

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM85786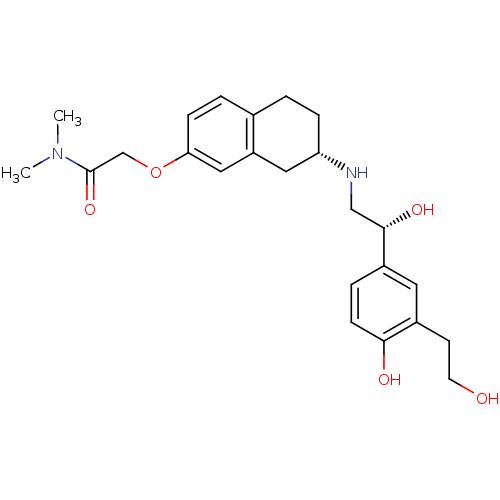 (KUR-1246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 25.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 666-71 (2001) BindingDB Entry DOI: 10.7270/Q2D798Z1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018671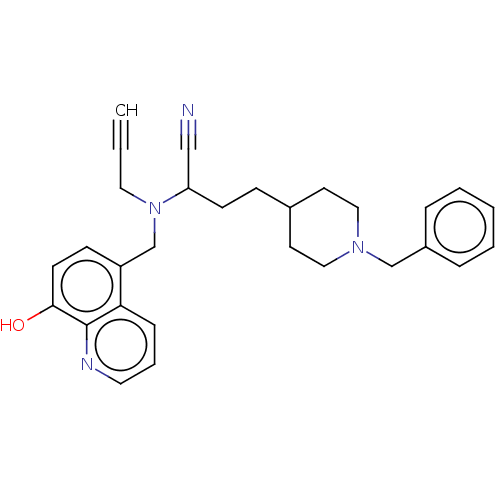 (CHEMBL3291019) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Reversible inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM85786 (KUR-1246) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 297: 666-71 (2001) BindingDB Entry DOI: 10.7270/Q2D798Z1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282377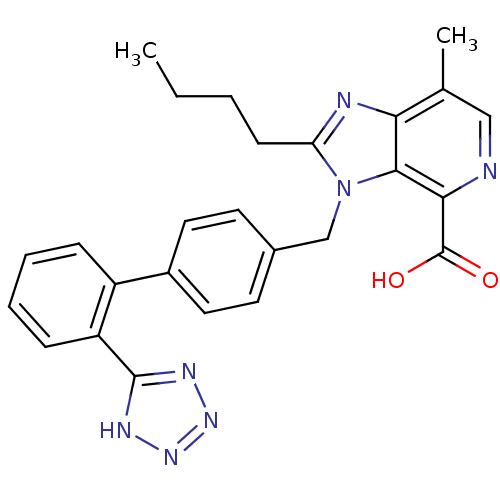 (2-Butyl-7-methyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex | Bioorg Med Chem Lett 4: 35-40 (1994) Article DOI: 10.1016/S0960-894X(01)81118-5 BindingDB Entry DOI: 10.7270/Q21Z44CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282379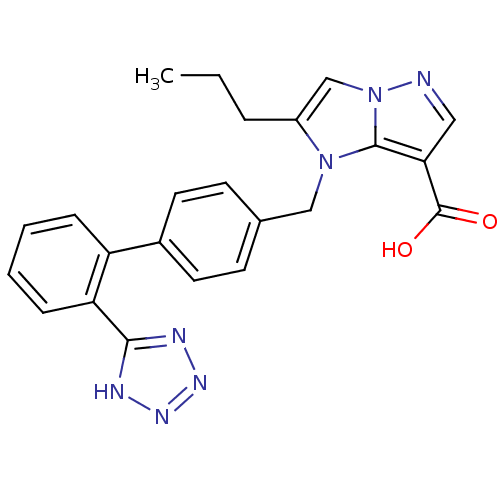 (2-Propyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex | Bioorg Med Chem Lett 4: 35-40 (1994) Article DOI: 10.1016/S0960-894X(01)81118-5 BindingDB Entry DOI: 10.7270/Q21Z44CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282373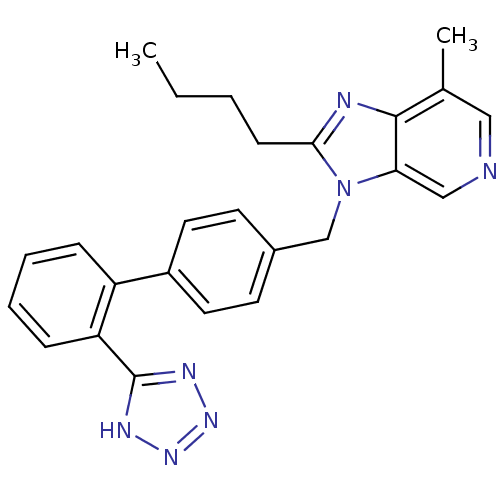 (2-Butyl-7-methyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex | Bioorg Med Chem Lett 4: 35-40 (1994) Article DOI: 10.1016/S0960-894X(01)81118-5 BindingDB Entry DOI: 10.7270/Q21Z44CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM36551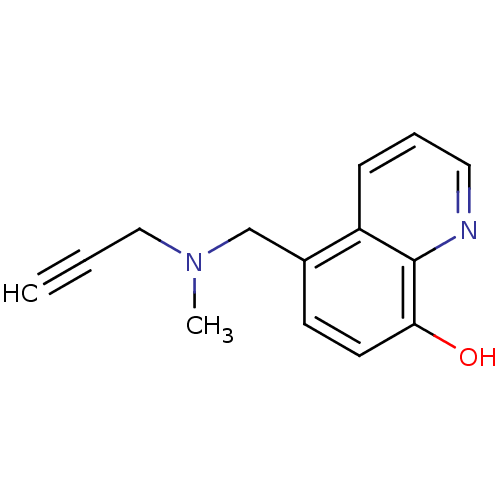 (Chelator, M30 | US9034303, M30) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50240609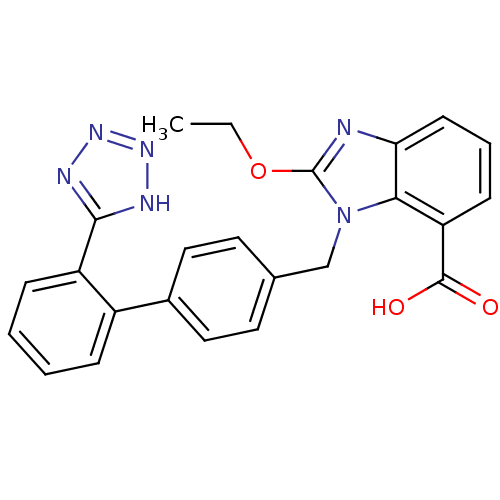 (2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex | Bioorg Med Chem Lett 4: 35-40 (1994) Article DOI: 10.1016/S0960-894X(01)81118-5 BindingDB Entry DOI: 10.7270/Q21Z44CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282374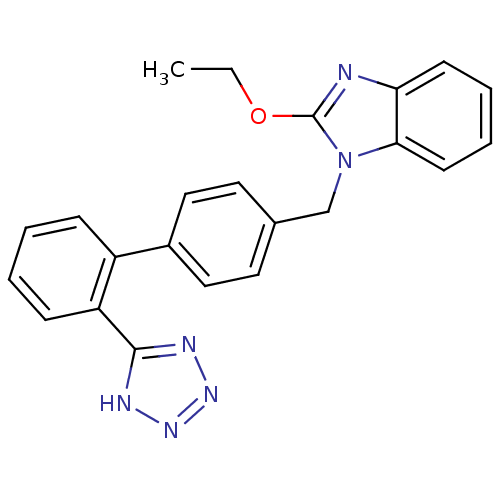 (2-Ethoxy-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex | Bioorg Med Chem Lett 4: 35-40 (1994) Article DOI: 10.1016/S0960-894X(01)81118-5 BindingDB Entry DOI: 10.7270/Q21Z44CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282376 (2-Ethylsulfanyl-4-methyl-1-[2'-(1H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex | Bioorg Med Chem Lett 4: 35-40 (1994) Article DOI: 10.1016/S0960-894X(01)81118-5 BindingDB Entry DOI: 10.7270/Q21Z44CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate preincubated for 240 mins | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate preincubated for 240 mins | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282378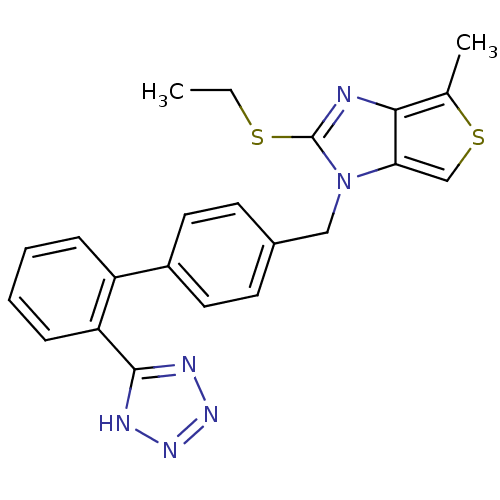 (2-Ethylsulfanyl-4-methyl-1-[2'-(1H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex | Bioorg Med Chem Lett 4: 35-40 (1994) Article DOI: 10.1016/S0960-894X(01)81118-5 BindingDB Entry DOI: 10.7270/Q21Z44CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM36551 (Chelator, M30 | US9034303, M30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50018671 (CHEMBL3291019) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018671 (CHEMBL3291019) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate preincubated for 60 mins | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018672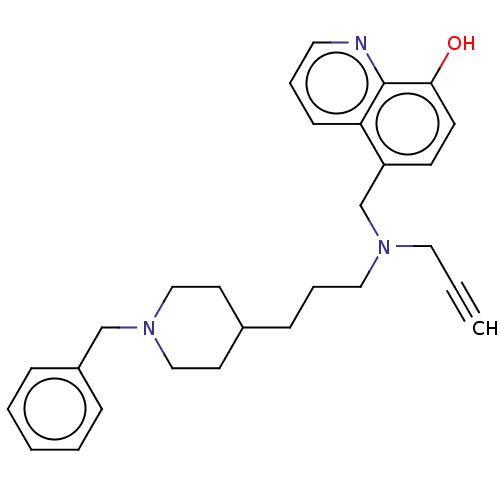 (CHEMBL3291020) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate preincubated for 60 mins | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282375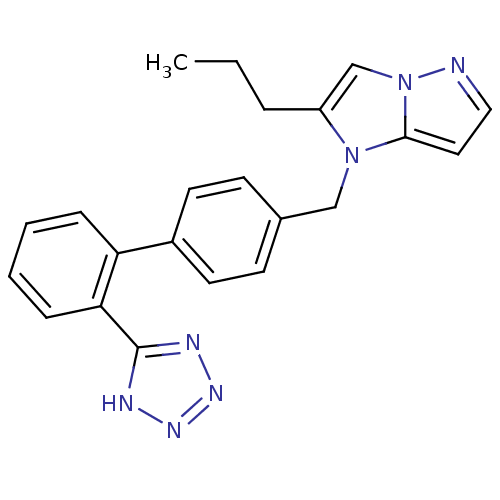 (2-Propyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex | Bioorg Med Chem Lett 4: 35-40 (1994) Article DOI: 10.1016/S0960-894X(01)81118-5 BindingDB Entry DOI: 10.7270/Q21Z44CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate preincubated for 30 mins | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50018672 (CHEMBL3291020) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018673 (CHEMBL3291018) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018674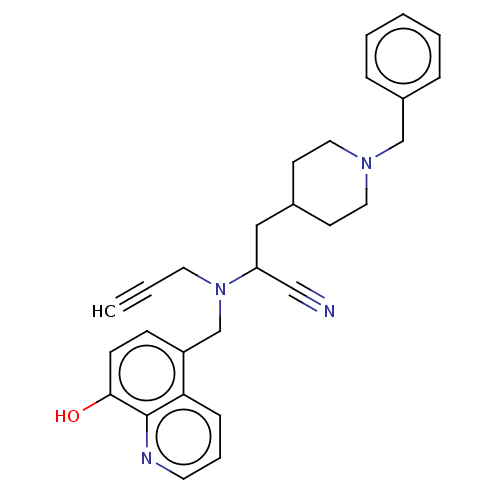 (CHEMBL3291017) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50018674 (CHEMBL3291017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate preincubated for 30 mins | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50018674 (CHEMBL3291017) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50018674 (CHEMBL3291017) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50018675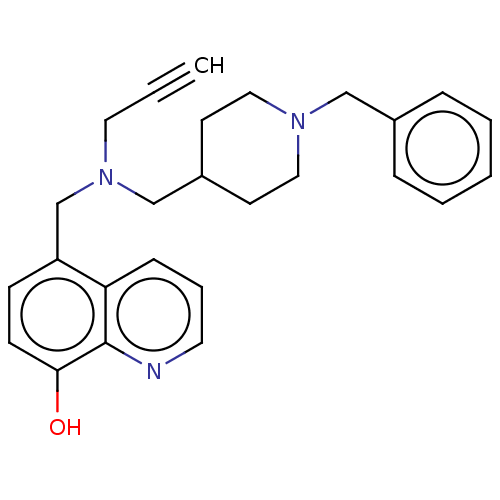 (CHEMBL3291016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50018676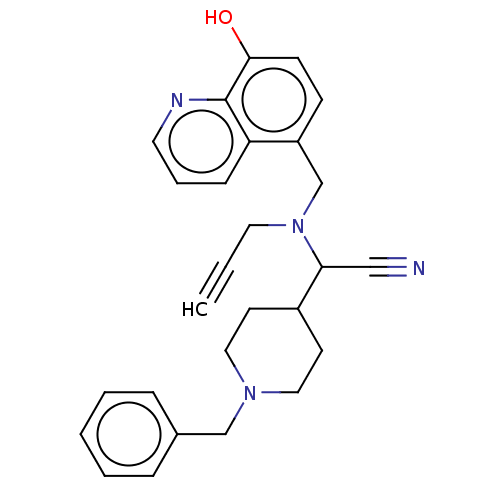 (CHEMBL3291015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018676 (CHEMBL3291015) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50018673 (CHEMBL3291018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018675 (CHEMBL3291016) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50018675 (CHEMBL3291016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM50009999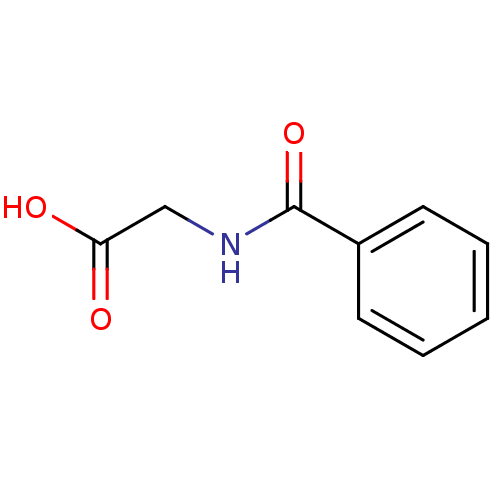 (CHEMBL461 | N-benzoylglycine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Women's Medical University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in OAT-expressing OK cells | Br J Pharmacol 135: 555-63 (2002) Article DOI: 10.1038/sj.bjp.0704482 BindingDB Entry DOI: 10.7270/Q21J9C29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50018676 (CHEMBL3291015) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM50420239 (CHEMBL500596) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Women's Medical University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in OAT1-expressing OK cells | Br J Pharmacol 135: 555-63 (2002) Article DOI: 10.1038/sj.bjp.0704482 BindingDB Entry DOI: 10.7270/Q21J9C29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM50328021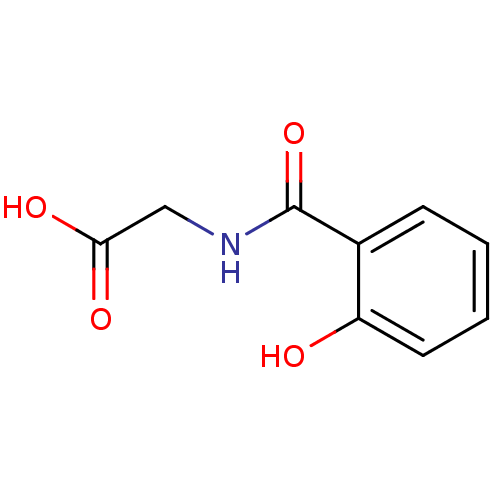 ((2-Hydroxy-benzoylamino)-acetic acid | 2-(2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Women's Medical University Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in OAT-expressing OK cells | Br J Pharmacol 135: 555-63 (2002) Article DOI: 10.1038/sj.bjp.0704482 BindingDB Entry DOI: 10.7270/Q21J9C29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50018672 (CHEMBL3291020) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50018676 (CHEMBL3291015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |