-Amino-ethylidene)-piperidin-1-yl]-2-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human muscarinic acetylcholine receptor M3 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249758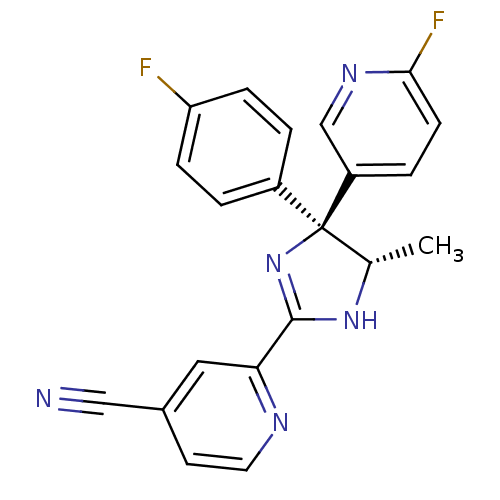 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPYY5 receptor | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129392 ((R)-1-[4-(2-Amino-ethylidene)-piperidin-1-yl]-2-((...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50129387 ((R)-1-(4-Amino-piperidin-1-yl)-2-cyclopentyl-2-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human muscarinic acetylcholine receptor M3 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249833 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50095662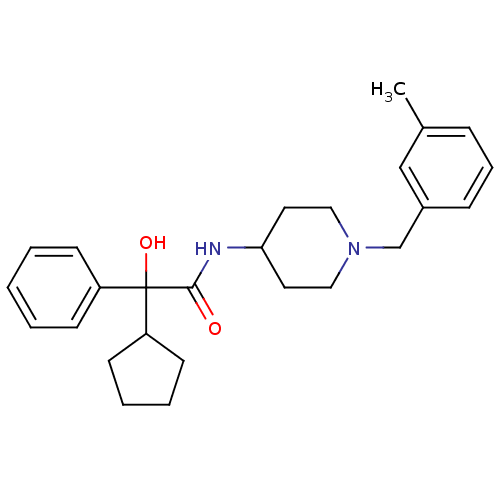 (2-Cyclopentyl-2-hydroxy-N-[1-(3-methyl-benzyl)-pip...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-NMS binding to human muscarinic acetylcholine receptor M1 expressed in CHO cells | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50095657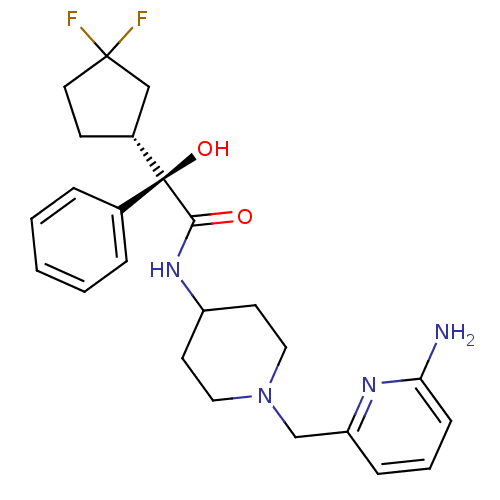 ((R)-N-[1-(6-Amino-pyridin-2-ylmethyl)-piperidin-4-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-NMS binding to human muscarinic acetylcholine receptor M1 expressed in CHO cells | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM86231 (ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129397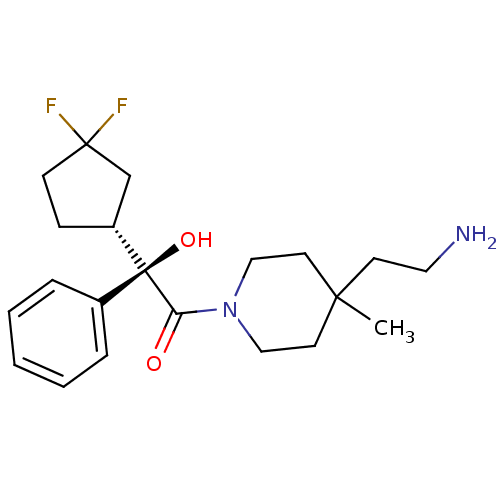 ((R)-1-[4-(2-Amino-ethyl)-4-methyl-piperidin-1-yl]-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50095657 ((R)-N-[1-(6-Amino-pyridin-2-ylmethyl)-piperidin-4-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM82372 (CAS_22254-24-6 | Ipratropium | NSC_3746) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50194643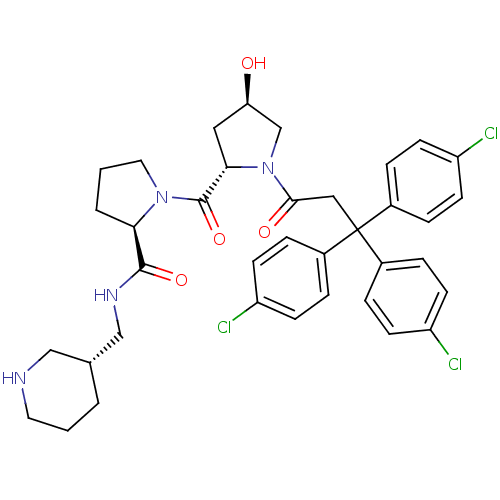 ((2R)-1-((2S,4R)-4-hydroxy-1-[3,3,3-tris(4-chloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to human cloned M3 receptor expressed in CHO cells | J Med Chem 49: 5653-63 (2006) Article DOI: 10.1021/jm051205r BindingDB Entry DOI: 10.7270/Q2F76C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM85787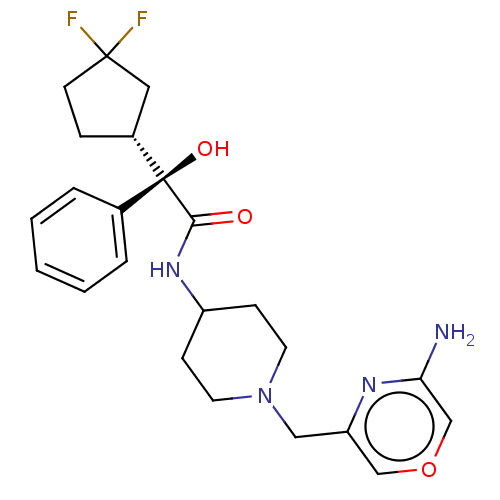 (Compound A) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50129410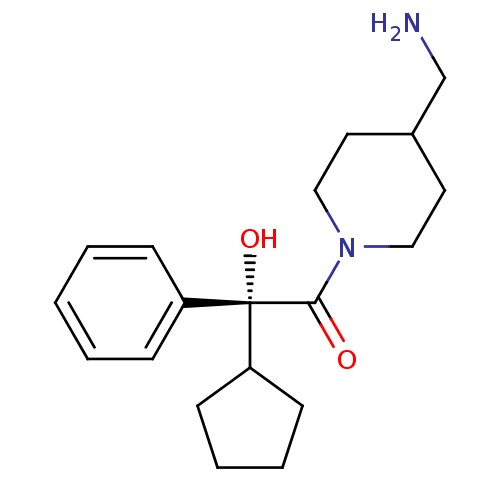 ((R)-1-(4-Aminomethyl-piperidin-1-yl)-2-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human muscarinic acetylcholine receptor M3 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM82372 (CAS_22254-24-6 | Ipratropium | NSC_3746) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129410 ((R)-1-(4-Aminomethyl-piperidin-1-yl)-2-cyclopentyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50095656 (2-(3,3-Difluoro-cyclopentyl)-2-hydroxy-N-[1-(3-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit binding of [3H]-NMS was determined by receptor binding assay using membranes from chinese hamster ovary (CHO) cells expressing clo... | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129399 ((R)-1-[4-(2-Amino-ethyl)-piperidin-1-yl]-2-cyclope...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50095680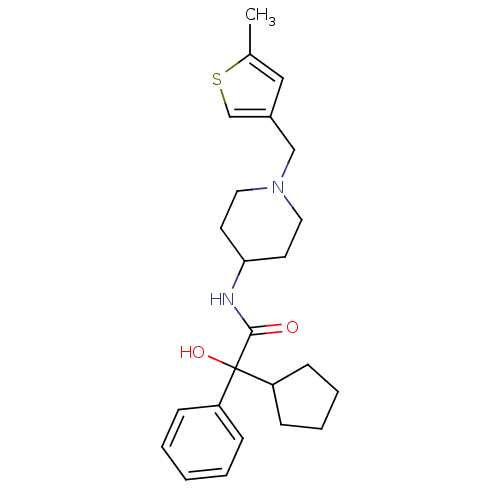 (2-Cyclopentyl-2-hydroxy-N-[1-(5-methyl-thiophen-3-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-NMS binding to human muscarinic acetylcholine receptor M1 expressed in CHO cells | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50129409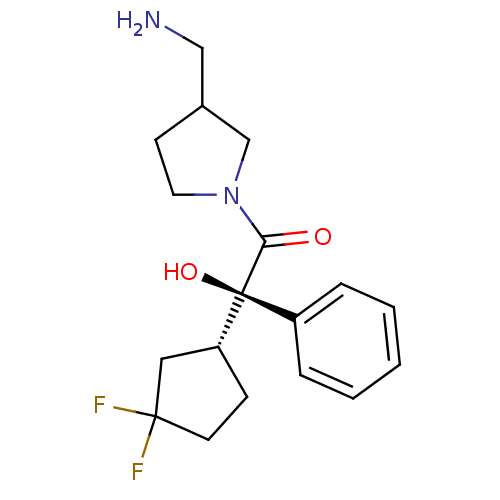 ((R)-1-(3-Aminomethyl-pyrrolidin-1-yl)-2-((R)-3,3-d...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Muscarinic acetylcholine receptor M1 in transfected CHO cells | Bioorg Med Chem Lett 13: 2167-72 (2003) BindingDB Entry DOI: 10.7270/Q29G5M68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50109650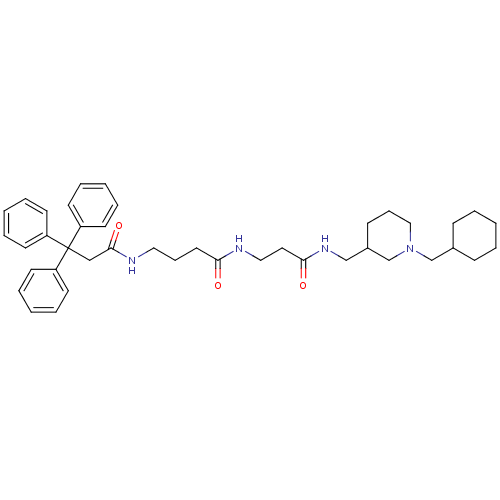 (CHEMBL369062 | N-{2-[(1-Cyclohexylmethyl-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity (Ki) against binding of [3H]NMS using membranes from CHO cells expressing cloned human Muscarinic acetylcholine receptor M3 | J Med Chem 45: 984-7 (2002) BindingDB Entry DOI: 10.7270/Q2P26ZVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50095662 (2-Cyclopentyl-2-hydroxy-N-[1-(3-methyl-benzyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-NMS binding to human muscarinic acetylcholine receptor M3 expressed in CHO cells | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50109647 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 790-7 (2001) BindingDB Entry DOI: 10.7270/Q24T6GX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50109647 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity (Ki) against binding of [3H]NMS using membranes from CHO cells expressing cloned human Muscarinic acetylcholine receptor M5 | J Med Chem 45: 984-7 (2002) BindingDB Entry DOI: 10.7270/Q2P26ZVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50095670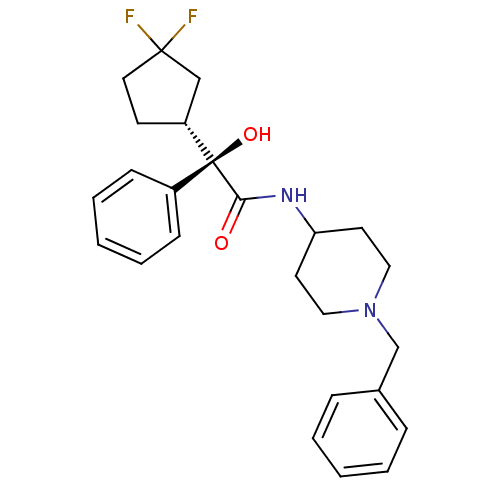 (CHEMBL147653 | N-(1-Benzyl-piperidin-4-yl)-2-(3,3-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-NMS binding to human muscarinic acetylcholine receptor M1 expressed in CHO cells | J Med Chem 43: 5017-29 (2001) BindingDB Entry DOI: 10.7270/Q2HM57P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1277 total ) | Next | Last >> |