

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin receptor type B (Sus scrofa) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287890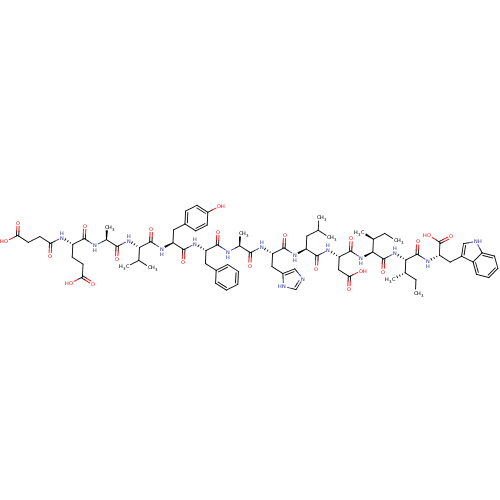 (CHEMBL427778 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50274347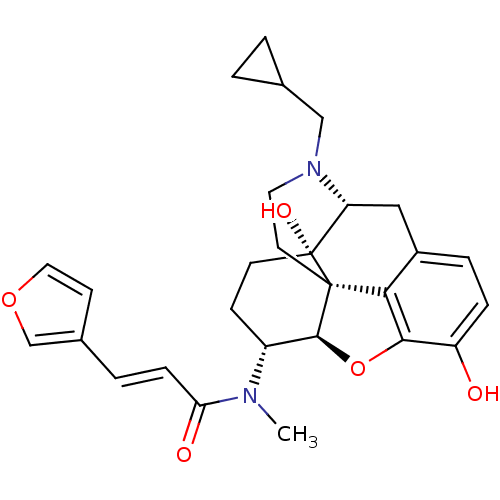 ((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287885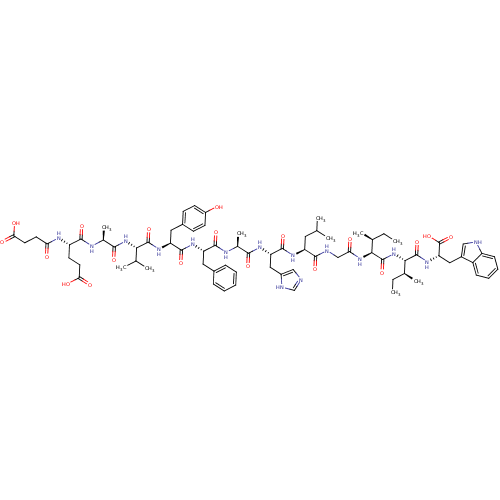 (CHEMBL405377 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287883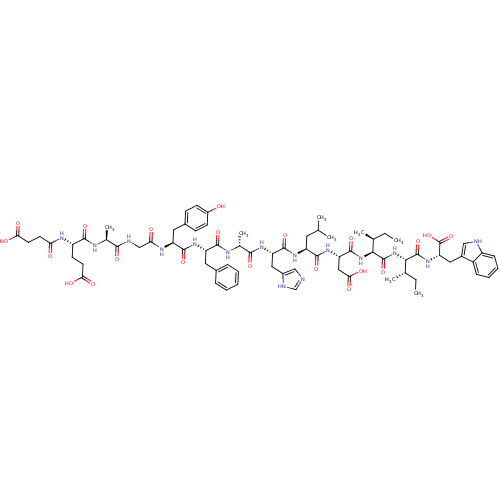 (CHEMBL412003 | Suc-Glu-Ala-Gly-Tyr-Phe-Ala-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50071433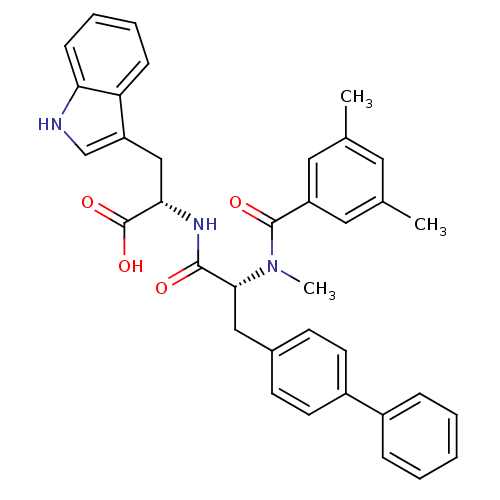 ((S)-2-{(R)-3-Biphenyl-4-yl-2-[(3,5-dimethyl-benzoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287882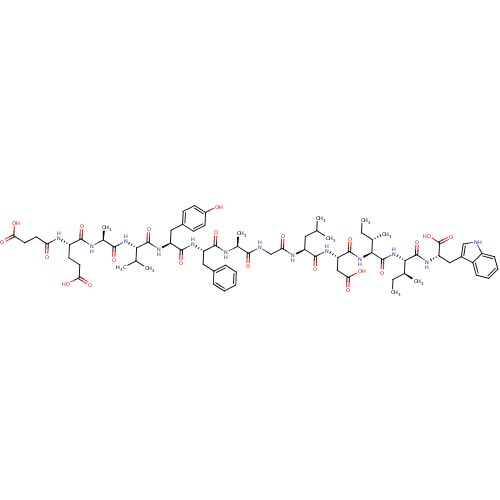 (CHEMBL412065 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-Gly-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230035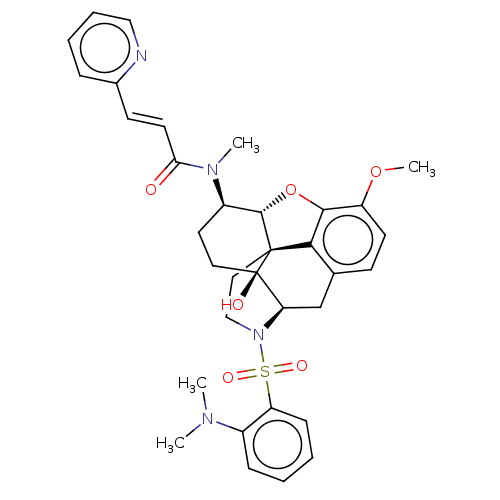 (CHEMBL4091544) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230164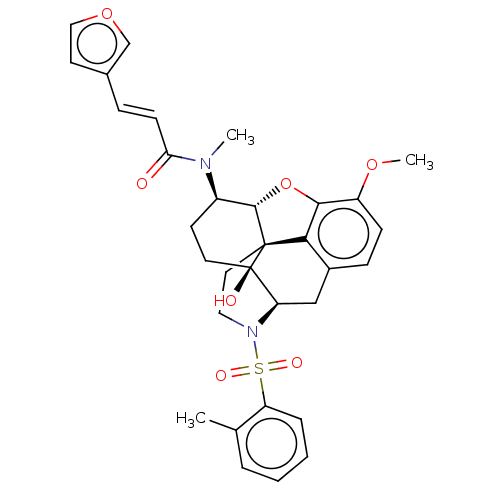 (CHEMBL4091834 | US10377763, Example 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230139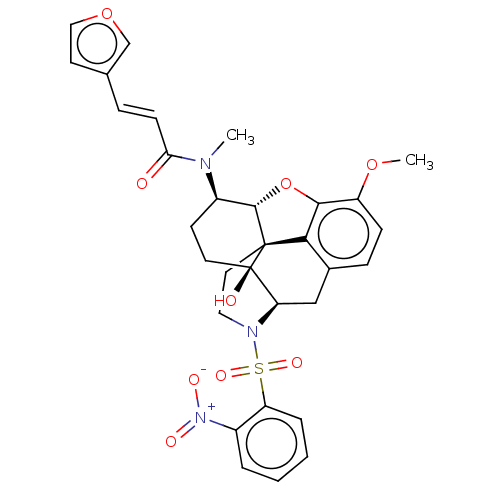 (CHEMBL4105072 | US10377763, Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230138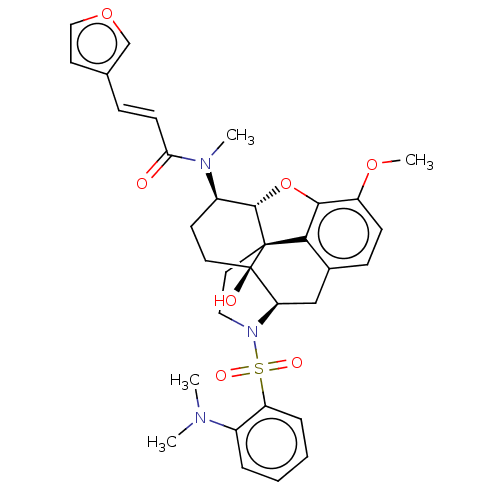 (CHEMBL4094318) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230161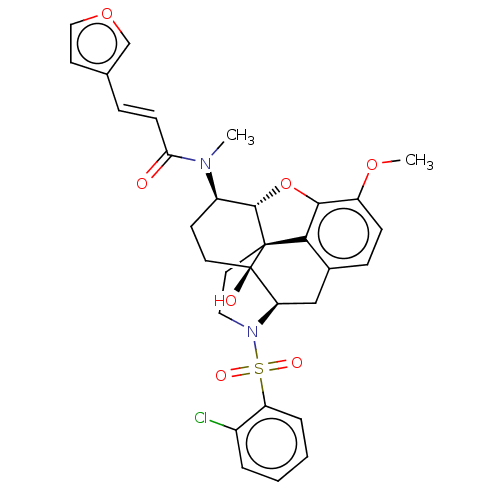 (CHEMBL4089496) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230158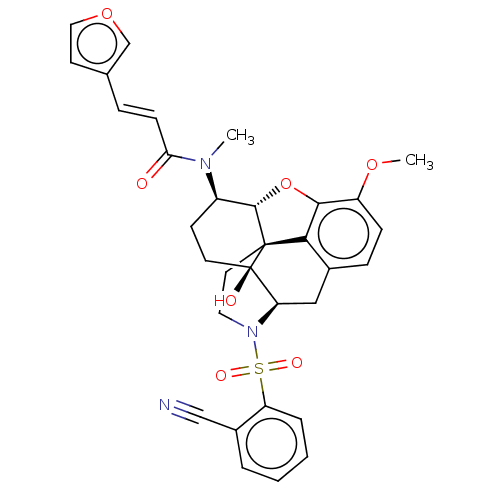 (CHEMBL4073947 | US10377763, Example 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230134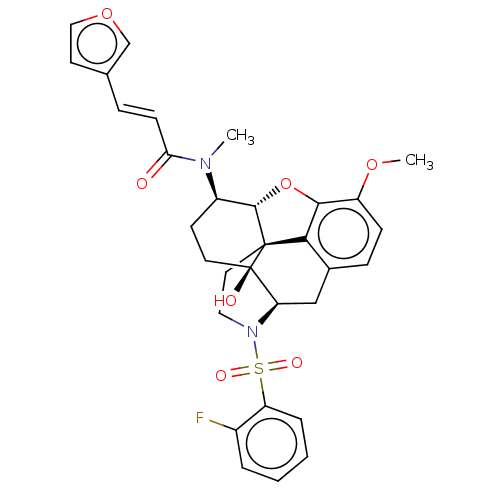 (CHEMBL4087046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230144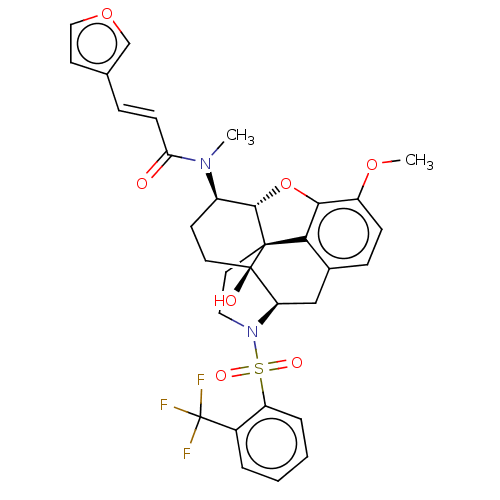 (CHEMBL4081763 | US10377763, Example 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230141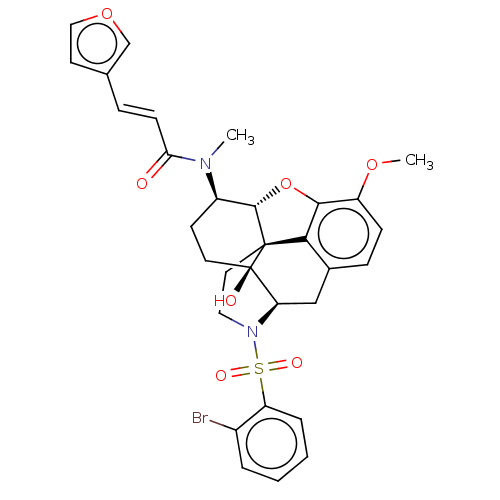 (CHEMBL4083587 | US10377763, Example 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230167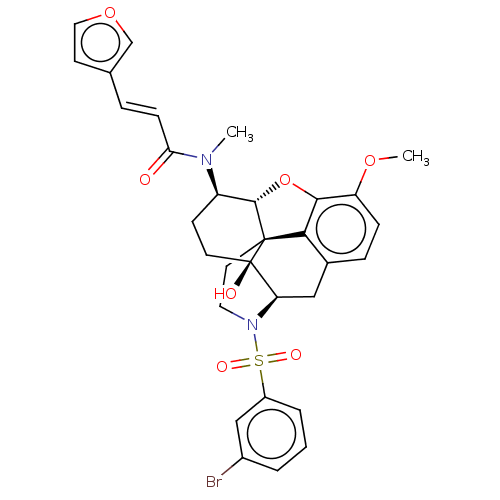 (CHEMBL4071220 | US10377763, Example 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230160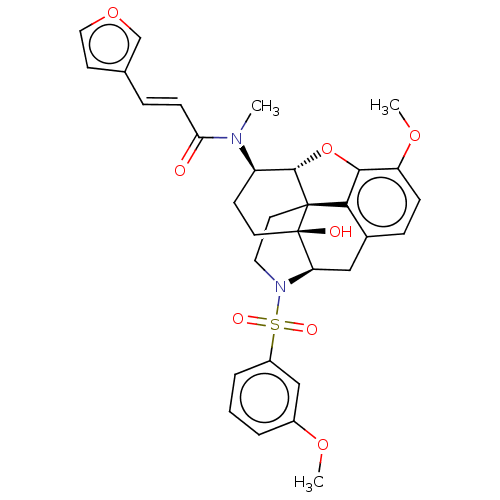 (CHEMBL4065120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078977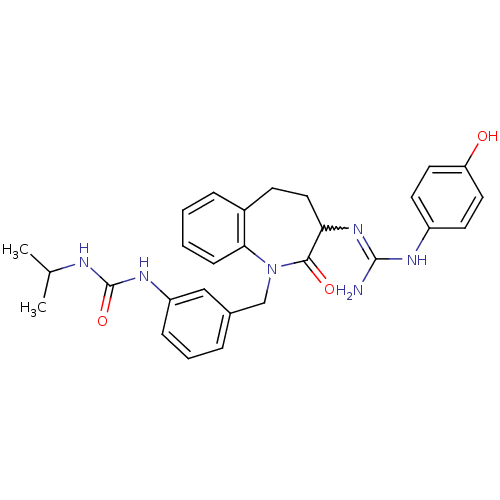 (1-(3-{3-[N'-(4-Hydroxy-phenyl)-guanidino]-2-oxo-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230156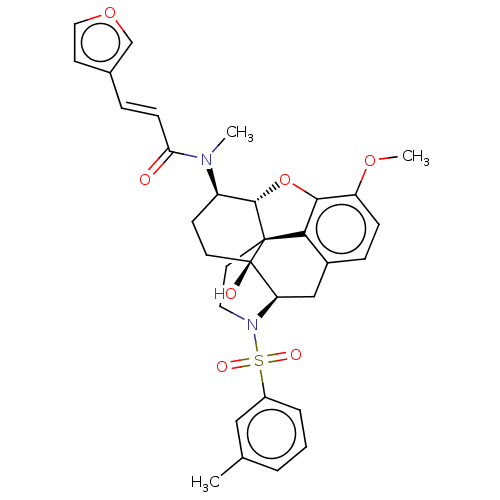 (CHEMBL4097697 | US10377763, Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230136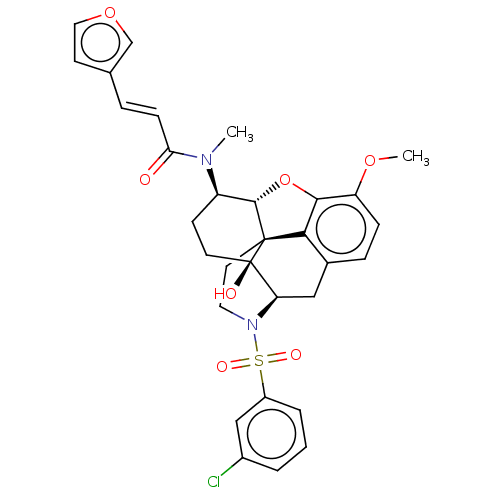 (CHEMBL4068322 | US10377763, Example 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230166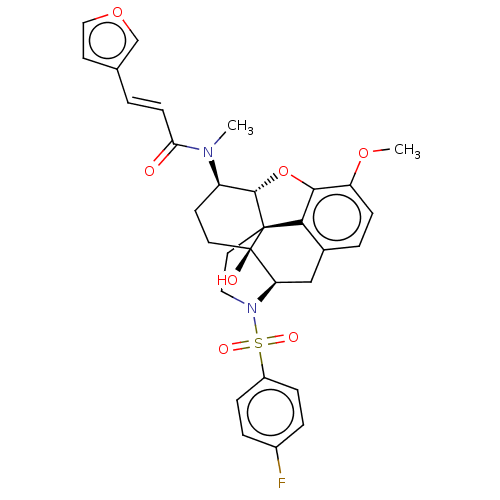 (CHEMBL4079180) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230142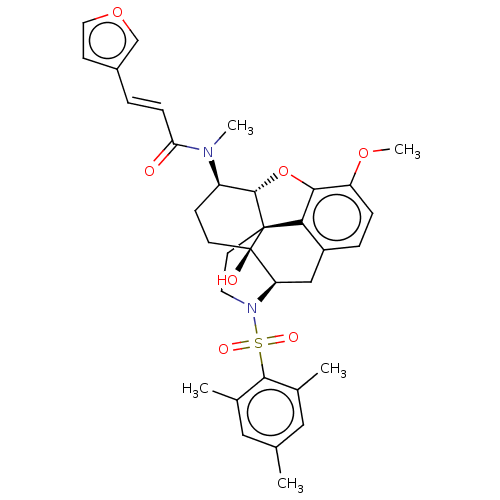 (CHEMBL4099280 | US10377763, Example 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230029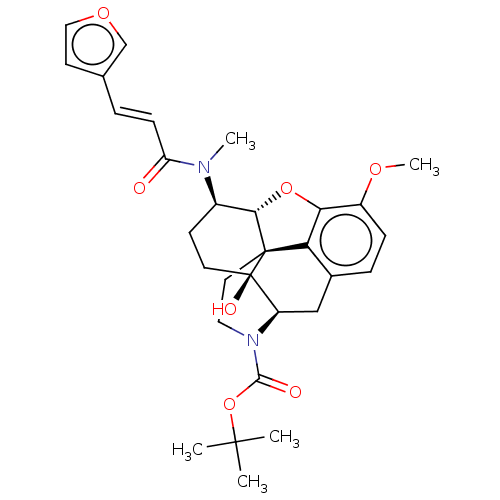 (CHEMBL4071362) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50526575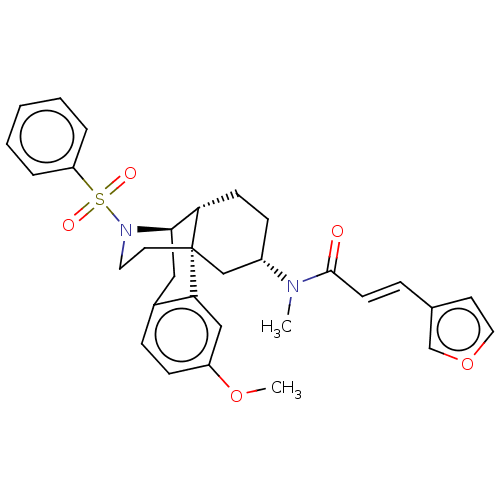 (CHEMBL4456012) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium mobilization preincubated for 1... | Bioorg Med Chem 27: 1747-1758 (2019) Article DOI: 10.1016/j.bmc.2019.03.010 BindingDB Entry DOI: 10.7270/Q2GB27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287878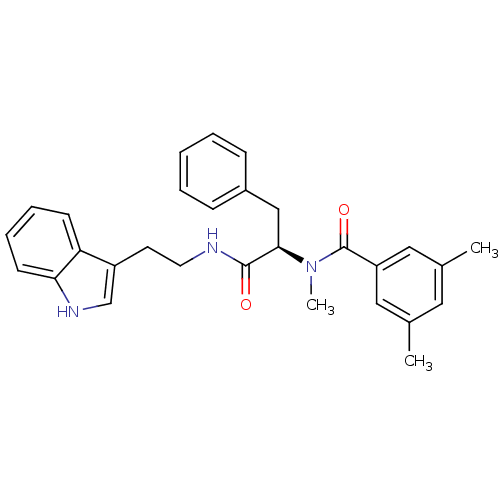 (CGP-49941 | CHEMBL305615 | N-{(R)-1-[2-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078965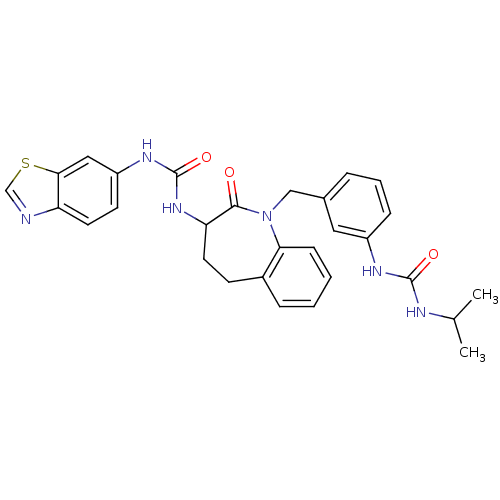 (1-Benzothiazol-6-yl-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230145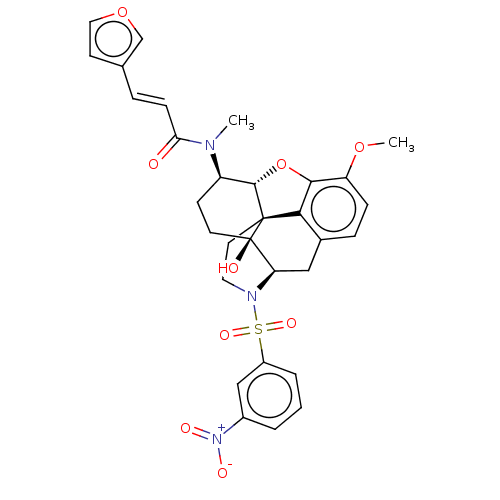 (CHEMBL4080833) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230148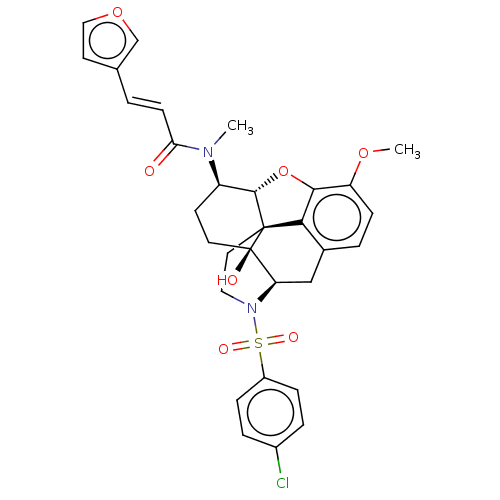 (CHEMBL4101590) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287888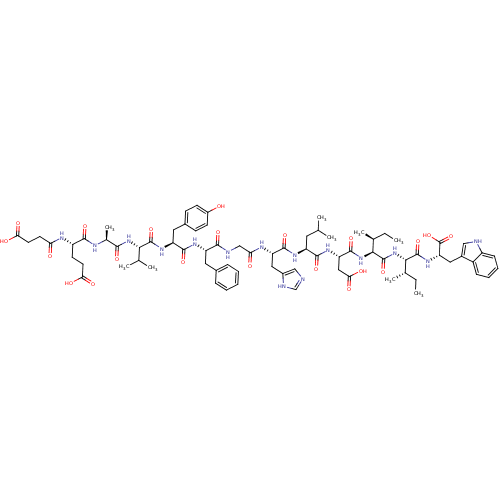 (CHEMBL407559 | Suc-Glu-Ala-Val-Tyr-Phe-Gly-His-Leu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50526577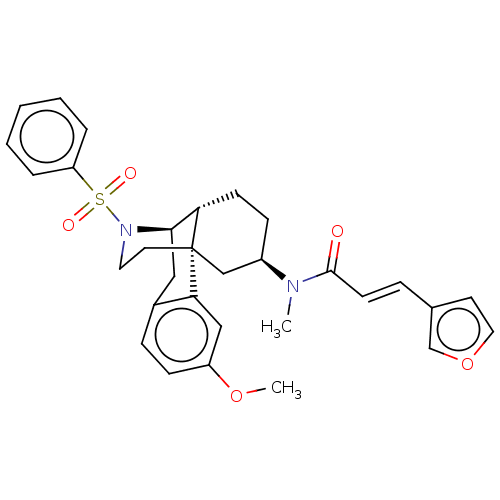 (CHEMBL4457194) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium mobilization preincubated for 1... | Bioorg Med Chem 27: 1747-1758 (2019) Article DOI: 10.1016/j.bmc.2019.03.010 BindingDB Entry DOI: 10.7270/Q2GB27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50287886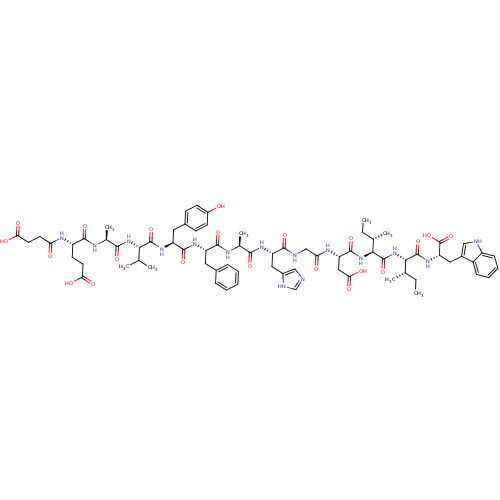 (CHEMBL405796 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated | Bioorg Med Chem Lett 6: 2323-2328 (1996) Article DOI: 10.1016/0960-894X(96)00421-0 BindingDB Entry DOI: 10.7270/Q21J9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230168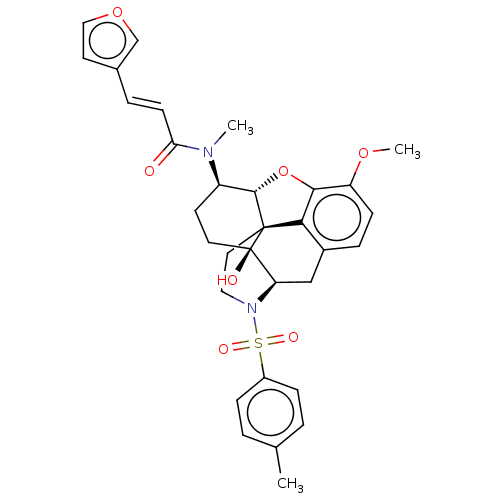 (CHEMBL4079662 | US10377763, Example 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50526573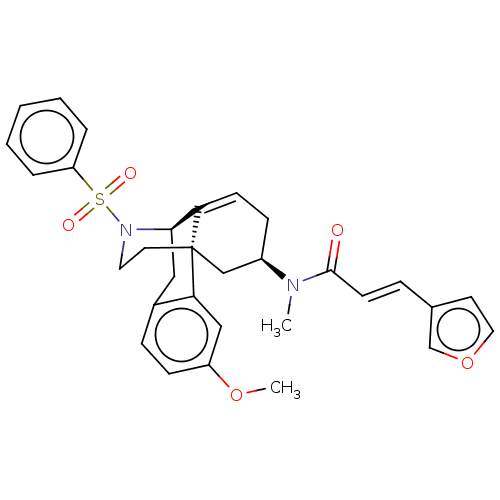 (CHEMBL4448949) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium mobilization preincubated for 1... | Bioorg Med Chem 27: 1747-1758 (2019) Article DOI: 10.1016/j.bmc.2019.03.010 BindingDB Entry DOI: 10.7270/Q2GB27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230155 (CHEMBL4094746 | US10377763, Example 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50274347 ((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230146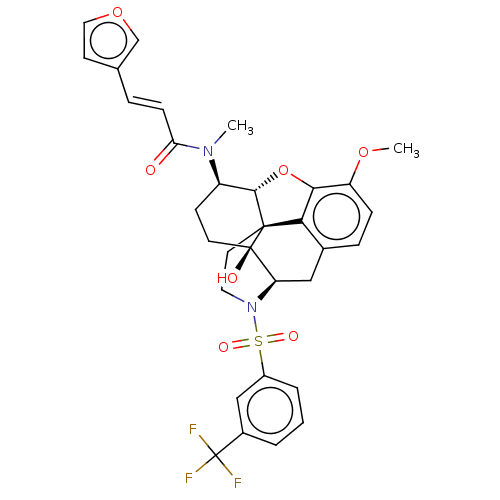 (CHEMBL4099781) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230163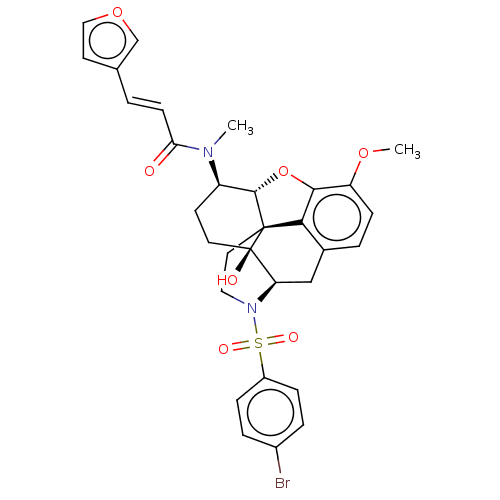 (CHEMBL4092034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230162 (CHEMBL4084072) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079009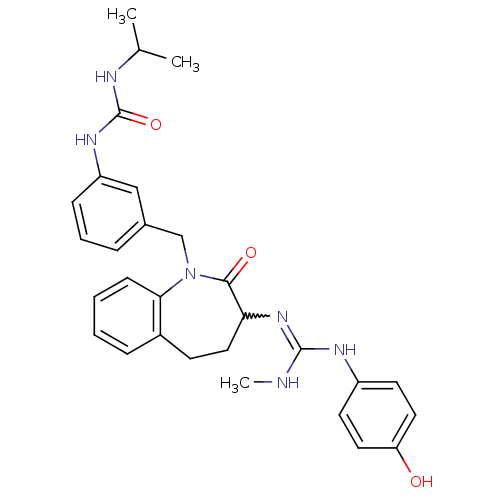 (1-(3-{3-[N'-(4-Hydroxy-phenyl)-N''-methyl-guanidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230165 (CHEMBL4062575) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230137 (CHEMBL4073714) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50526576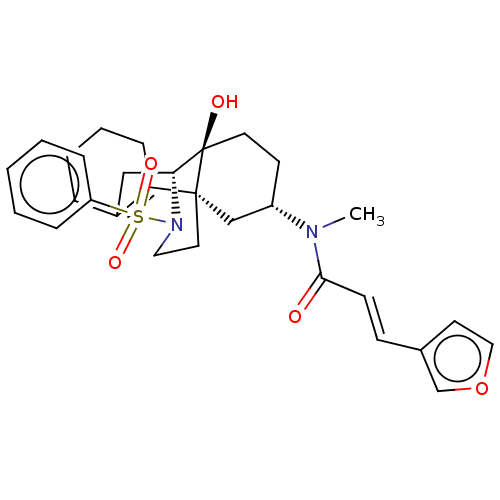 (CHEMBL4588327) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium mobilization preincubated for 1... | Bioorg Med Chem 27: 1747-1758 (2019) Article DOI: 10.1016/j.bmc.2019.03.010 BindingDB Entry DOI: 10.7270/Q2GB27HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078970 (1-(2-Fluoro-phenyl)-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230154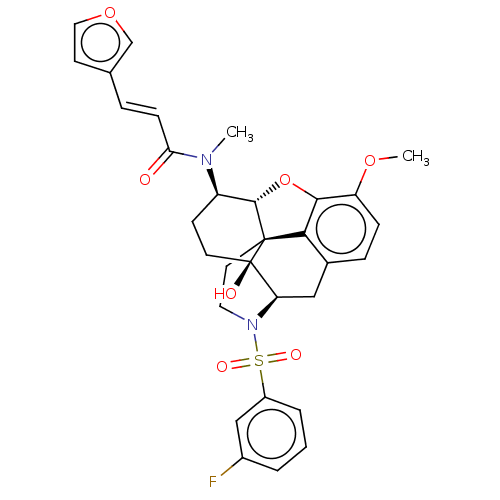 (CHEMBL4097196 | US10377763, Example 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079015 (1-(2,4-Difluoro-phenyl)-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079012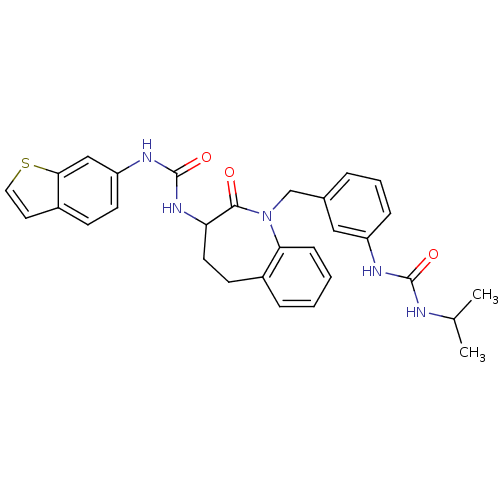 (1-Benzo[b]thiophen-6-yl-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079016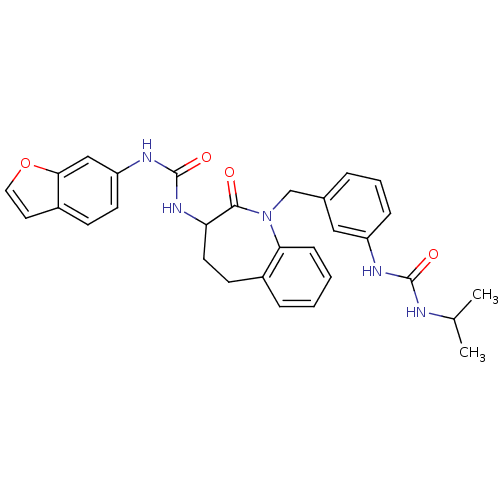 (1-Benzofuran-6-yl-3-{1-[3-(3-isopropyl-ureido)-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230031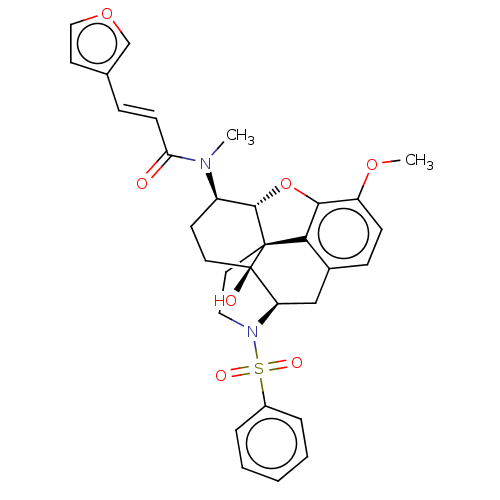 (CHEMBL4080914) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1117 total ) | Next | Last >> |