

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERbeta-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation count... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM29608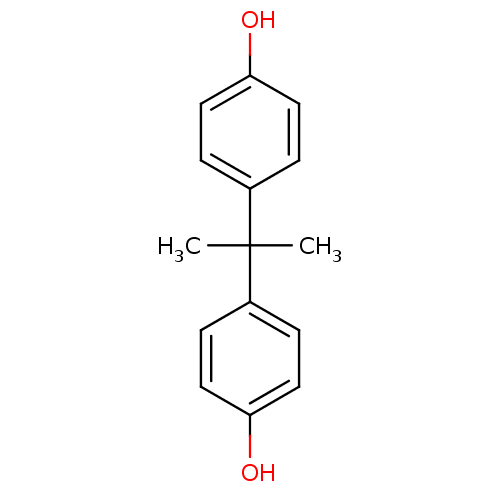 (Bisphenol A (BPA) | Diphenylolpropane | US9688816,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Binding affinity to human ERRgamma | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM20607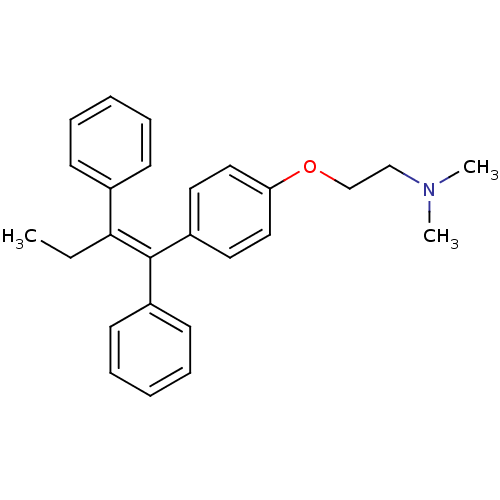 ((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Binding affinity to human ERRgamma | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20607 ((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERbeta-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation count... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50261553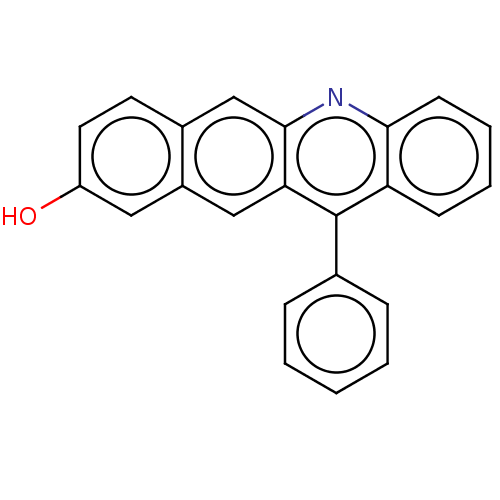 (CHEMBL4087989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERbeta-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation count... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261553 (CHEMBL4087989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERalpha-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation coun... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20607 ((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERalpha-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation coun... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261555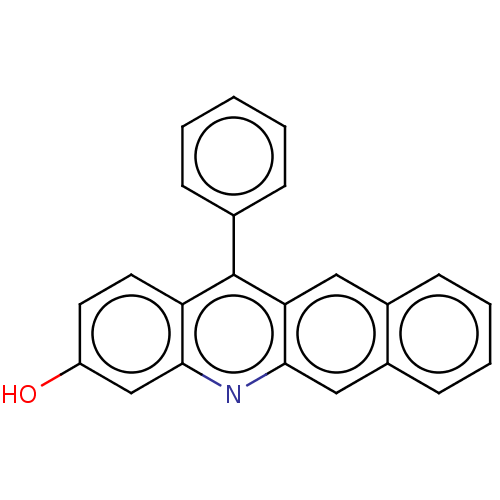 (CHEMBL4088694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50261555 (CHEMBL4088694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERbeta-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation count... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20607 ((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 341 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261551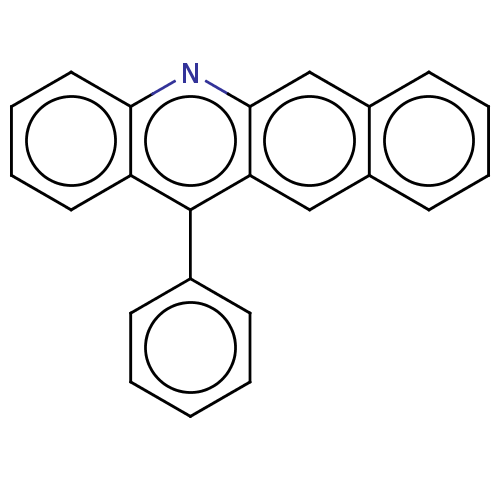 (CHEMBL4094003) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 915 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERalpha-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation coun... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50261554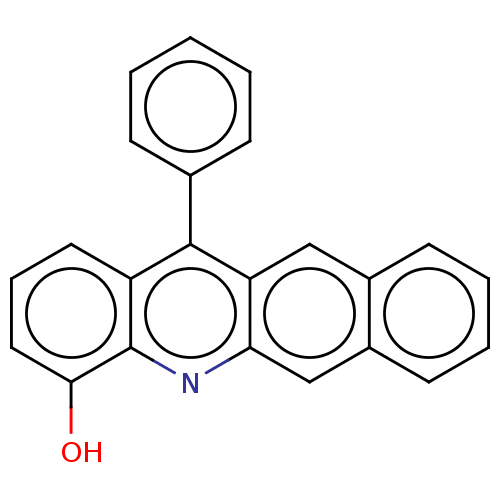 (CHEMBL4092300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 939 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERbeta-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation count... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261554 (CHEMBL4092300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20607 ((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERbeta (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene ... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261556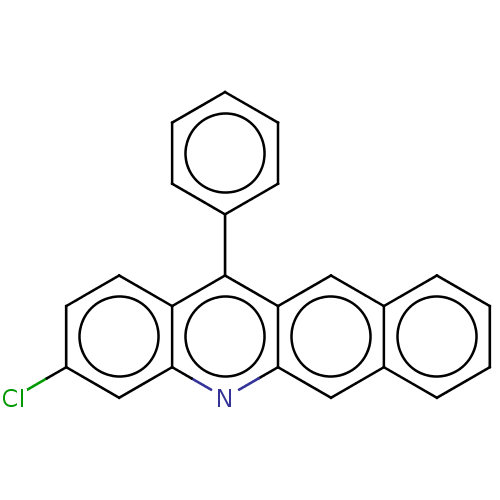 (CHEMBL4105062) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261557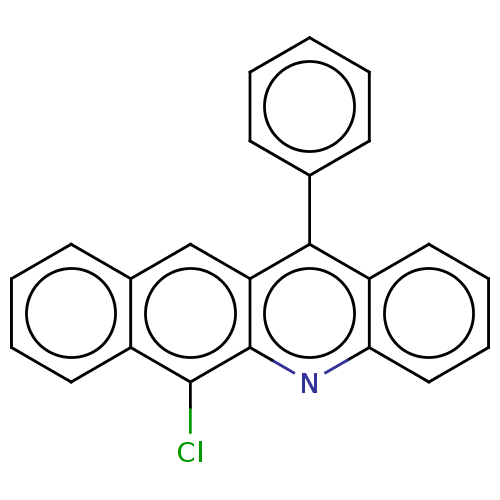 (CHEMBL4071813) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50261551 (CHEMBL4094003) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERbeta-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation count... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50261556 (CHEMBL4105062) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERbeta-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation count... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50261557 (CHEMBL4071813) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERbeta-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation count... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261558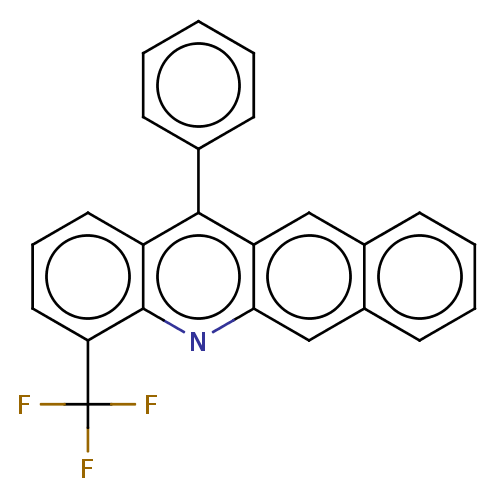 (CHEMBL4071506) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261552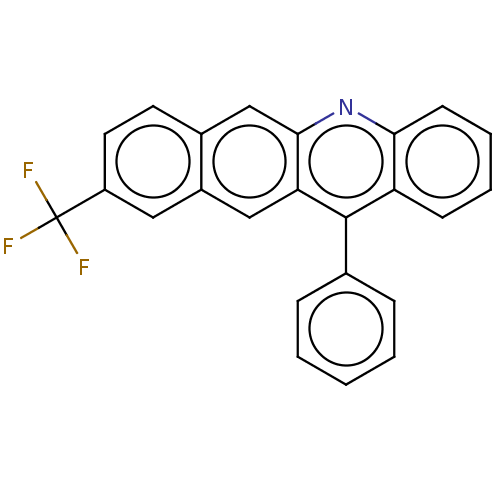 (CHEMBL4066690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Displacement of [3H]E2 from GST-fused ERalpha-LBD (unknown origin) expressed in Escherichia coli BL21 incubated for 1 hr by liquid scintillation coun... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261552 (CHEMBL4066690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261551 (CHEMBL4094003) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Antagonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261555 (CHEMBL4088694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Agonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene as... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras-related protein Rap-1A (Homo sapiens (Human)) | BDBM50310364 (CHEMBL1077126 | YESSOTOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to RAP1A in human RBC membrane by surface plasmon resonance analysis | Bioorg Med Chem Lett 20: 6443-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.080 BindingDB Entry DOI: 10.7270/Q2R212C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras-related protein Rap-1A (Homo sapiens (Human)) | BDBM50310364 (CHEMBL1077126 | YESSOTOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to RAP1A in human RBC membrane by western blot analysis | Bioorg Med Chem Lett 20: 6443-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.080 BindingDB Entry DOI: 10.7270/Q2R212C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras-related protein Rap-1A (Homo sapiens (Human)) | BDBM50330178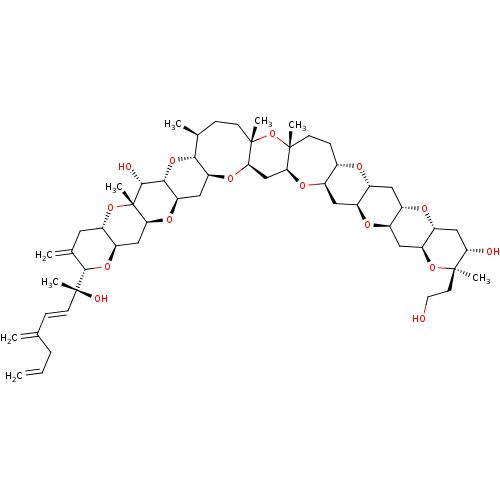 (CHEMBL1269578 | desulfo-Yessotoxin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to RAP1A in human RBC membrane by surface plasmon resonance analysis with inverted configuration | Bioorg Med Chem Lett 20: 6443-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.080 BindingDB Entry DOI: 10.7270/Q2R212C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycophorin-A (Homo sapiens (Human)) | BDBM50310365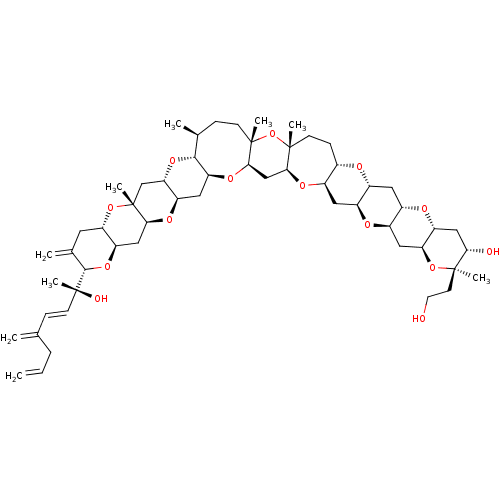 (CHEMBL1077121 | desulfated yessotoxin) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to transmembrane alpha-helix of glycophorin A by surface plasmon resonance method | Bioorg Med Chem Lett 18: 6115-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.020 BindingDB Entry DOI: 10.7270/Q2P55PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycophorin-A (Homo sapiens (Human)) | BDBM50246050 ((2R,3S,5R,6S)-5-(benzyloxy)-6-(benzyloxymethyl)-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.40E+6 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to transmembrane alpha-helix of glycophorin A by surface plasmon resonance method | Bioorg Med Chem Lett 18: 6115-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.020 BindingDB Entry DOI: 10.7270/Q2P55PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycophorin-A (Homo sapiens (Human)) | BDBM50246051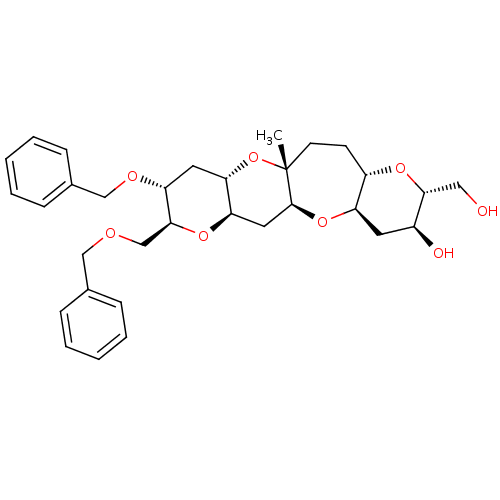 ((2R,3S,4aR,5aS,6aR,8S,9R,10aS,11aR,13aS)-9-Benzylo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to transmembrane alpha-helix of glycophorin A by surface plasmon resonance method | Bioorg Med Chem Lett 18: 6115-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.020 BindingDB Entry DOI: 10.7270/Q2P55PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261553 (CHEMBL4087989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Agonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene as... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycophorin-A (Homo sapiens (Human)) | BDBM50310364 (CHEMBL1077126 | YESSOTOXIN) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to transmembrane alpha-helix of glycophorin A by surface plasmon resonance method | Bioorg Med Chem Lett 18: 6115-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.020 BindingDB Entry DOI: 10.7270/Q2P55PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50246052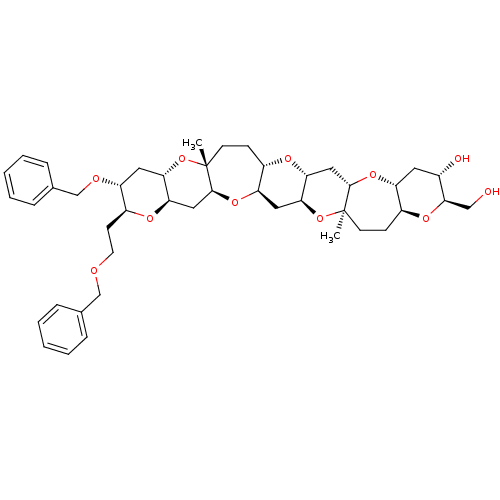 ((1S,3R,5S,7R,9S,10R,12S,14R,17S,19R,21S,23R,25S,26...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to recombinant PDE4D by surface plasmon resonance assay | Bioorg Med Chem Lett 19: 2824-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.103 BindingDB Entry DOI: 10.7270/Q2C53KZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50310369 ((2R,3S,4aR,5aS,6aR,8S,9R,10aS,11aR,13aS)-9-Benzylo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to recombinant PDE4D by surface plasmon resonance assay | Bioorg Med Chem Lett 19: 2824-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.103 BindingDB Entry DOI: 10.7270/Q2C53KZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50310368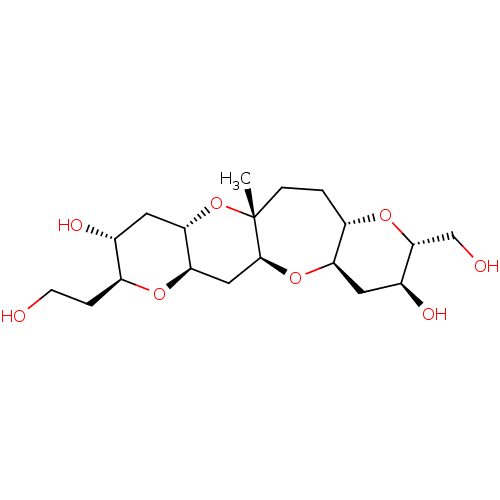 ((2R,3S,4aR,5aS,6aR,8S,9R,10aS,11aR,13aS)-8-(2-Hydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to recombinant PDE4D by surface plasmon resonance assay | Bioorg Med Chem Lett 19: 2824-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.103 BindingDB Entry DOI: 10.7270/Q2C53KZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50310367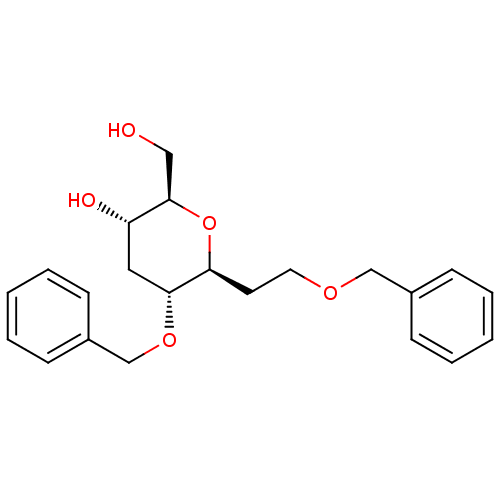 ((2R,3S,5R,6S)-5-(benzyloxy)-6-(2-(benzyloxy)ethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to recombinant PDE4D by surface plasmon resonance assay | Bioorg Med Chem Lett 19: 2824-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.103 BindingDB Entry DOI: 10.7270/Q2C53KZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50310366 (Brevetoxin | CHEMBL1077122) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to recombinant PDE4D by surface plasmon resonance assay | Bioorg Med Chem Lett 19: 2824-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.103 BindingDB Entry DOI: 10.7270/Q2C53KZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50310365 (CHEMBL1077121 | desulfated yessotoxin) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to recombinant PDE4D by surface plasmon resonance assay | Bioorg Med Chem Lett 19: 2824-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.103 BindingDB Entry DOI: 10.7270/Q2C53KZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50310364 (CHEMBL1077126 | YESSOTOXIN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to recombinant PDE4D by surface plasmon resonance assay | Bioorg Med Chem Lett 19: 2824-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.103 BindingDB Entry DOI: 10.7270/Q2C53KZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50261554 (CHEMBL4092300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Agonist activity at full length ERalpha (unknown origin) expressed in human HeLa cells incubated for 24 hrs by ERE-driven luciferase reporter gene as... | Bioorg Med Chem 25: 5216-5237 (2017) Article DOI: 10.1016/j.bmc.2017.07.067 BindingDB Entry DOI: 10.7270/Q26H4KVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycophorin-A (Homo sapiens (Human)) | BDBM50246052 ((1S,3R,5S,7R,9S,10R,12S,14R,17S,19R,21S,23R,25S,26...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Binding affinity to transmembrane alpha-helix of glycophorin A by surface plasmon resonance method | Bioorg Med Chem Lett 18: 6115-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.020 BindingDB Entry DOI: 10.7270/Q2P55PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||