

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50554340 (CHEMBL4788866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human AMCase expressed in CHO-K1 cells assessed as reduction in chitinolytic activity using 4-methylu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554340 (CHEMBL4788866) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243745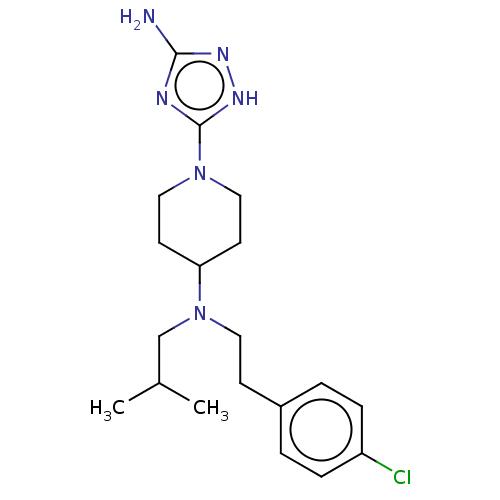 (CHEMBL4076989) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554340 (CHEMBL4788866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50554340 (CHEMBL4788866) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length C-terminal his-tagged mouse CHIT1 expressed in CHO-K1 cells assessed as reduction in chitinolytic activity usin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50243745 (CHEMBL4076989) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50037079 (CHEMBL3355781) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50037080 (CHEMBL3355780) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50037079 (CHEMBL3355781) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H][Ile5,6]deltorphin-2 from DOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50359546 (CHEMBL1927270) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541950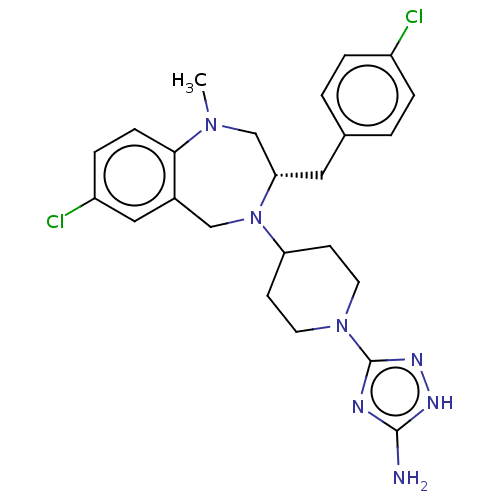 (CHEMBL4643001) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membranes | Bioorg Med Chem 22: 4803-9 (2014) Article DOI: 10.1016/j.bmc.2014.06.056 BindingDB Entry DOI: 10.7270/Q2251KT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50053573 (CHEMBL3327220) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membranes | Bioorg Med Chem 22: 4803-9 (2014) Article DOI: 10.1016/j.bmc.2014.06.056 BindingDB Entry DOI: 10.7270/Q2251KT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554344 (CHEMBL4756869) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554342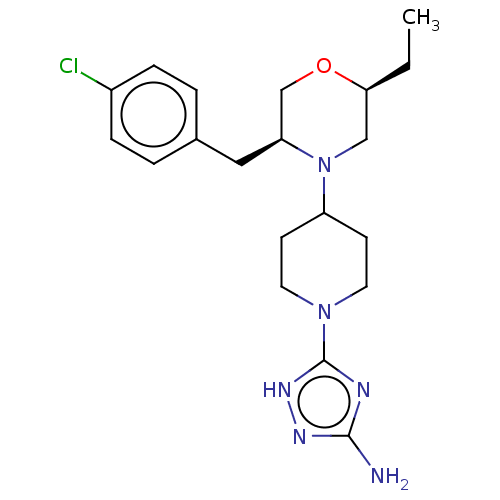 (CHEMBL4776610) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541949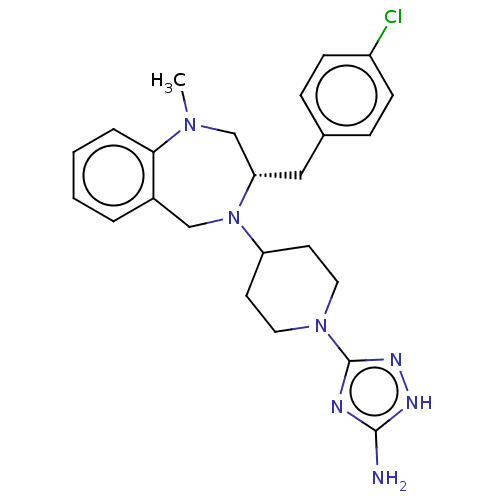 (CHEMBL4635305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50554344 (CHEMBL4756869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human AMCase expressed in CHO-K1 cells assessed as reduction in chitinolytic activity using 4-methylu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541930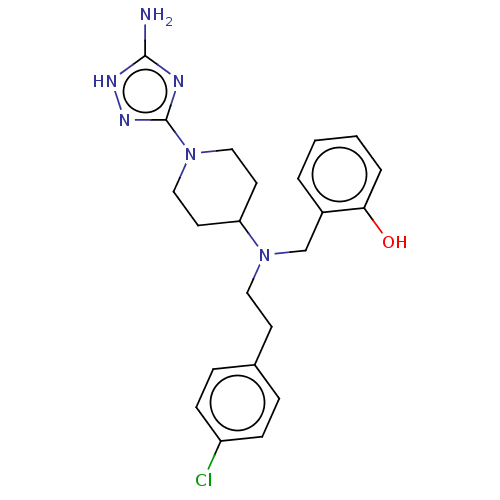 (CHEMBL4637417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541931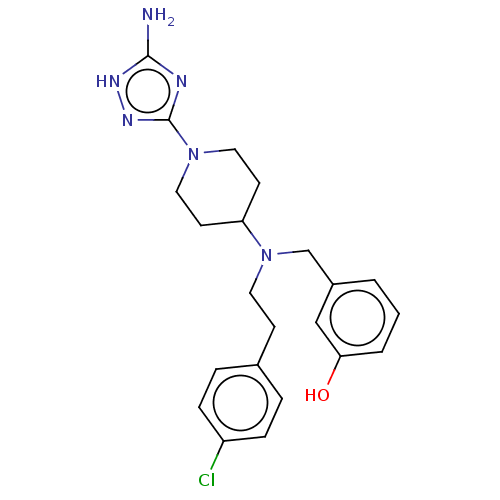 (CHEMBL4634943) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50037081 (CHEMBL3355779) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554343 (CHEMBL4794014) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50359546 (CHEMBL1927270) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]nor-BNI from KOR in Dunkin Hartley guinea pig membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50053572 (CHEMBL3327221) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membranes | Bioorg Med Chem 22: 4803-9 (2014) Article DOI: 10.1016/j.bmc.2014.06.056 BindingDB Entry DOI: 10.7270/Q2251KT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243795 (CHEMBL4077644) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50037082 (CHEMBL3355778) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50554343 (CHEMBL4794014) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human AMCase expressed in CHO-K1 cells assessed as reduction in chitinolytic activity using 4-methylu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243685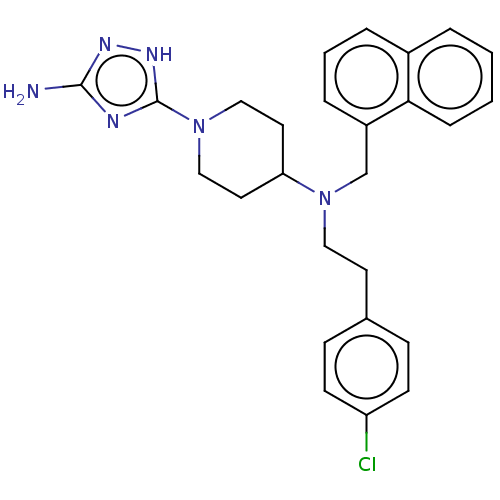 (CHEMBL4084573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243795 (CHEMBL4077644) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50037081 (CHEMBL3355779) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H][Ile5,6]deltorphin-2 from DOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541933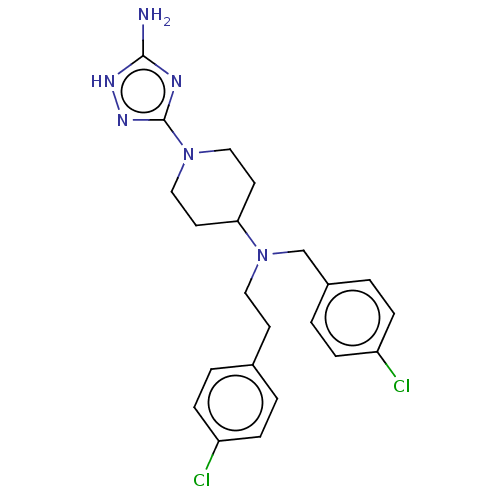 (CHEMBL4646309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum | Bioorg Med Chem Lett 23: 6673-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.041 BindingDB Entry DOI: 10.7270/Q2542RJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541934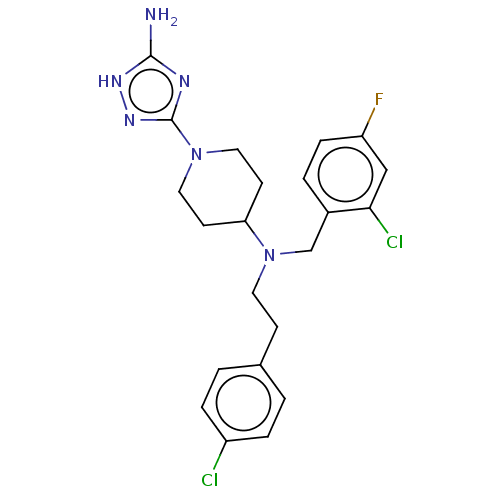 (CHEMBL4632547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243799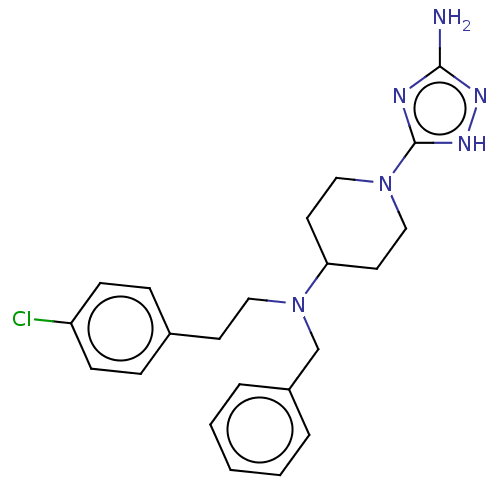 (CHEMBL4077482) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541936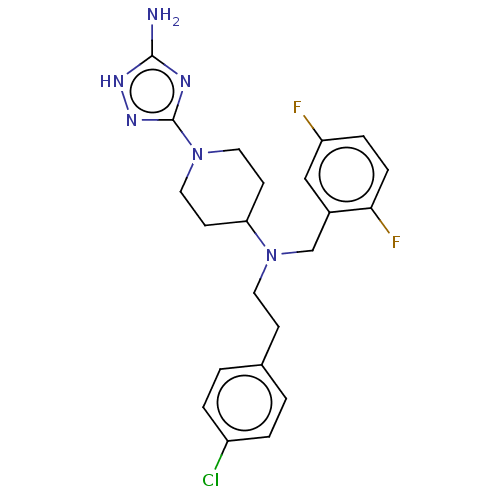 (CHEMBL4646247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243799 (CHEMBL4077482) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554346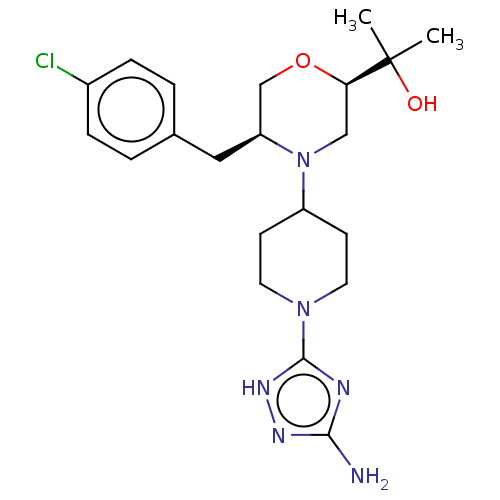 (CHEMBL4764430) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541932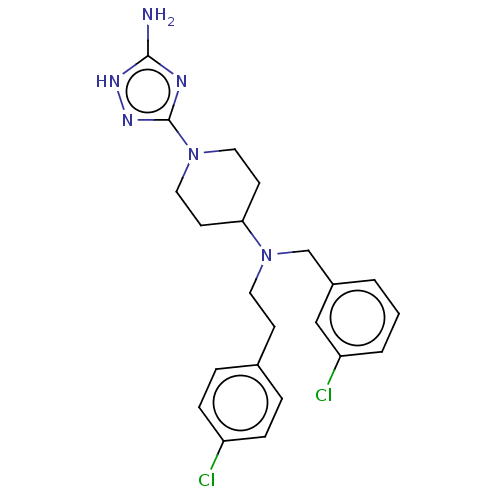 (CHEMBL4649081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243685 (CHEMBL4084573) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554345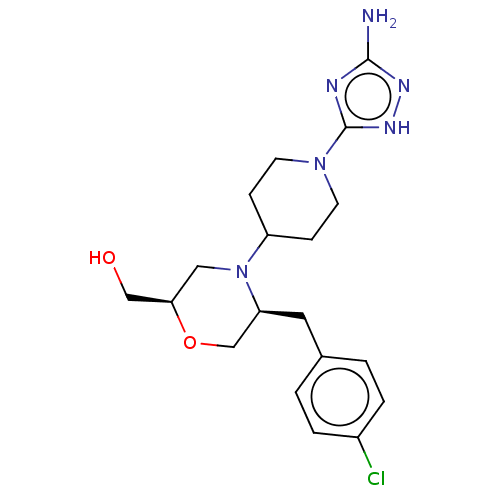 (CHEMBL4789376) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50554345 (CHEMBL4789376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human AMCase expressed in CHO-K1 cells assessed as reduction in chitinolytic activity using 4-methylu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541939 (CHEMBL4648962) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541938 (CHEMBL4640857) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human AMCase expressed in CHO-K1 cells assessed as reduction in chitinolytic activity using 4-methylu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50554346 (CHEMBL4764430) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human AMCase expressed in CHO-K1 cells assessed as reduction in chitinolytic activity using 4-methylu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50541938 (CHEMBL4640857) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitin... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554347 (CHEMBL4755139) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554340 (CHEMBL4788866) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554348 (CHEMBL4749391) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1337 total ) | Next | Last >> |