 Found 66 hits with Last Name = 'okha-mokube' and Initial = 'fm'
Found 66 hits with Last Name = 'okha-mokube' and Initial = 'fm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50038210
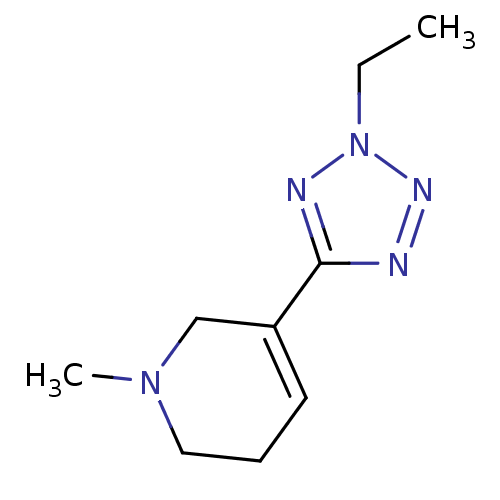
(5-(2-Ethyl-2H-tetrazol-5-yl)-1-methyl-1,2,3,6-tetr...)Show InChI InChI=1S/C9H15N5/c1-3-14-11-9(10-12-14)8-5-4-6-13(2)7-8/h5H,3-4,6-7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 296 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50061705

((R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino...)Show SMILES CO\N=C(/C#N)[C@H]1CN2CCC1CC2 |wU:6.5,(10.77,-7.56,;9.23,-7.56,;8.14,-8.66,;8.14,-10.2,;9.41,-10.96,;10.69,-11.69,;6.8,-10.98,;6.8,-12.53,;5.46,-13.28,;4.14,-12.53,;4.14,-10.98,;5.46,-10.2,;4.95,-11.51,;5.89,-12.1,)| Show InChI InChI=1S/C10H15N3O/c1-14-12-10(6-11)9-7-13-4-2-8(9)3-5-13/h8-9H,2-5,7H2,1H3/b12-10+/t9-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50119648
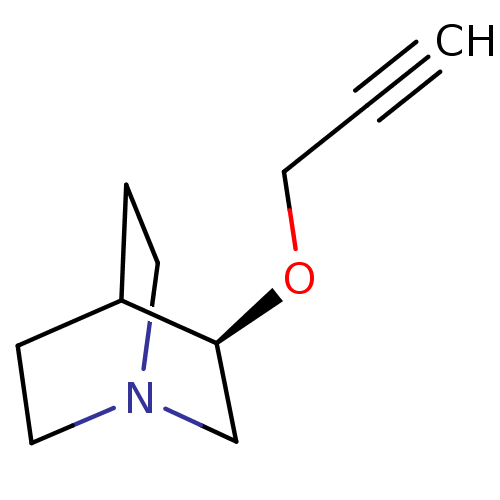
(Talsaclidine)Show SMILES C#CCO[C@H]1CN2CCC1CC2 |wU:4.3,(10.21,-8.05,;8.84,-8.8,;7.52,-9.57,;6.13,-8.8,;4.75,-9.57,;4.75,-11.19,;3.37,-11.99,;2.03,-11.19,;2.03,-9.66,;3.37,-8.88,;3.81,-10.1,;3,-10.66,)| Show InChI InChI=1S/C10H15NO/c1-2-7-12-10-8-11-5-3-9(10)4-6-11/h1,9-10H,3-8H2/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 849 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 121 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 205 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50061705

((R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino...)Show SMILES CO\N=C(/C#N)[C@H]1CN2CCC1CC2 |wU:6.5,(10.77,-7.56,;9.23,-7.56,;8.14,-8.66,;8.14,-10.2,;9.41,-10.96,;10.69,-11.69,;6.8,-10.98,;6.8,-12.53,;5.46,-13.28,;4.14,-12.53,;4.14,-10.98,;5.46,-10.2,;4.95,-11.51,;5.89,-12.1,)| Show InChI InChI=1S/C10H15N3O/c1-14-12-10(6-11)9-7-13-4-2-8(9)3-5-13/h8-9H,2-5,7H2,1H3/b12-10+/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50119648

(Talsaclidine)Show SMILES C#CCO[C@H]1CN2CCC1CC2 |wU:4.3,(10.21,-8.05,;8.84,-8.8,;7.52,-9.57,;6.13,-8.8,;4.75,-9.57,;4.75,-11.19,;3.37,-11.99,;2.03,-11.19,;2.03,-9.66,;3.37,-8.88,;3.81,-10.1,;3,-10.66,)| Show InChI InChI=1S/C10H15NO/c1-2-7-12-10-8-11-5-3-9(10)4-6-11/h1,9-10H,3-8H2/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M3 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119623
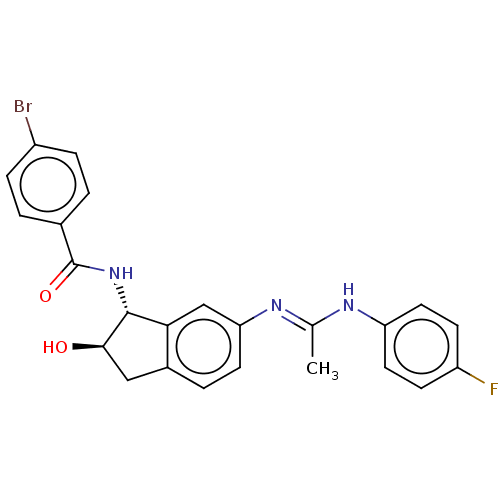
(CHEMBL3618436)Show SMILES C\C(Nc1ccc(F)cc1)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C24H21BrFN3O2/c1-14(27-19-10-7-18(26)8-11-19)28-20-9-4-16-12-22(30)23(21(16)13-20)29-24(31)15-2-5-17(25)6-3-15/h2-11,13,22-23,30H,12H2,1H3,(H,27,28)(H,29,31)/t22-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119626
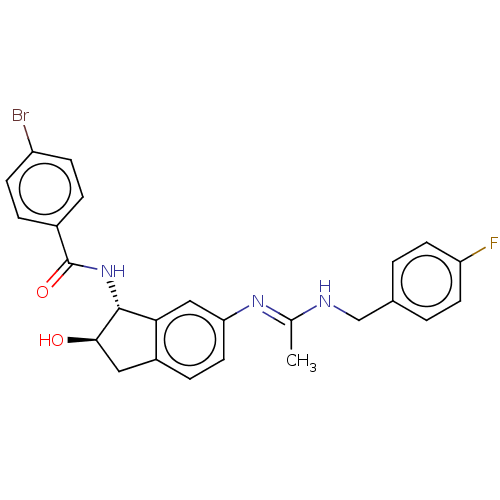
(CHEMBL3618440)Show SMILES C\C(NCc1ccc(F)cc1)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C25H23BrFN3O2/c1-15(28-14-16-2-9-20(27)10-3-16)29-21-11-6-18-12-23(31)24(22(18)13-21)30-25(32)17-4-7-19(26)8-5-17/h2-11,13,23-24,31H,12,14H2,1H3,(H,28,29)(H,30,32)/t23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 115 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119630
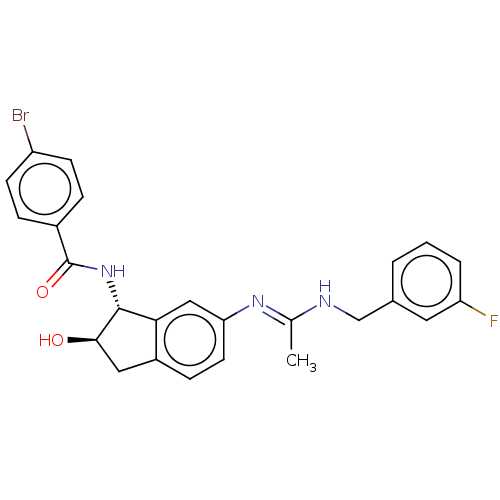
(CHEMBL3618444)Show SMILES C\C(NCc1cccc(F)c1)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C25H23BrFN3O2/c1-15(28-14-16-3-2-4-20(27)11-16)29-21-10-7-18-12-23(31)24(22(18)13-21)30-25(32)17-5-8-19(26)9-6-17/h2-11,13,23-24,31H,12,14H2,1H3,(H,28,29)(H,30,32)/t23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 355 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119632
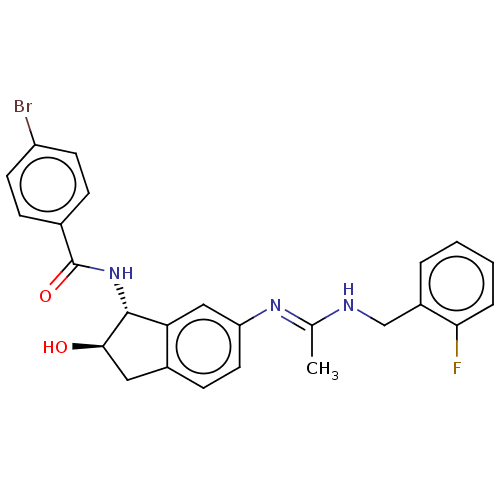
(CHEMBL3618445)Show SMILES C\C(NCc1ccccc1F)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C25H23BrFN3O2/c1-15(28-14-18-4-2-3-5-22(18)27)29-20-11-8-17-12-23(31)24(21(17)13-20)30-25(32)16-6-9-19(26)10-7-16/h2-11,13,23-24,31H,12,14H2,1H3,(H,28,29)(H,30,32)/t23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119636
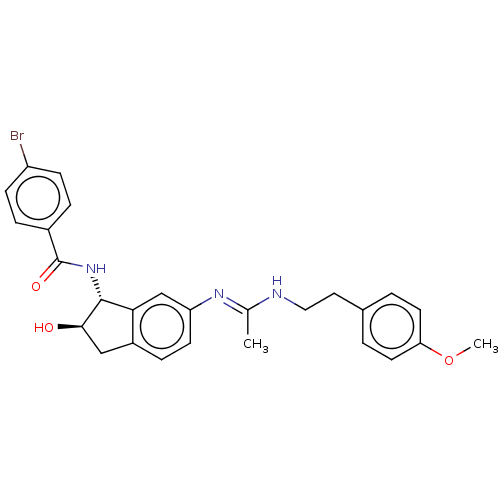
(CHEMBL3616497)Show SMILES COc1ccc(CCN\C(C)=N\c2ccc3C[C@@H](O)[C@H](NC(=O)c4ccc(Br)cc4)c3c2)cc1 |r| Show InChI InChI=1S/C27H28BrN3O3/c1-17(29-14-13-18-3-11-23(34-2)12-4-18)30-22-10-7-20-15-25(32)26(24(20)16-22)31-27(33)19-5-8-21(28)9-6-19/h3-12,16,25-26,32H,13-15H2,1-2H3,(H,29,30)(H,31,33)/t25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119637
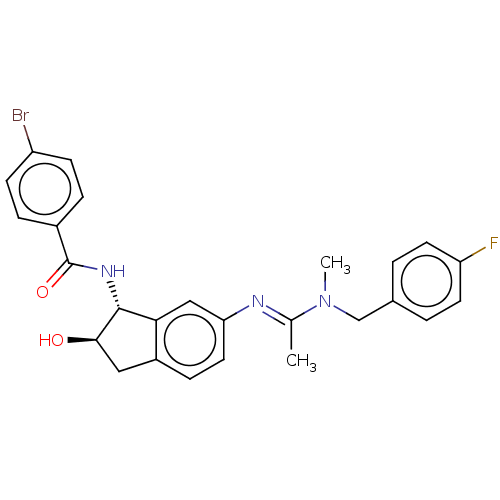
(CHEMBL3618447)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C26H25BrFN3O2/c1-16(31(2)15-17-3-10-21(28)11-4-17)29-22-12-7-19-13-24(32)25(23(19)14-22)30-26(33)18-5-8-20(27)9-6-18/h3-12,14,24-25,32H,13,15H2,1-2H3,(H,30,33)/b29-16+/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119639
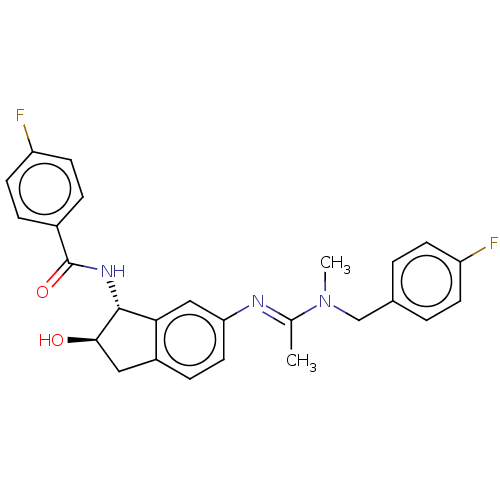
(CHEMBL3618449)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(F)cc3)c2c1 |r| Show InChI InChI=1S/C26H25F2N3O2/c1-16(31(2)15-17-3-8-20(27)9-4-17)29-22-12-7-19-13-24(32)25(23(19)14-22)30-26(33)18-5-10-21(28)11-6-18/h3-12,14,24-25,32H,13,15H2,1-2H3,(H,30,33)/b29-16+/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 392 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119641
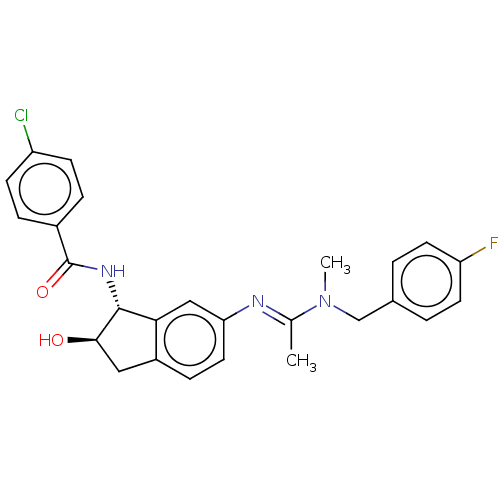
(CHEMBL3618450)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Cl)cc3)c2c1 |r| Show InChI InChI=1S/C26H25ClFN3O2/c1-16(31(2)15-17-3-10-21(28)11-4-17)29-22-12-7-19-13-24(32)25(23(19)14-22)30-26(33)18-5-8-20(27)9-6-18/h3-12,14,24-25,32H,13,15H2,1-2H3,(H,30,33)/b29-16+/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 405 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119644
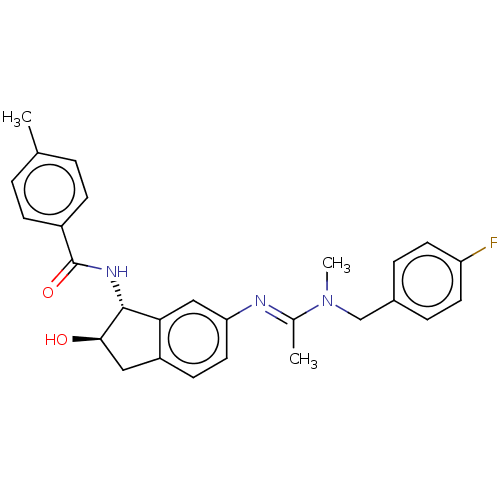
(CHEMBL3616499)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(C)cc3)c2c1 |r| Show InChI InChI=1S/C27H28FN3O2/c1-17-4-8-20(9-5-17)27(33)30-26-24-15-23(13-10-21(24)14-25(26)32)29-18(2)31(3)16-19-6-11-22(28)12-7-19/h4-13,15,25-26,32H,14,16H2,1-3H3,(H,30,33)/b29-18+/t25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 235 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119645

(CHEMBL3616500)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(cc3)C(F)(F)F)c2c1 |r| Show InChI InChI=1S/C27H25F4N3O2/c1-16(34(2)15-17-3-10-21(28)11-4-17)32-22-12-7-19-13-24(35)25(23(19)14-22)33-26(36)18-5-8-20(9-6-18)27(29,30)31/h3-12,14,24-25,35H,13,15H2,1-2H3,(H,33,36)/b32-16+/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 393 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119646
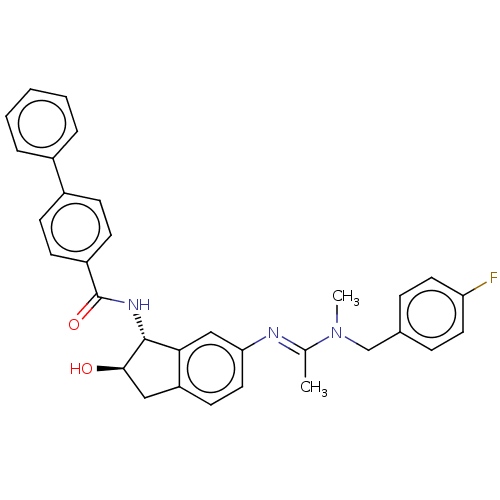
(CHEMBL3616501)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(cc3)-c3ccccc3)c2c1 |r| Show InChI InChI=1S/C32H30FN3O2/c1-21(36(2)20-22-8-15-27(33)16-9-22)34-28-17-14-26-18-30(37)31(29(26)19-28)35-32(38)25-12-10-24(11-13-25)23-6-4-3-5-7-23/h3-17,19,30-31,37H,18,20H2,1-2H3,(H,35,38)/b34-21+/t30-,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119647
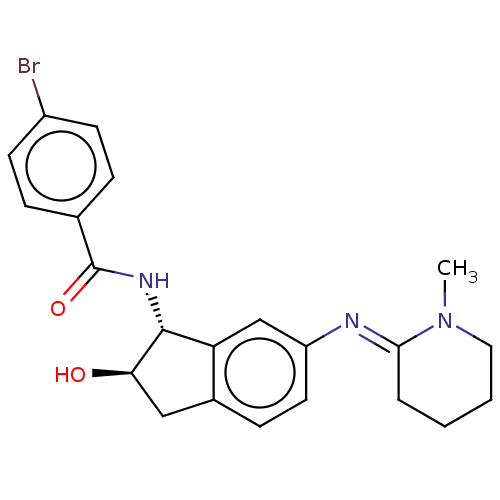
(CHEMBL3616504)Show SMILES CN1CCCC\C1=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C22H24BrN3O2/c1-26-11-3-2-4-20(26)24-17-10-7-15-12-19(27)21(18(15)13-17)25-22(28)14-5-8-16(23)9-6-14/h5-10,13,19,21,27H,2-4,11-12H2,1H3,(H,25,28)/b24-20+/t19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50061705

((R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino...)Show SMILES CO\N=C(/C#N)[C@H]1CN2CCC1CC2 |wU:6.5,(10.77,-7.56,;9.23,-7.56,;8.14,-8.66,;8.14,-10.2,;9.41,-10.96,;10.69,-11.69,;6.8,-10.98,;6.8,-12.53,;5.46,-13.28,;4.14,-12.53,;4.14,-10.98,;5.46,-10.2,;4.95,-11.51,;5.89,-12.1,)| Show InChI InChI=1S/C10H15N3O/c1-14-12-10(6-11)9-7-13-4-2-8(9)3-5-13/h8-9H,2-5,7H2,1H3/b12-10+/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 214 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50119648

(Talsaclidine)Show SMILES C#CCO[C@H]1CN2CCC1CC2 |wU:4.3,(10.21,-8.05,;8.84,-8.8,;7.52,-9.57,;6.13,-8.8,;4.75,-9.57,;4.75,-11.19,;3.37,-11.99,;2.03,-11.19,;2.03,-9.66,;3.37,-8.88,;3.81,-10.1,;3,-10.66,)| Show InChI InChI=1S/C10H15NO/c1-2-7-12-10-8-11-5-3-9(10)4-6-11/h1,9-10H,3-8H2/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 844 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 229 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M4 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M5 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50061705

((R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino...)Show SMILES CO\N=C(/C#N)[C@H]1CN2CCC1CC2 |wU:6.5,(10.77,-7.56,;9.23,-7.56,;8.14,-8.66,;8.14,-10.2,;9.41,-10.96,;10.69,-11.69,;6.8,-10.98,;6.8,-12.53,;5.46,-13.28,;4.14,-12.53,;4.14,-10.98,;5.46,-10.2,;4.95,-11.51,;5.89,-12.1,)| Show InChI InChI=1S/C10H15N3O/c1-14-12-10(6-11)9-7-13-4-2-8(9)3-5-13/h8-9H,2-5,7H2,1H3/b12-10+/t9-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M5 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50119648

(Talsaclidine)Show SMILES C#CCO[C@H]1CN2CCC1CC2 |wU:4.3,(10.21,-8.05,;8.84,-8.8,;7.52,-9.57,;6.13,-8.8,;4.75,-9.57,;4.75,-11.19,;3.37,-11.99,;2.03,-11.19,;2.03,-9.66,;3.37,-8.88,;3.81,-10.1,;3,-10.66,)| Show InChI InChI=1S/C10H15NO/c1-2-7-12-10-8-11-5-3-9(10)4-6-11/h1,9-10H,3-8H2/t10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 422 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M5 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 142 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M5 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119623

(CHEMBL3618436)Show SMILES C\C(Nc1ccc(F)cc1)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C24H21BrFN3O2/c1-14(27-19-10-7-18(26)8-11-19)28-20-9-4-16-12-22(30)23(21(16)13-20)29-24(31)15-2-5-17(25)6-3-15/h2-11,13,22-23,30H,12H2,1H3,(H,27,28)(H,29,31)/t22-,23-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119624
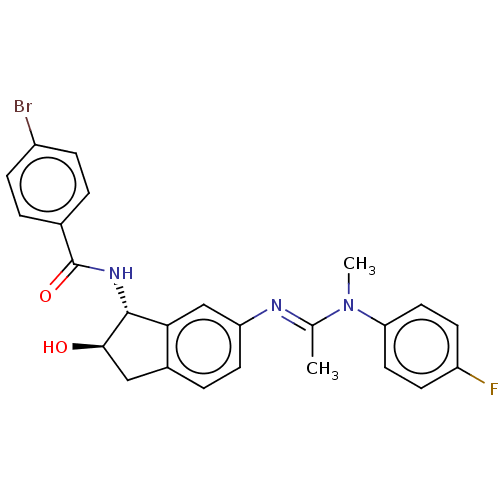
(CHEMBL3618437)Show SMILES CN(\C(C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C25H23BrFN3O2/c1-15(30(2)21-11-8-19(27)9-12-21)28-20-10-5-17-13-23(31)24(22(17)14-20)29-25(32)16-3-6-18(26)7-4-16/h3-12,14,23-24,31H,13H2,1-2H3,(H,29,32)/b28-15+/t23-,24-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 118 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119625
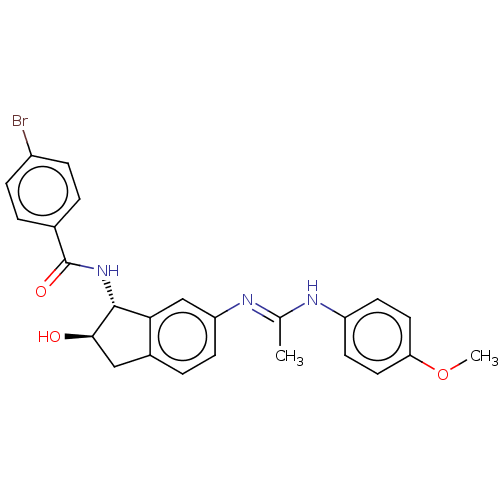
(CHEMBL3618439)Show SMILES COc1ccc(N\C(C)=N\c2ccc3C[C@@H](O)[C@H](NC(=O)c4ccc(Br)cc4)c3c2)cc1 |r| Show InChI InChI=1S/C25H24BrN3O3/c1-15(27-19-9-11-21(32-2)12-10-19)28-20-8-5-17-13-23(30)24(22(17)14-20)29-25(31)16-3-6-18(26)7-4-16/h3-12,14,23-24,30H,13H2,1-2H3,(H,27,28)(H,29,31)/t23-,24-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 105 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119626

(CHEMBL3618440)Show SMILES C\C(NCc1ccc(F)cc1)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C25H23BrFN3O2/c1-15(28-14-16-2-9-20(27)10-3-16)29-21-11-6-18-12-23(31)24(22(18)13-21)30-25(32)17-4-7-19(26)8-5-17/h2-11,13,23-24,31H,12,14H2,1H3,(H,28,29)(H,30,32)/t23-,24-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119627
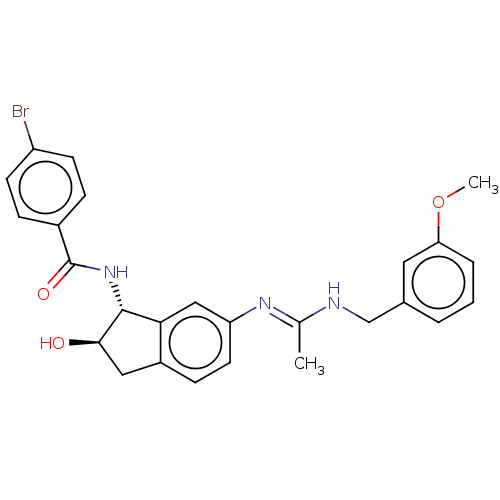
(CHEMBL3618441)Show SMILES COc1cccc(CN\C(C)=N\c2ccc3C[C@@H](O)[C@H](NC(=O)c4ccc(Br)cc4)c3c2)c1 |r| Show InChI InChI=1S/C26H26BrN3O3/c1-16(28-15-17-4-3-5-22(12-17)33-2)29-21-11-8-19-13-24(31)25(23(19)14-21)30-26(32)18-6-9-20(27)10-7-18/h3-12,14,24-25,31H,13,15H2,1-2H3,(H,28,29)(H,30,32)/t24-,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 198 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
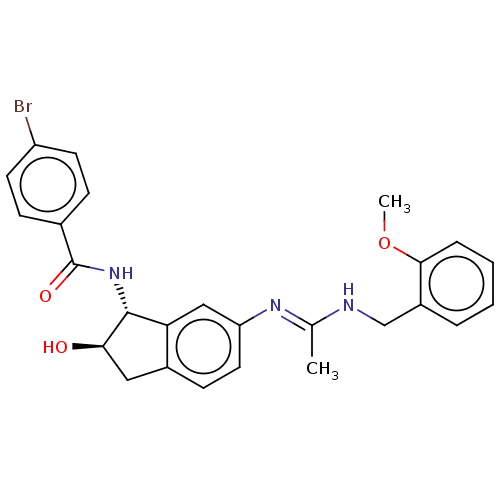
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119628
(CHEMBL3618442)Show SMILES COc1ccccc1CN\C(C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C26H26BrN3O3/c1-16(28-15-19-5-3-4-6-24(19)33-2)29-21-12-9-18-13-23(31)25(22(18)14-21)30-26(32)17-7-10-20(27)11-8-17/h3-12,14,23,25,31H,13,15H2,1-2H3,(H,28,29)(H,30,32)/t23-,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 572 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
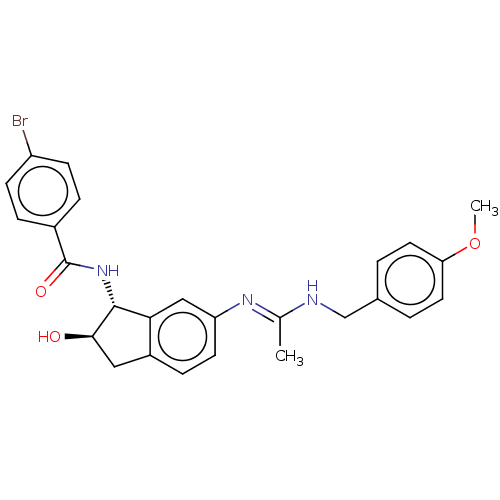
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119629
(CHEMBL3618443)Show SMILES COc1ccc(CN\C(C)=N\c2ccc3C[C@@H](O)[C@H](NC(=O)c4ccc(Br)cc4)c3c2)cc1 |r| Show InChI InChI=1S/C26H26BrN3O3/c1-16(28-15-17-3-11-22(33-2)12-4-17)29-21-10-7-19-13-24(31)25(23(19)14-21)30-26(32)18-5-8-20(27)9-6-18/h3-12,14,24-25,31H,13,15H2,1-2H3,(H,28,29)(H,30,32)/t24-,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 837 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119630

(CHEMBL3618444)Show SMILES C\C(NCc1cccc(F)c1)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C25H23BrFN3O2/c1-15(28-14-16-3-2-4-20(27)11-16)29-21-10-7-18-12-23(31)24(22(18)13-21)30-25(32)17-5-8-19(26)9-6-17/h2-11,13,23-24,31H,12,14H2,1H3,(H,28,29)(H,30,32)/t23-,24-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119632

(CHEMBL3618445)Show SMILES C\C(NCc1ccccc1F)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C25H23BrFN3O2/c1-15(28-14-18-4-2-3-5-22(18)27)29-20-11-8-17-12-23(31)24(21(17)13-20)30-25(32)16-6-9-19(26)10-7-16/h2-11,13,23-24,31H,12,14H2,1H3,(H,28,29)(H,30,32)/t23-,24-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
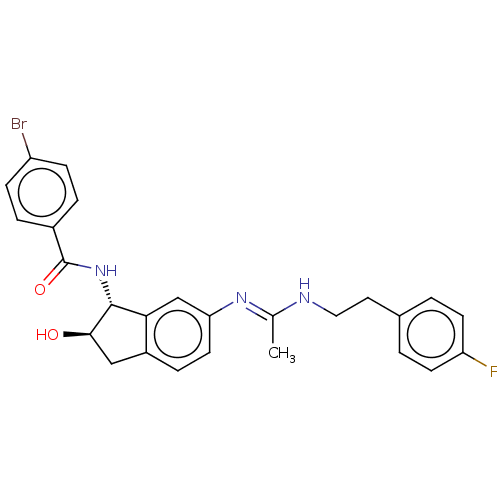
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119635
(CHEMBL3618446)Show SMILES C\C(NCCc1ccc(F)cc1)=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C26H25BrFN3O2/c1-16(29-13-12-17-2-9-21(28)10-3-17)30-22-11-6-19-14-24(32)25(23(19)15-22)31-26(33)18-4-7-20(27)8-5-18/h2-11,15,24-25,32H,12-14H2,1H3,(H,29,30)(H,31,33)/t24-,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 183 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119636

(CHEMBL3616497)Show SMILES COc1ccc(CCN\C(C)=N\c2ccc3C[C@@H](O)[C@H](NC(=O)c4ccc(Br)cc4)c3c2)cc1 |r| Show InChI InChI=1S/C27H28BrN3O3/c1-17(29-14-13-18-3-11-23(34-2)12-4-18)30-22-10-7-20-15-25(32)26(24(20)16-22)31-27(33)19-5-8-21(28)9-6-19/h3-12,16,25-26,32H,13-15H2,1-2H3,(H,29,30)(H,31,33)/t25-,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 426 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119637

(CHEMBL3618447)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C26H25BrFN3O2/c1-16(31(2)15-17-3-10-21(28)11-4-17)29-22-12-7-19-13-24(32)25(23(19)14-22)30-26(33)18-5-8-20(27)9-6-18/h3-12,14,24-25,32H,13,15H2,1-2H3,(H,30,33)/b29-16+/t24-,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 154 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
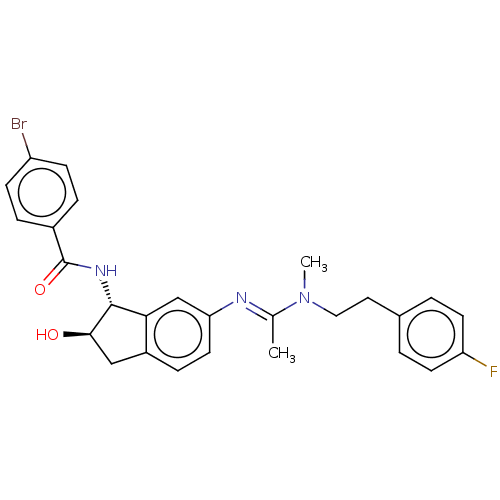
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119638
(CHEMBL3618448)Show SMILES CN(CCc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C27H27BrFN3O2/c1-17(32(2)14-13-18-3-10-22(29)11-4-18)30-23-12-7-20-15-25(33)26(24(20)16-23)31-27(34)19-5-8-21(28)9-6-19/h3-12,16,25-26,33H,13-15H2,1-2H3,(H,31,34)/b30-17+/t25-,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119639

(CHEMBL3618449)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(F)cc3)c2c1 |r| Show InChI InChI=1S/C26H25F2N3O2/c1-16(31(2)15-17-3-8-20(27)9-4-17)29-22-12-7-19-13-24(32)25(23(19)14-22)30-26(33)18-5-10-21(28)11-6-18/h3-12,14,24-25,32H,13,15H2,1-2H3,(H,30,33)/b29-16+/t24-,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 392 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119641

(CHEMBL3618450)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Cl)cc3)c2c1 |r| Show InChI InChI=1S/C26H25ClFN3O2/c1-16(31(2)15-17-3-10-21(28)11-4-17)29-22-12-7-19-13-24(32)25(23(19)14-22)30-26(33)18-5-8-20(27)9-6-18/h3-12,14,24-25,32H,13,15H2,1-2H3,(H,30,33)/b29-16+/t24-,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119643
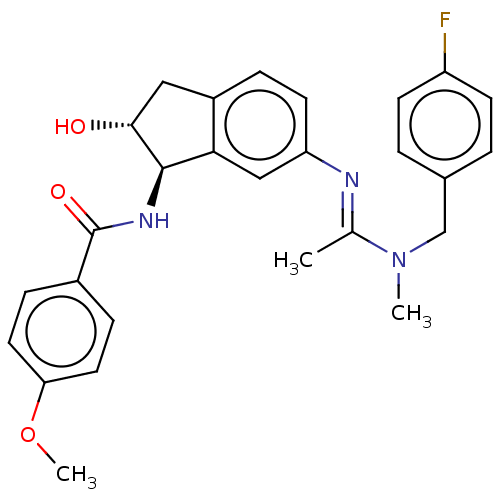
(CHEMBL3616498)Show SMILES COc1ccc(cc1)C(=O)N[C@H]1[C@H](O)Cc2ccc(cc12)\N=C(/C)N(C)Cc1ccc(F)cc1 |r| Show InChI InChI=1S/C27H28FN3O3/c1-17(31(2)16-18-4-9-21(28)10-5-18)29-22-11-6-20-14-25(32)26(24(20)15-22)30-27(33)19-7-12-23(34-3)13-8-19/h4-13,15,25-26,32H,14,16H2,1-3H3,(H,30,33)/b29-17+/t25-,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119644

(CHEMBL3616499)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(C)cc3)c2c1 |r| Show InChI InChI=1S/C27H28FN3O2/c1-17-4-8-20(9-5-17)27(33)30-26-24-15-23(13-10-21(24)14-25(26)32)29-18(2)31(3)16-19-6-11-22(28)12-7-19/h4-13,15,25-26,32H,14,16H2,1-3H3,(H,30,33)/b29-18+/t25-,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119645

(CHEMBL3616500)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(cc3)C(F)(F)F)c2c1 |r| Show InChI InChI=1S/C27H25F4N3O2/c1-16(34(2)15-17-3-10-21(28)11-4-17)32-22-12-7-19-13-24(35)25(23(19)14-22)33-26(36)18-5-8-20(9-6-18)27(29,30)31/h3-12,14,24-25,35H,13,15H2,1-2H3,(H,33,36)/b32-16+/t24-,25-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119646

(CHEMBL3616501)Show SMILES CN(Cc1ccc(F)cc1)C(\C)=N\c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(cc3)-c3ccccc3)c2c1 |r| Show InChI InChI=1S/C32H30FN3O2/c1-21(36(2)20-22-8-15-27(33)16-9-22)34-28-17-14-26-18-30(37)31(29(26)19-28)35-32(38)25-12-10-24(11-13-25)23-6-4-3-5-7-23/h3-17,19,30-31,37H,18,20H2,1-2H3,(H,35,38)/b34-21+/t30-,31-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50119647

(CHEMBL3616504)Show SMILES CN1CCCC\C1=N/c1ccc2C[C@@H](O)[C@H](NC(=O)c3ccc(Br)cc3)c2c1 |r| Show InChI InChI=1S/C22H24BrN3O2/c1-26-11-3-2-4-20(26)24-17-10-7-15-12-19(27)21(18(15)13-17)25-22(28)14-5-8-16(23)9-6-14/h5-10,13,19,21,27H,2-4,11-12H2,1H3,(H,25,28)/b24-20+/t19-,21-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50061705

((R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino...)Show SMILES CO\N=C(/C#N)[C@H]1CN2CCC1CC2 |wU:6.5,(10.77,-7.56,;9.23,-7.56,;8.14,-8.66,;8.14,-10.2,;9.41,-10.96,;10.69,-11.69,;6.8,-10.98,;6.8,-12.53,;5.46,-13.28,;4.14,-12.53,;4.14,-10.98,;5.46,-10.2,;4.95,-11.51,;5.89,-12.1,)| Show InChI InChI=1S/C10H15N3O/c1-14-12-10(6-11)9-7-13-4-2-8(9)3-5-13/h8-9H,2-5,7H2,1H3/b12-10+/t9-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 64 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data