

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430060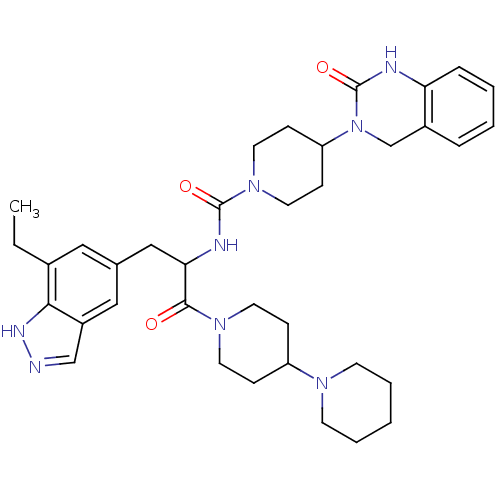 (CHEMBL2336421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50273292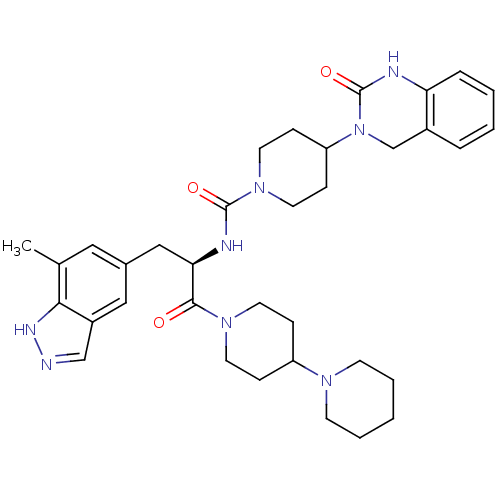 ((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research Curated by PDSP Ki Database | Br J Pharmacol 115: 622-8 (1995) Article DOI: 10.1111/j.1476-5381.1995.tb14977.x BindingDB Entry DOI: 10.7270/Q2BR8QP0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50273292 ((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [I125]CGRP from human CGRP receptor in SK-N-MC cells | J Med Chem 51: 4858-61 (2008) Article DOI: 10.1021/jm800546t BindingDB Entry DOI: 10.7270/Q2N016BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430061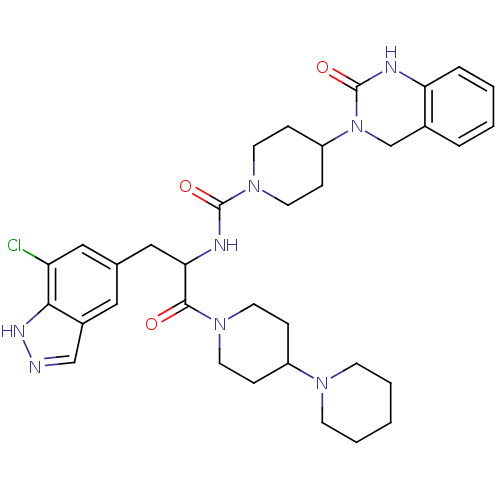 (CHEMBL2336422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50273292 ((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268484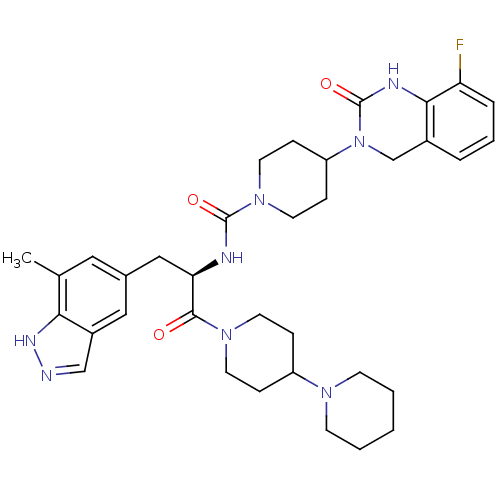 ((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [I125]CGRP from human CGRP receptor in SK-N-MC cells | J Med Chem 51: 4858-61 (2008) Article DOI: 10.1021/jm800546t BindingDB Entry DOI: 10.7270/Q2N016BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268484 ((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430062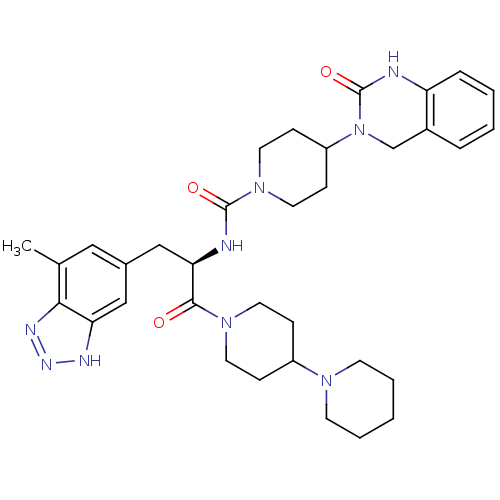 (CHEMBL2336411) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430066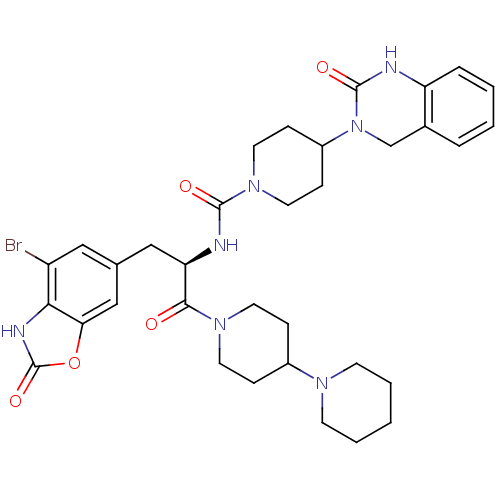 (CHEMBL2336416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430058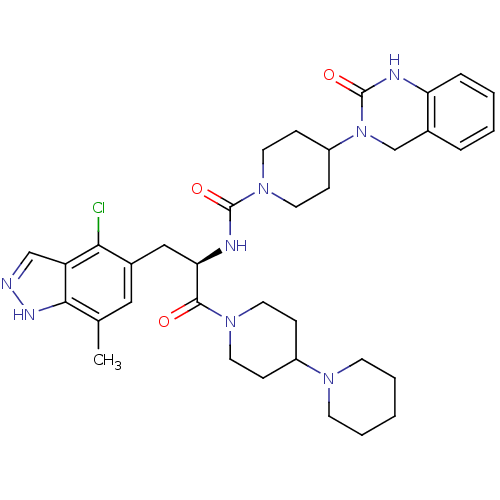 (CHEMBL2336418) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430057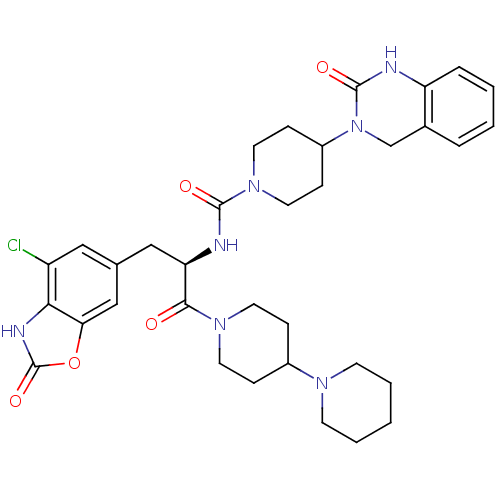 (CHEMBL2336417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498218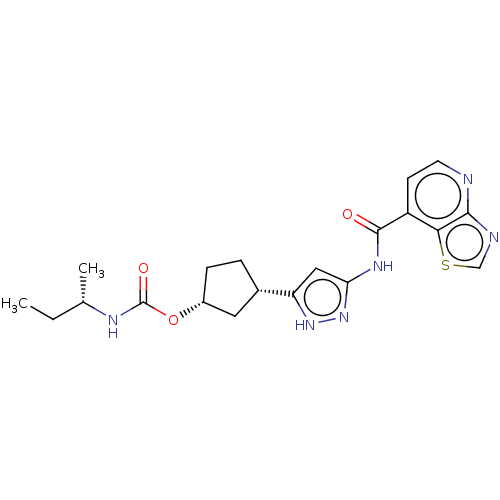 (US11014911, Example 43 | US11718603, Example 43) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498218 (US11014911, Example 43 | US11718603, Example 43) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370133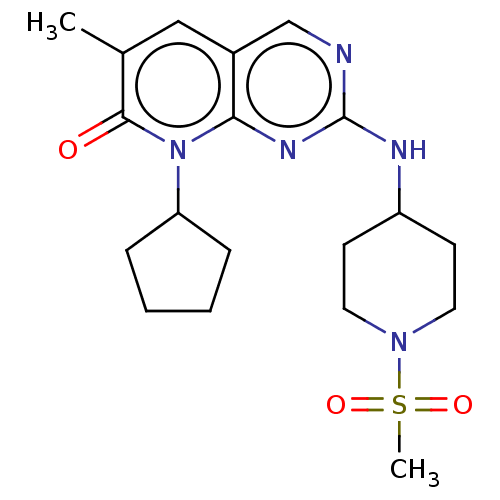 (US10233188, Example 22 | US10800783, Example 22 | ...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430059 (CHEMBL2336419) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498217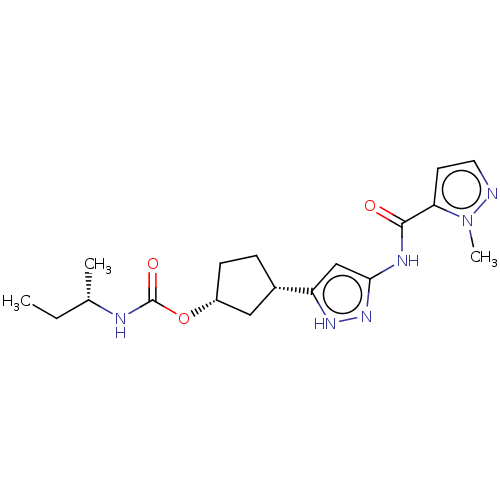 (US11014911, Example 42 | US11718603, Example 42) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498217 (US11014911, Example 42 | US11718603, Example 42) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430065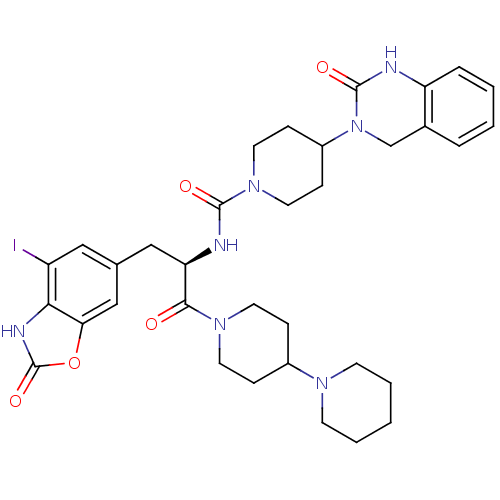 (CHEMBL2336415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370149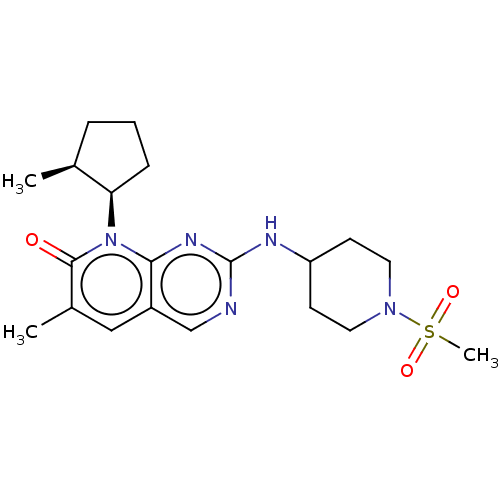 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370115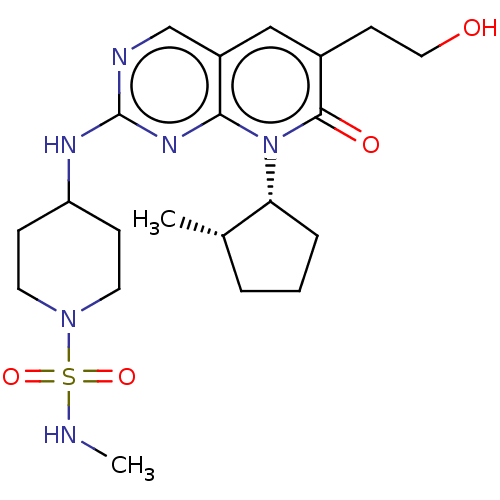 (4-({6-(2-hydroxyethyl)-8-[(1R,2S)-2-methylcyclopen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370115 (4-({6-(2-hydroxyethyl)-8-[(1R,2S)-2-methylcyclopen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498324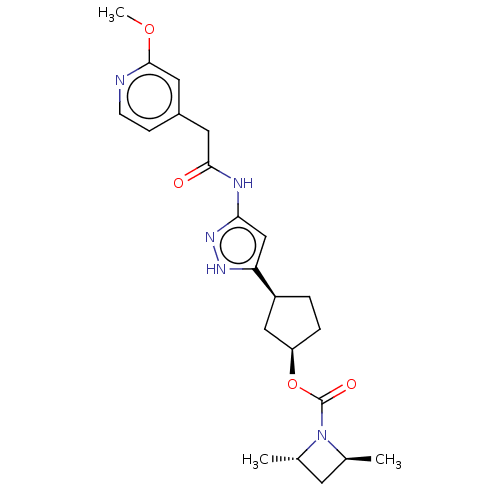 (US11014911, Example 149 | US11718603, Example 149) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370115 (4-({6-(2-hydroxyethyl)-8-[(1R,2S)-2-methylcyclopen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The mobility shift assay electrophoretically separates the fluorescently labeled peptides (substrate and phosphorylated product) following the kinase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The mobility shift assay electrophoretically separates the fluorescently labeled peptides (substrate and phosphorylated product) following the kinase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM370149 (US10233188, Example 37 | US10800783, Example 37 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KK9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498324 (US11014911, Example 149 | US11718603, Example 149) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM467013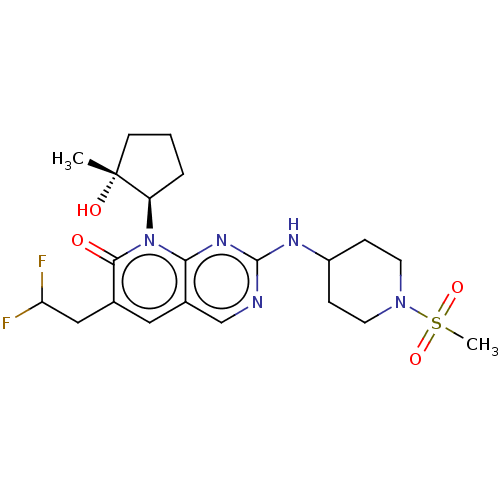 ((-)-6-(2,2-difluoroethyl)-8-[(1R*,2R*)-2-hydroxy-2...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498321 (US11014911, Example 146 | US11718603, Example 146) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498778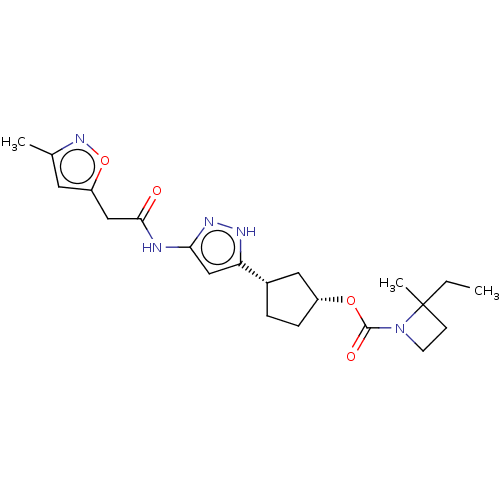 ((1R,3S)-3-(3-{[(3-methyl-1,2- oxazol-5-yl)acetyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | CHEMBL5273346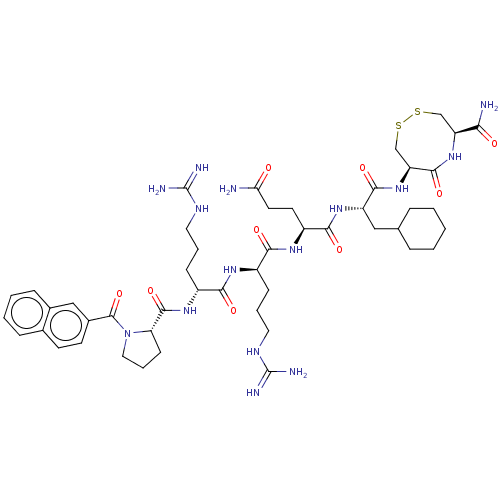 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498778 ((1R,3S)-3-(3-{[(3-methyl-1,2- oxazol-5-yl)acetyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498321 (US11014911, Example 146 | US11718603, Example 146) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370152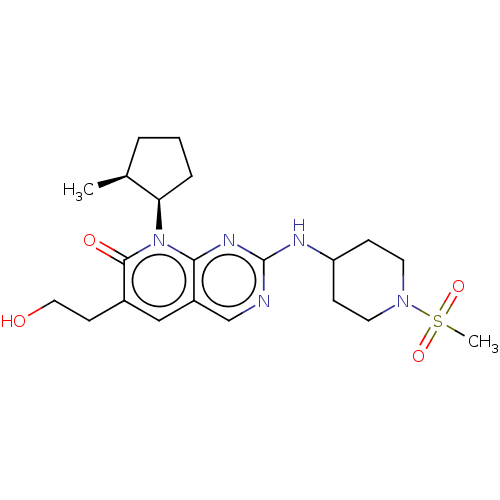 (US10233188, Example 40 | US10800783, Example 40 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498778 ((1R,3S)-3-(3-{[(3-methyl-1,2- oxazol-5-yl)acetyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370300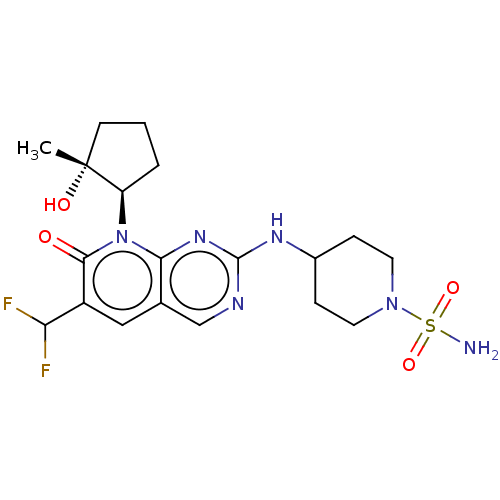 (BDBM467195 | US10233188, Example 187) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370197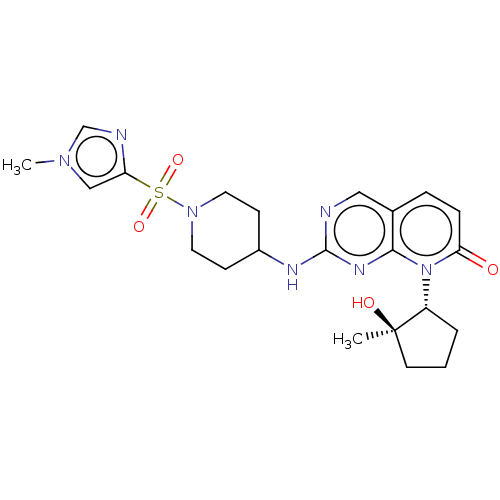 (US10233188, Example 84 | US10800783, Example 84 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | J Med Chem 45: 1477-86 (2002) BindingDB Entry DOI: 10.7270/Q2FX7CSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370197 (US10233188, Example 84 | US10800783, Example 84 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370205 (US10233188, Example 92 | US10800783, Example 92 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370121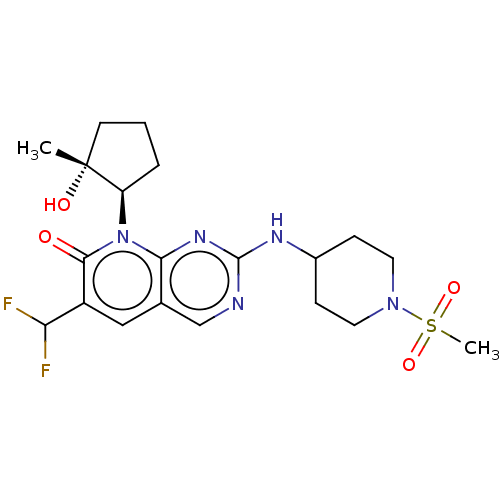 (6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370152 (US10233188, Example 40 | US10800783, Example 40 | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D1 (Homo sapiens (Human)-Mus musculus (mouse)) | BDBM370162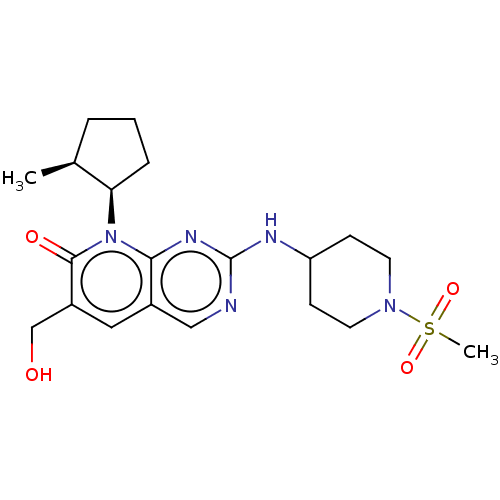 (US10233188, Example 50 | US10800783, Example 50 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK6/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of human P2Y14R | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM370300 (BDBM467195 | US10233188, Example 187) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of the CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a flu... | US Patent US10800783 (2020) BindingDB Entry DOI: 10.7270/Q24X5BVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498316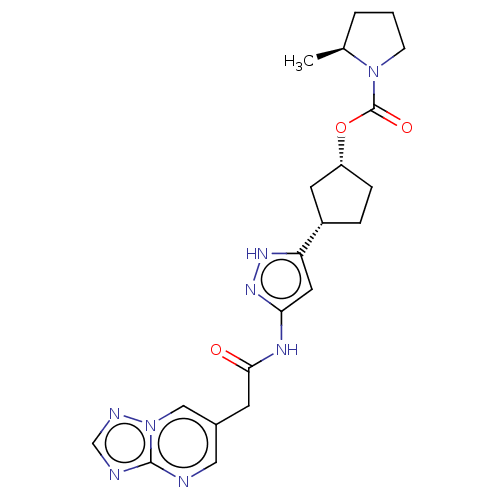 (US11014911, Example 141 | US11718603, Example 141) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498295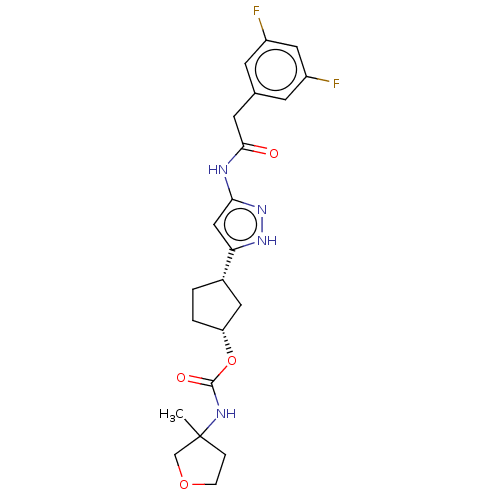 (US11014911, Example 120 | US11014911, Example 121 ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM498778 ((1R,3S)-3-(3-{[(3-methyl-1,2- oxazol-5-yl)acetyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of CDK2/Cyclin E1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluores... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 13075 total ) | Next | Last >> |