 Found 31 hits with Last Name = 'okerberg' and Initial = 'c'
Found 31 hits with Last Name = 'okerberg' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133601
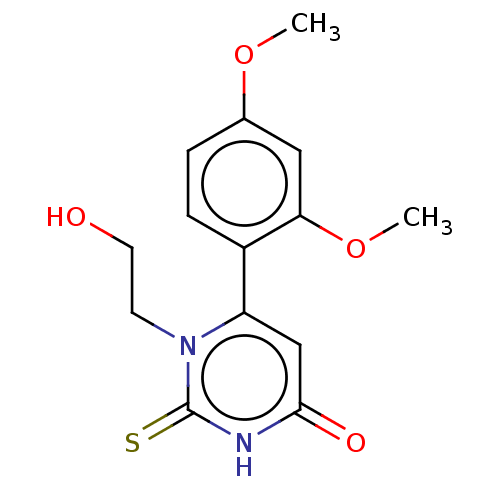
(CHEMBL3633251)Show InChI InChI=1S/C14H16N2O4S/c1-19-9-3-4-10(12(7-9)20-2)11-8-13(18)15-14(21)16(11)5-6-17/h3-4,7-8,17H,5-6H2,1-2H3,(H,15,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133602
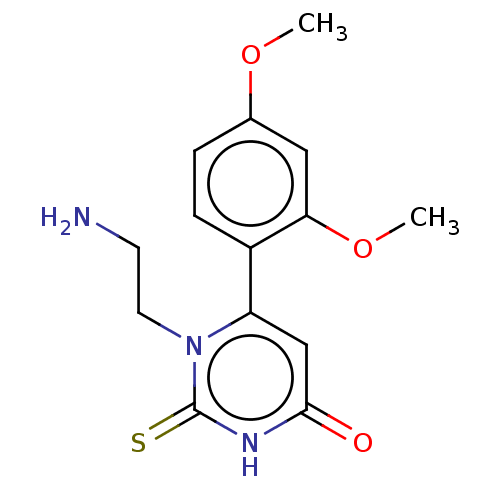
(CHEMBL3633250)Show InChI InChI=1S/C14H17N3O3S/c1-19-9-3-4-10(12(7-9)20-2)11-8-13(18)16-14(21)17(11)6-5-15/h3-4,7-8H,5-6,15H2,1-2H3,(H,16,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133596
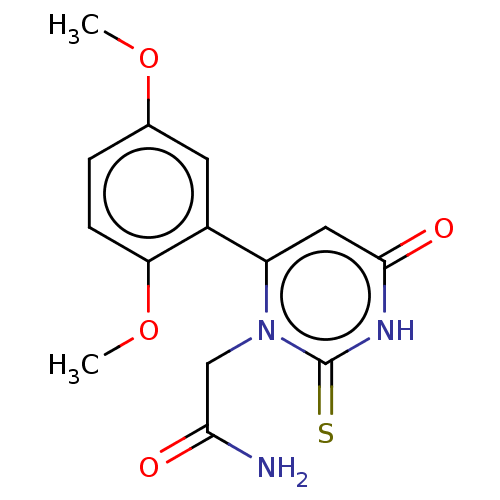
(CHEMBL3633459)Show SMILES COc1ccc(OC)c(c1)-c1cc(=O)[nH]c(=S)n1CC(N)=O Show InChI InChI=1S/C14H15N3O4S/c1-20-8-3-4-11(21-2)9(5-8)10-6-13(19)16-14(22)17(10)7-12(15)18/h3-6H,7H2,1-2H3,(H2,15,18)(H,16,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133603
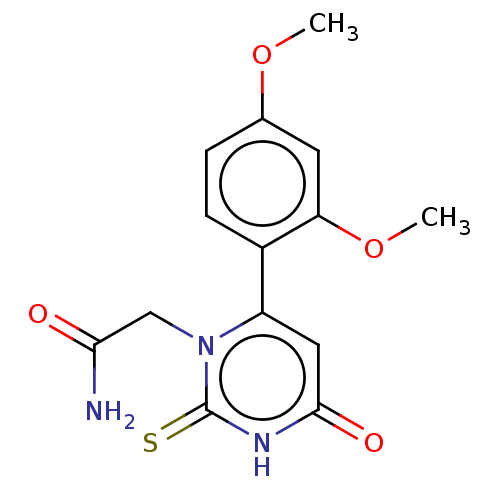
(CHEMBL3633248)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CC(N)=O Show InChI InChI=1S/C14H15N3O4S/c1-20-8-3-4-9(11(5-8)21-2)10-6-13(19)16-14(22)17(10)7-12(15)18/h3-6H,7H2,1-2H3,(H2,15,18)(H,16,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133600
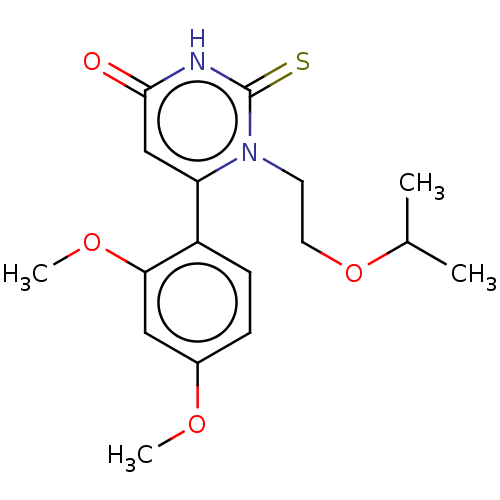
(CHEMBL3633457)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CCOC(C)C Show InChI InChI=1S/C17H22N2O4S/c1-11(2)23-8-7-19-14(10-16(20)18-17(19)24)13-6-5-12(21-3)9-15(13)22-4/h5-6,9-11H,7-8H2,1-4H3,(H,18,20,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133602

(CHEMBL3633250)Show InChI InChI=1S/C14H17N3O3S/c1-19-9-3-4-10(12(7-9)20-2)11-8-13(18)16-14(21)17(11)6-5-15/h3-4,7-8H,5-6,15H2,1-2H3,(H,16,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133604
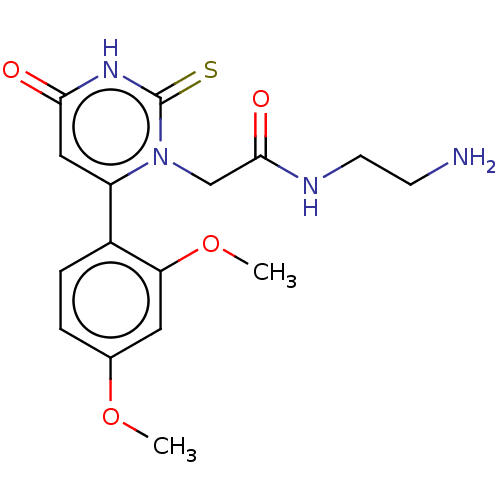
(CHEMBL3633249)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CC(=O)NCCN Show InChI InChI=1S/C16H20N4O4S/c1-23-10-3-4-11(13(7-10)24-2)12-8-14(21)19-16(25)20(12)9-15(22)18-6-5-17/h3-4,7-8H,5-6,9,17H2,1-2H3,(H,18,22)(H,19,21,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133596

(CHEMBL3633459)Show SMILES COc1ccc(OC)c(c1)-c1cc(=O)[nH]c(=S)n1CC(N)=O Show InChI InChI=1S/C14H15N3O4S/c1-20-8-3-4-11(21-2)9(5-8)10-6-13(19)16-14(22)17(10)7-12(15)18/h3-6H,7H2,1-2H3,(H2,15,18)(H,16,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133601

(CHEMBL3633251)Show InChI InChI=1S/C14H16N2O4S/c1-19-9-3-4-10(12(7-9)20-2)11-8-13(18)15-14(21)16(11)5-6-17/h3-4,7-8,17H,5-6H2,1-2H3,(H,15,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133603

(CHEMBL3633248)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CC(N)=O Show InChI InChI=1S/C14H15N3O4S/c1-20-8-3-4-9(11(5-8)21-2)10-6-13(19)16-14(22)17(10)7-12(15)18/h3-6H,7H2,1-2H3,(H2,15,18)(H,16,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133597
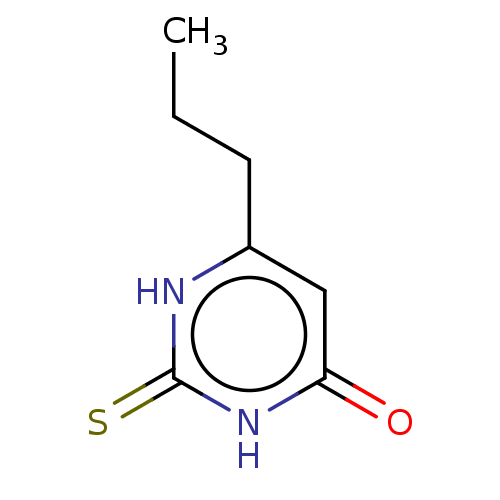
(CHEBI:8502 | Propacil | Propylthiouracil | Prothyr...)Show InChI InChI=1S/C7H10N2OS/c1-2-3-5-4-6(10)9-7(11)8-5/h4H,2-3H2,1H3,(H2,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Thyroid peroxidase
(Homo sapiens (Human)) | BDBM50133597

(CHEBI:8502 | Propacil | Propylthiouracil | Prothyr...)Show InChI InChI=1S/C7H10N2OS/c1-2-3-5-4-6(10)9-7(11)8-5/h4H,2-3H2,1H3,(H2,8,9,10,11) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TPO (unknown origin) using Amplex Red as substrate assessed as formation of resorufin measured every 20 secs by spectrophotometric anal... |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133597

(CHEBI:8502 | Propacil | Propylthiouracil | Prothyr...)Show InChI InChI=1S/C7H10N2OS/c1-2-3-5-4-6(10)9-7(11)8-5/h4H,2-3H2,1H3,(H2,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133598
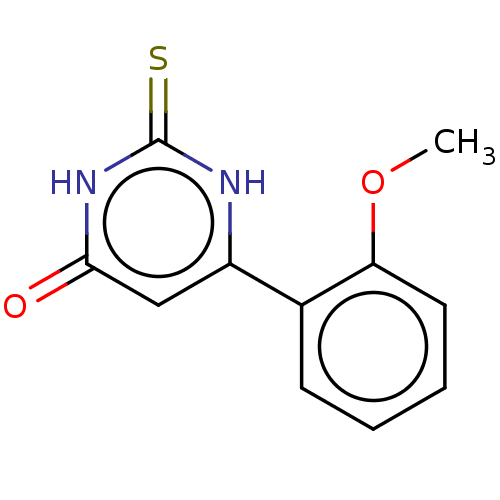
(CHEMBL3633458)Show InChI InChI=1S/C11H10N2O2S/c1-15-9-5-3-2-4-7(9)8-6-10(14)13-11(16)12-8/h2-6H,1H3,(H2,12,13,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50133600

(CHEMBL3633457)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)[nH]c(=S)n1CCOC(C)C Show InChI InChI=1S/C17H22N2O4S/c1-11(2)23-8-7-19-14(10-16(20)18-17(19)24)13-6-5-12(21-3)9-15(13)22-4/h5-6,9-11H,7-8H2,1-4H3,(H,18,20,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of CYP2D6 in human liver microsomes |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of CYP2C19 in human liver microsomes |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of CYP2C9 in human liver microsomes |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of CYP2C8 in human liver microsomes |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of CYP2B6 in human liver microsomes |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of CYP1A2 in human liver microsomes |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 in human liver microsomes by LC-MS/MS analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C19 (S)-Mephenytoin 4'-hydroxylase activity in human liver microsomes by LC-MS/MS analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C9 in human liver microsomes by LC-MS/MS analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2C8 amodiaquine-N-deethylase activity in human liver microsomes by LC-MS/MS analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2B6 bupropion hydroxylase activity in human liver microsomes by LC-MS/MS analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP1A2 phenacetin O-deethylase activity in human liver microsomes by LC-MS/MS analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50133595

(CHEMBL3633460)Show InChI InChI=1S/C13H12ClN3O3S/c1-20-10-3-2-7(14)4-8(10)9-5-12(19)16-13(21)17(9)6-11(15)18/h2-5H,6H2,1H3,(H2,15,18)(H,16,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP2D6 dextromethorphan O-demethylase activity in human liver microsomes by LC-MS/MS analysis |
J Med Chem 58: 8513-28 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00963
BindingDB Entry DOI: 10.7270/Q2SQ926X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data