 Found 2254 hits with Last Name = 'olsson' and Initial = 'r'
Found 2254 hits with Last Name = 'olsson' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243536
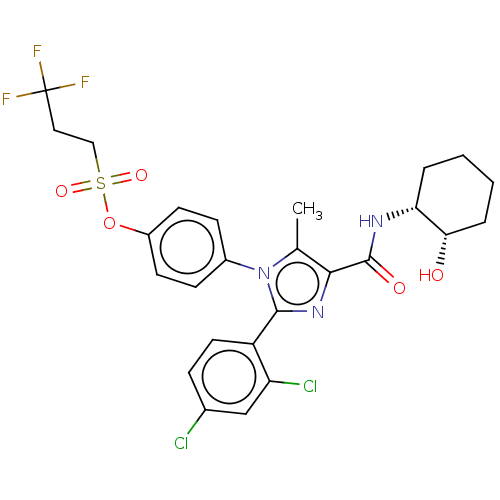
(CHEMBL4062749)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@@H]1CCCC[C@@H]1O |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-21-4-2-3-5-22(21)35)33-24(19-11-6-16(27)14-20(19)28)34(15)17-7-9-18(10-8-17)39-40(37,38)13-12-26(29,30)31/h6-11,14,21-22,35H,2-5,12-13H2,1H3,(H,32,36)/t21-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243536

(CHEMBL4062749)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@@H]1CCCC[C@@H]1O |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-21-4-2-3-5-22(21)35)33-24(19-11-6-16(27)14-20(19)28)34(15)17-7-9-18(10-8-17)39-40(37,38)13-12-26(29,30)31/h6-11,14,21-22,35H,2-5,12-13H2,1H3,(H,32,36)/t21-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570555
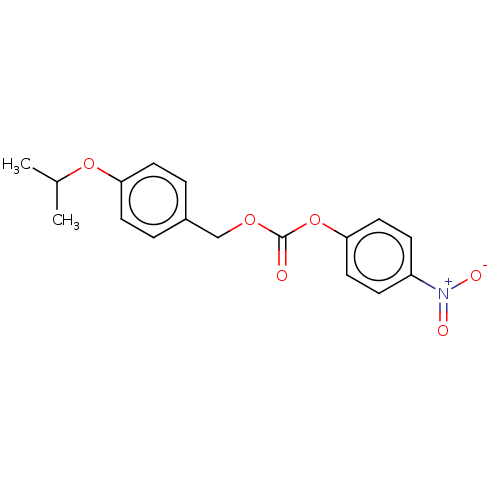
(US11440884, Example 18 | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)Oc2ccc(cc2)[N+]([O-])=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86187
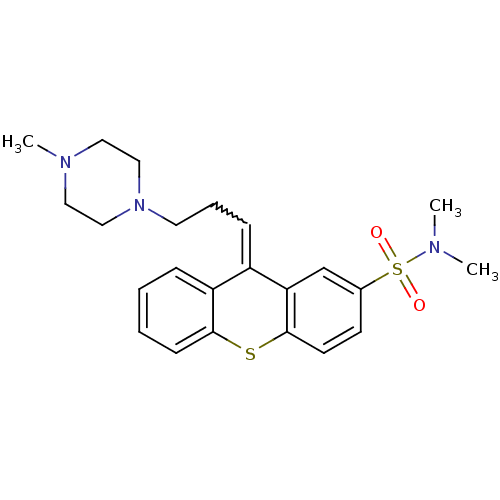
(CAS_22189-31-7 | NSC_5454 | THIOTHIXENE)Show SMILES CN(C)S(=O)(=O)c1ccc2Sc3ccccc3C(=CCCN3CCN(C)CC3)c2c1 |w:18.19| Show InChI InChI=1S/C23H29N3O2S2/c1-24(2)30(27,28)18-10-11-23-21(17-18)19(20-7-4-5-9-22(20)29-23)8-6-12-26-15-13-25(3)14-16-26/h4-5,7-11,17H,6,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570569
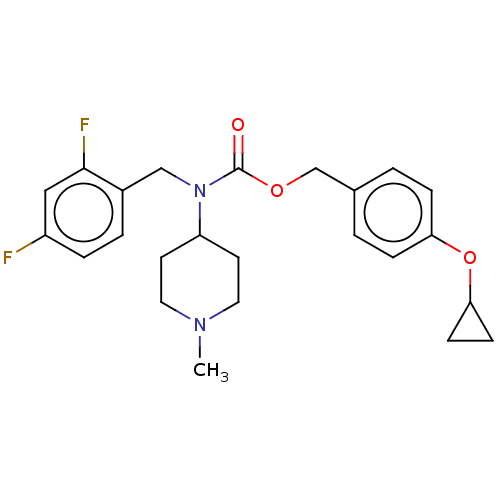
(US11440884, Example 30)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC2CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570568
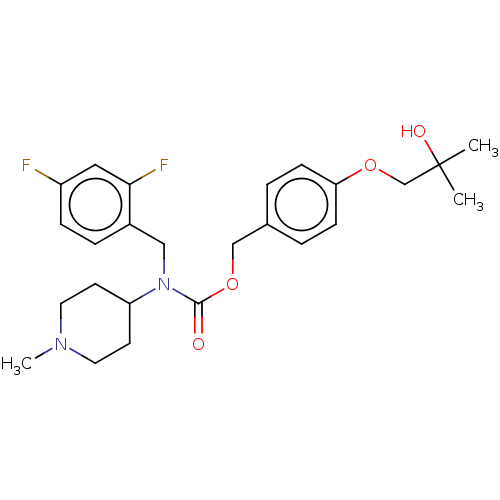
(US11440884, Example 29)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OCC(C)(C)O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570567
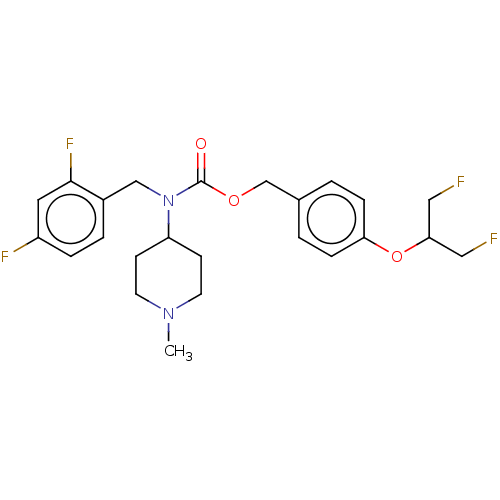
(US11440884, Example 28)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC(CF)CF)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM50207816
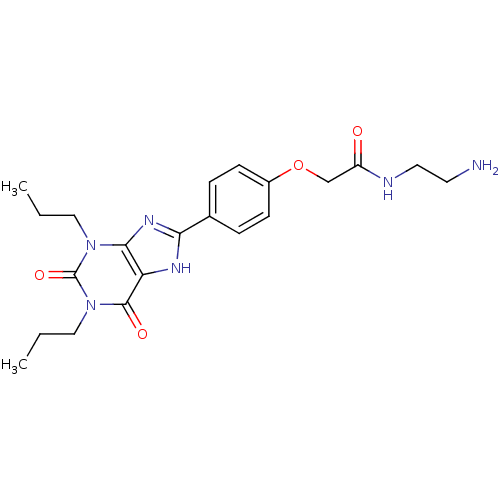
(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM50062858
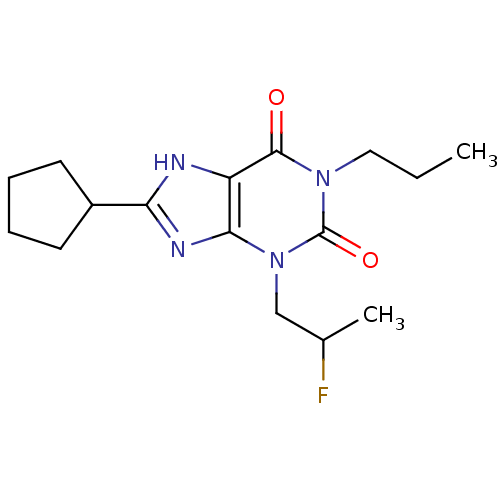
(8-Cyclopentyl-3-(2-fluoro-propyl)-1-propyl-3,7-dih...)Show InChI InChI=1S/C16H23FN4O2/c1-3-8-20-15(22)12-14(21(16(20)23)9-10(2)17)19-13(18-12)11-6-4-5-7-11/h10-11H,3-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CPX from Adenosine A1 receptor of bovine brain cerebral cortex membranes |
J Med Chem 41: 555-63 (1998)
Article DOI: 10.1021/jm9705465
BindingDB Entry DOI: 10.7270/Q2RX9B60 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243589
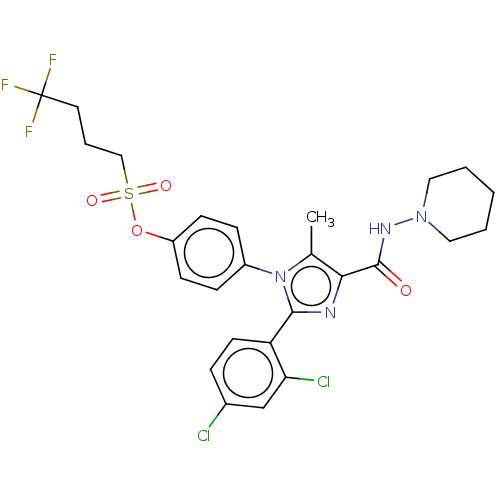
(CHEMBL4095223)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H27Cl2F3N4O4S/c1-17-23(25(36)33-34-13-3-2-4-14-34)32-24(21-11-6-18(27)16-22(21)28)35(17)19-7-9-20(10-8-19)39-40(37,38)15-5-12-26(29,30)31/h6-11,16H,2-5,12-15H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM81486
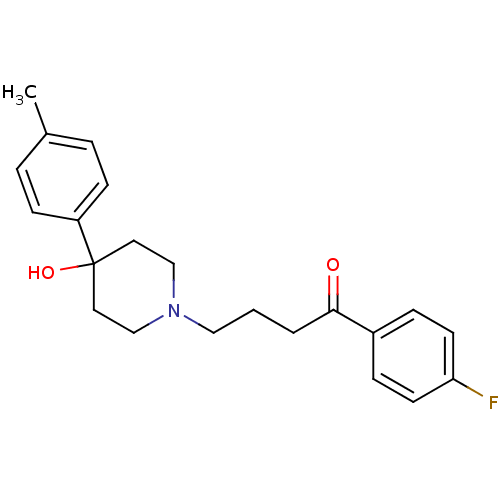
(CAS_1050-79-9 | MOPERONE | NSC_4249)Show SMILES Cc1ccc(cc1)C1(O)CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H26FNO2/c1-17-4-8-19(9-5-17)22(26)12-15-24(16-13-22)14-2-3-21(25)18-6-10-20(23)11-7-18/h4-11,26H,2-3,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM84734
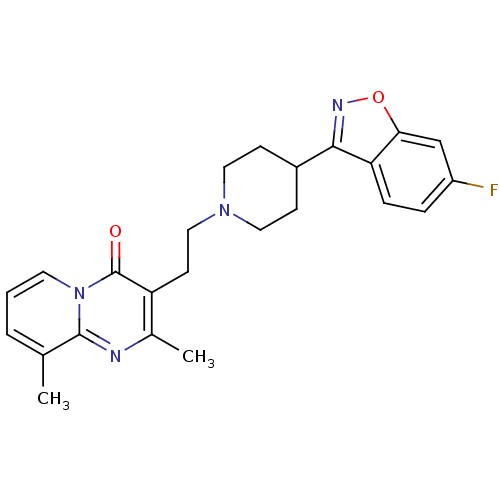
(CAS_129029-23-8 | NSC_71351 | Ocaperidone)Show SMILES Cc1cccn2c1nc(C)c(CCN1CCC(CC1)c1noc3cc(F)ccc13)c2=O Show InChI InChI=1S/C24H25FN4O2/c1-15-4-3-10-29-23(15)26-16(2)19(24(29)30)9-13-28-11-7-17(8-12-28)22-20-6-5-18(25)14-21(20)31-27-22/h3-6,10,14,17H,7-9,11-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50252513
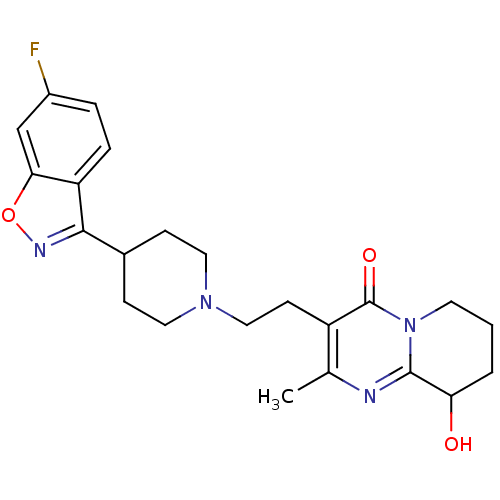
(3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1...)Show SMILES Cc1nc2C(O)CCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570571
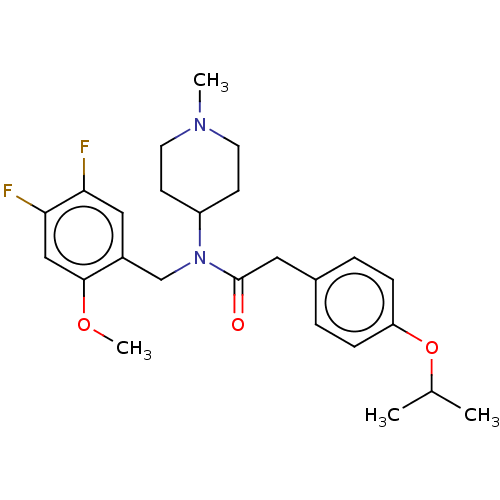
(US11440884, Example 32)Show SMILES COc1cc(F)c(F)cc1CN(C1CCN(C)CC1)C(=O)Cc1ccc(OC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570574
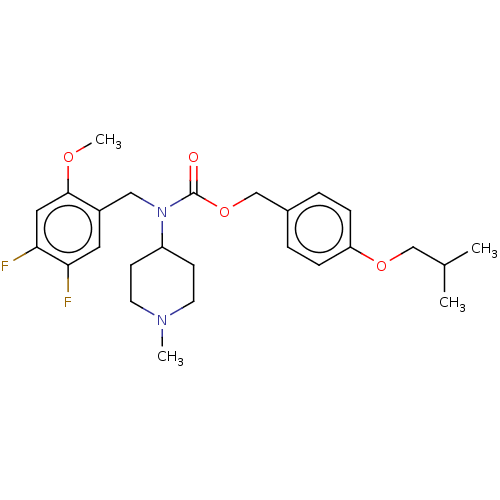
(US11440884, Example 35)Show SMILES COc1cc(F)c(F)cc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OCC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570602
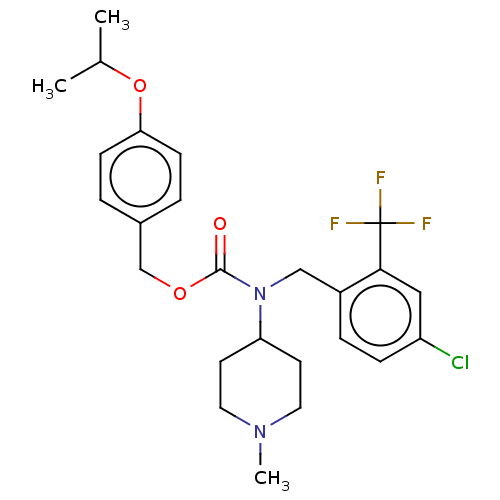
(US11440884, Example 54)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(Cl)cc2C(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570603
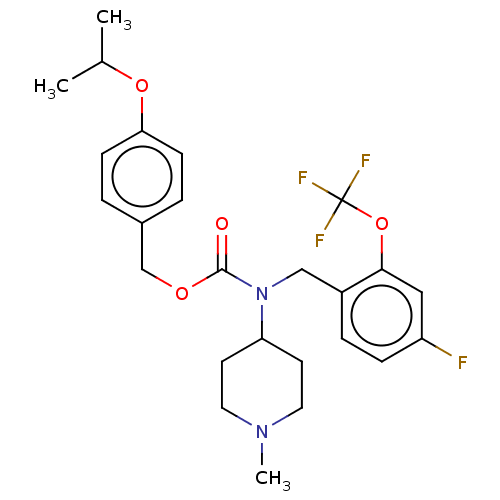
(US11440884, Example 55)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2OC(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570616
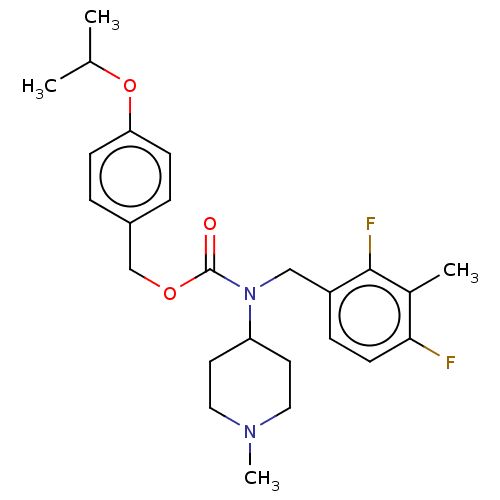
(US11440884, Example 67)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)c(C)c2F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570619
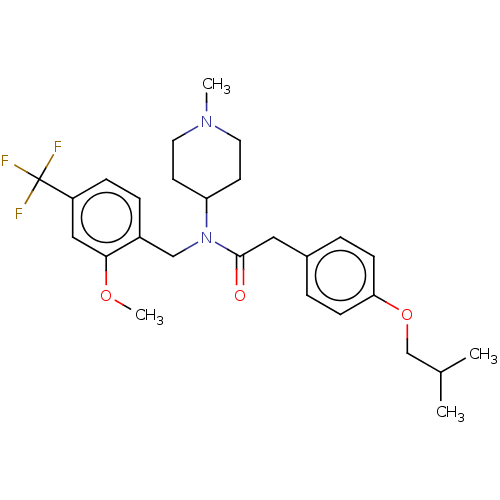
(US11440884, Example 70)Show SMILES COc1cc(ccc1CN(C1CCN(C)CC1)C(=O)Cc1ccc(OCC(C)C)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570621
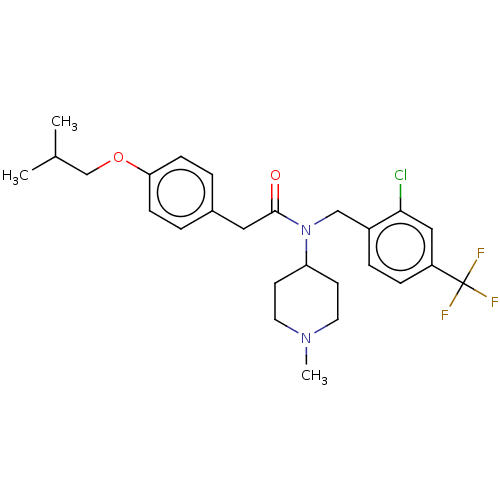
(US11440884, Example 71)Show SMILES CC(C)COc1ccc(CC(=O)N(Cc2ccc(cc2Cl)C(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570622
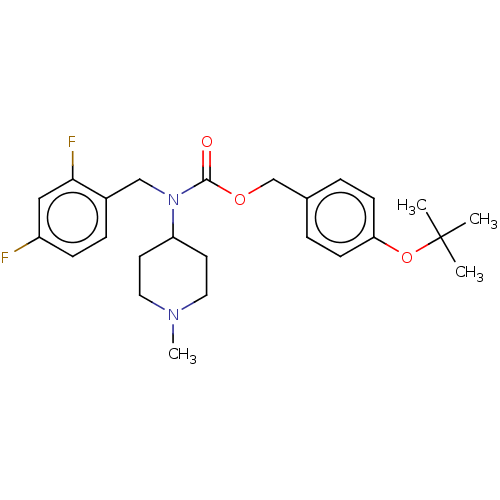
(US11440884, Example 72)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC(C)(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570627
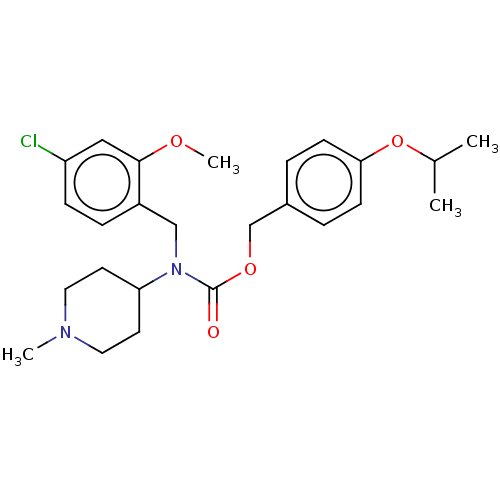
(US11440884, Example 76)Show SMILES COc1cc(Cl)ccc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570628

(US11440884, Example 77)Show SMILES COc1cc(ccc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OC(C)C)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570517
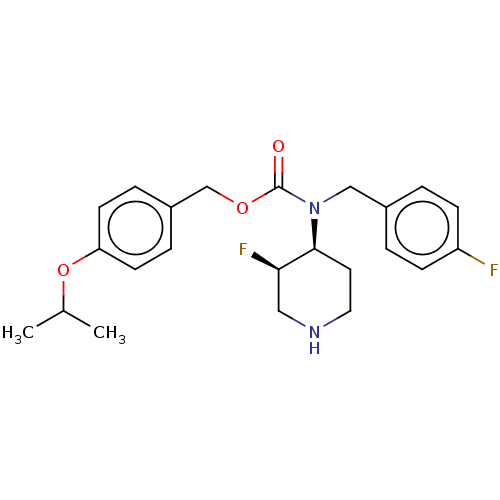
(US11440884, Example 3a | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@H]2CCNC[C@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570524
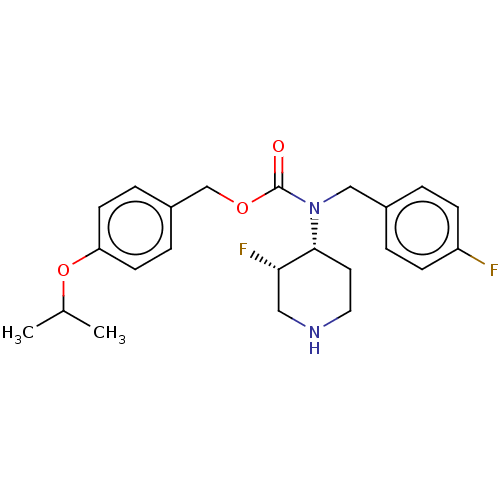
(US11440884, Example 3b | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@@H]2CCNC[C@@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570525
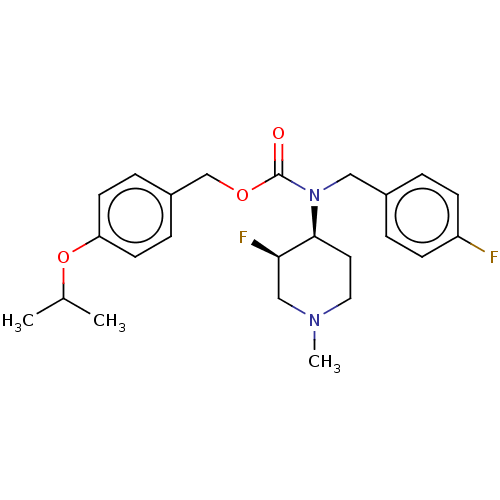
( [4-(propan-2-yloxy)phenyl]methyl N-[(3R,4S)-3-flu...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@H]2CCN(C)C[C@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570526
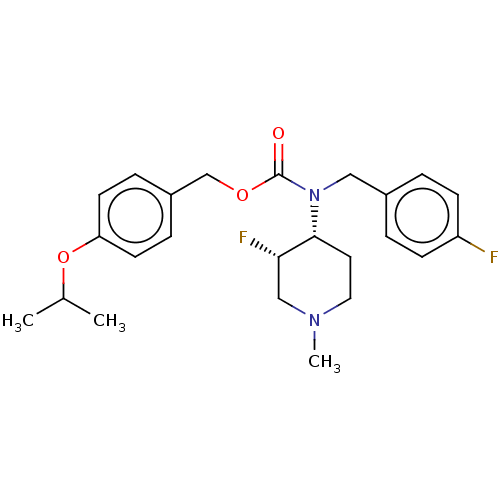
(US11440884, Example 4b | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@@H]2CCN(C)C[C@@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243625
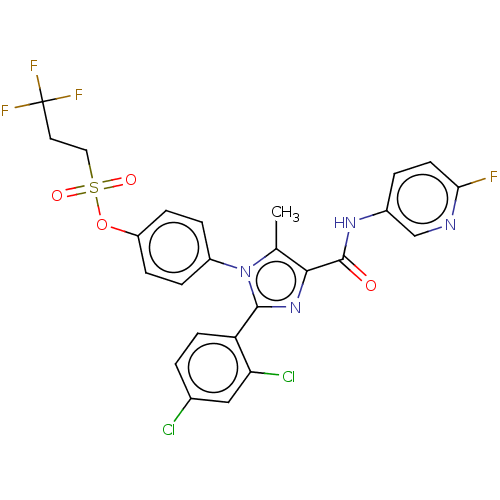
(CHEMBL4094098)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(F)nc1 Show InChI InChI=1S/C25H18Cl2F4N4O4S/c1-14-22(24(36)33-16-3-9-21(28)32-13-16)34-23(19-8-2-15(26)12-20(19)27)35(14)17-4-6-18(7-5-17)39-40(37,38)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243545
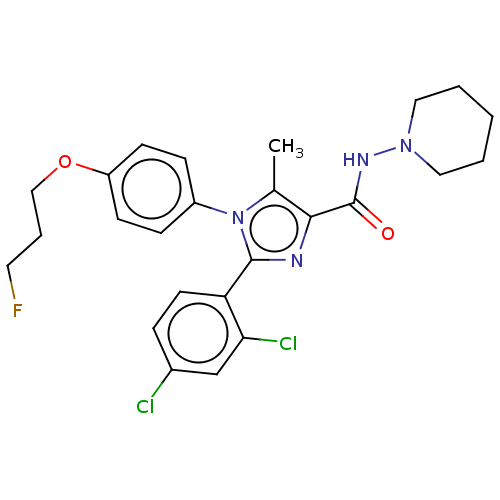
(CHEMBL4078689)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OCCCF)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H27Cl2FN4O2/c1-17-23(25(33)30-31-13-3-2-4-14-31)29-24(21-11-6-18(26)16-22(21)27)32(17)19-7-9-20(10-8-19)34-15-5-12-28/h6-11,16H,2-5,12-15H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243589

(CHEMBL4095223)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H27Cl2F3N4O4S/c1-17-23(25(36)33-34-13-3-2-4-14-34)32-24(21-11-6-18(27)16-22(21)28)35(17)19-7-9-20(10-8-19)39-40(37,38)15-5-12-26(29,30)31/h6-11,16H,2-5,12-15H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243608
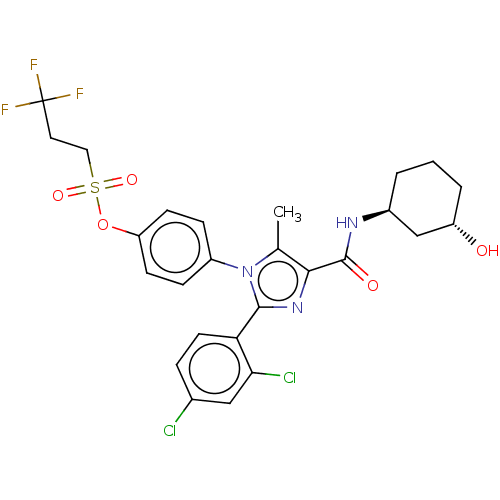
(CHEMBL4100882)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-17-3-2-4-19(35)14-17)33-24(21-10-5-16(27)13-22(21)28)34(15)18-6-8-20(9-7-18)39-40(37,38)12-11-26(29,30)31/h5-10,13,17,19,35H,2-4,11-12,14H2,1H3,(H,32,36)/t17-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243625

(CHEMBL4094098)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(F)nc1 Show InChI InChI=1S/C25H18Cl2F4N4O4S/c1-14-22(24(36)33-16-3-9-21(28)32-13-16)34-23(19-8-2-15(26)12-20(19)27)35(14)17-4-6-18(7-5-17)39-40(37,38)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243545

(CHEMBL4078689)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OCCCF)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H27Cl2FN4O2/c1-17-23(25(33)30-31-13-3-2-4-14-31)29-24(21-11-6-18(26)16-22(21)27)32(17)19-7-9-20(10-8-19)34-15-5-12-28/h6-11,16H,2-5,12-15H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243608

(CHEMBL4100882)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-17-3-2-4-19(35)14-17)33-24(21-10-5-16(27)13-22(21)28)34(15)18-6-8-20(9-7-18)39-40(37,38)12-11-26(29,30)31/h5-10,13,17,19,35H,2-4,11-12,14H2,1H3,(H,32,36)/t17-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243627
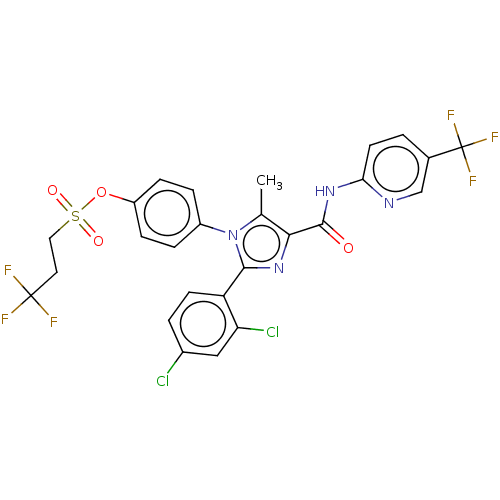
(CHEMBL4068708)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C26H18Cl2F6N4O4S/c1-14-22(24(39)36-21-9-2-15(13-35-21)26(32,33)34)37-23(19-8-3-16(27)12-20(19)28)38(14)17-4-6-18(7-5-17)42-43(40,41)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,35,36,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
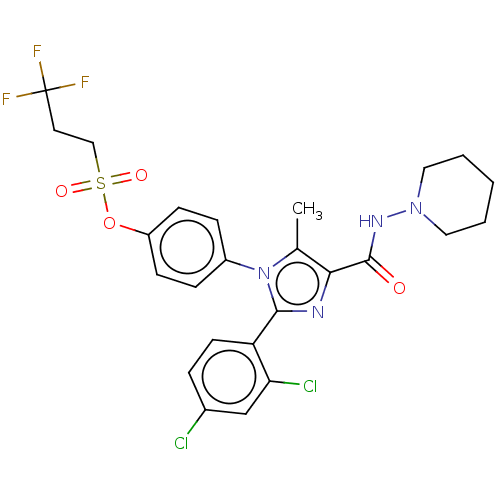
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243645
(CHEMBL4087520)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H25Cl2F3N4O4S/c1-16-22(24(35)32-33-12-3-2-4-13-33)31-23(20-10-5-17(26)15-21(20)27)34(16)18-6-8-19(9-7-18)38-39(36,37)14-11-25(28,29)30/h5-10,15H,2-4,11-14H2,1H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243626
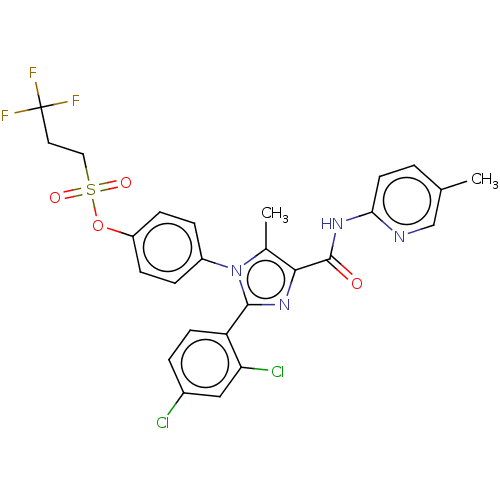
(CHEMBL4089821)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(C)cn1 Show InChI InChI=1S/C26H21Cl2F3N4O4S/c1-15-3-10-22(32-14-15)33-25(36)23-16(2)35(24(34-23)20-9-4-17(27)13-21(20)28)18-5-7-19(8-6-18)39-40(37,38)12-11-26(29,30)31/h3-10,13-14H,11-12H2,1-2H3,(H,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243588
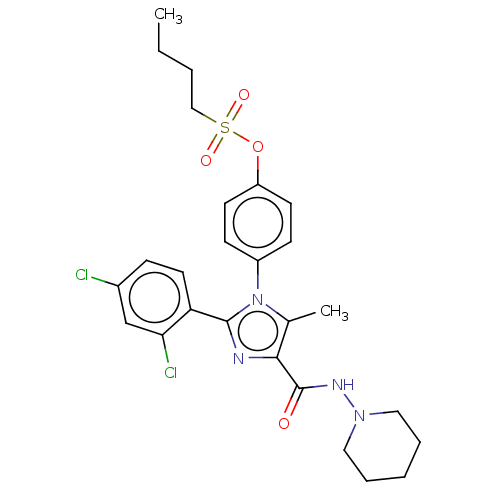
(CHEMBL4067800)Show SMILES CCCCS(=O)(=O)Oc1ccc(cc1)-n1c(C)c(nc1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H30Cl2N4O4S/c1-3-4-16-37(34,35)36-21-11-9-20(10-12-21)32-18(2)24(26(33)30-31-14-6-5-7-15-31)29-25(32)22-13-8-19(27)17-23(22)28/h8-13,17H,3-7,14-16H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243645

(CHEMBL4087520)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H25Cl2F3N4O4S/c1-16-22(24(35)32-33-12-3-2-4-13-33)31-23(20-10-5-17(26)15-21(20)27)34(16)18-6-8-19(9-7-18)38-39(36,37)14-11-25(28,29)30/h5-10,15H,2-4,11-14H2,1H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243588

(CHEMBL4067800)Show SMILES CCCCS(=O)(=O)Oc1ccc(cc1)-n1c(C)c(nc1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H30Cl2N4O4S/c1-3-4-16-37(34,35)36-21-11-9-20(10-12-21)32-18(2)24(26(33)30-31-14-6-5-7-15-31)29-25(32)22-13-8-19(27)17-23(22)28/h8-13,17H,3-7,14-16H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243627

(CHEMBL4068708)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C26H18Cl2F6N4O4S/c1-14-22(24(39)36-21-9-2-15(13-35-21)26(32,33)34)37-23(19-8-3-16(27)12-20(19)28)38(14)17-4-6-18(7-5-17)42-43(40,41)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,35,36,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570516
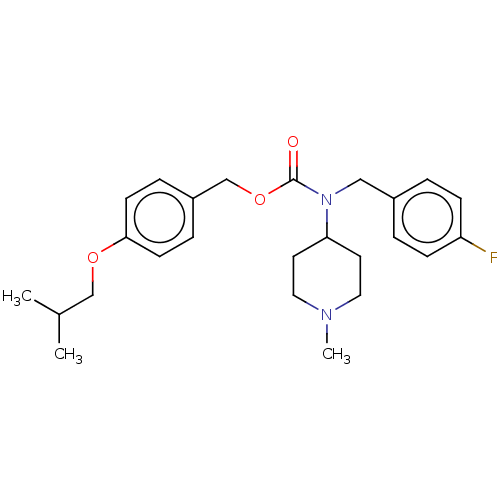
(US11440884, Example 2)Show SMILES CC(C)COc1ccc(COC(=O)N(Cc2ccc(F)cc2)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243626

(CHEMBL4089821)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(C)cn1 Show InChI InChI=1S/C26H21Cl2F3N4O4S/c1-15-3-10-22(32-14-15)33-25(36)23-16(2)35(24(34-23)20-9-4-17(27)13-21(20)28)18-5-7-19(8-6-18)39-40(37,38)12-11-26(29,30)31/h3-10,13-14H,11-12H2,1-2H3,(H,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570545
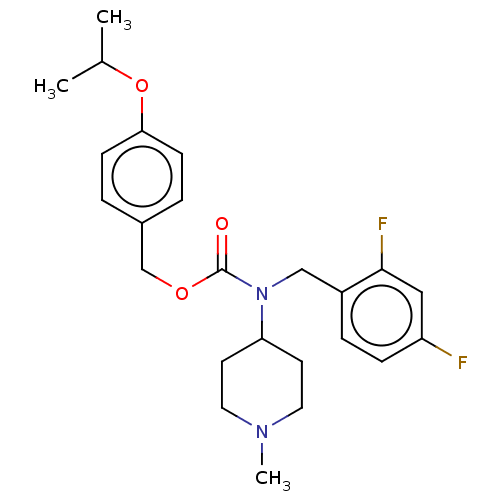
(US11440884, Example 13)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CPX from Adenosine A1 receptor of bovine brain cerebral cortex membranes |
J Med Chem 41: 555-63 (1998)
Article DOI: 10.1021/jm9705465
BindingDB Entry DOI: 10.7270/Q2RX9B60 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CPX from Adenosine A1 receptor of bovine brain cerebral cortex membranes |
J Med Chem 41: 555-63 (1998)
Article DOI: 10.1021/jm9705465
BindingDB Entry DOI: 10.7270/Q2RX9B60 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570587

(US11440884, Example 39)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OCCF)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570566
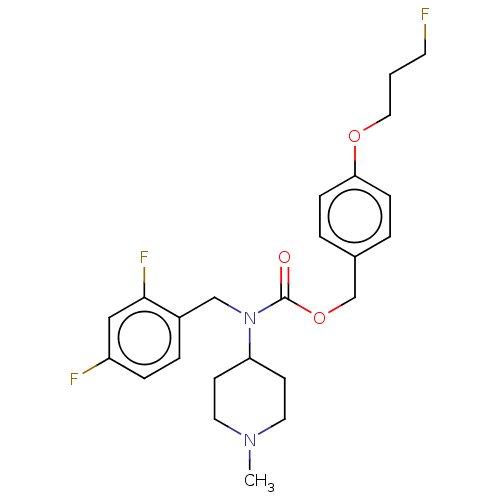
(US11440884, Example 27)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OCCCF)cc1 |$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;F;;$| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data