

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31172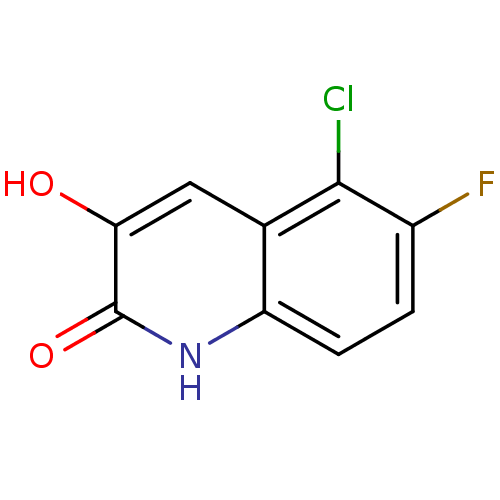 (3-hydroxyquinolin-2(1H)-one, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAAO expressed in Escherichia coli assessed as H2O2 production from D-serine degradation after 30 mins by fluorescenc... | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAAO expressed in Escherichia coli assessed as H2O2 production from D-serine degradation after 30 mins by fluorescenc... | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260725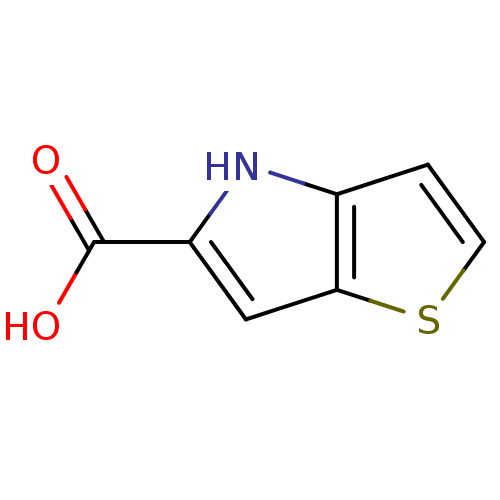 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAAO expressed in Escherichia coli assessed as H2O2 production from D-serine degradation after 30 mins by fluorescenc... | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM23174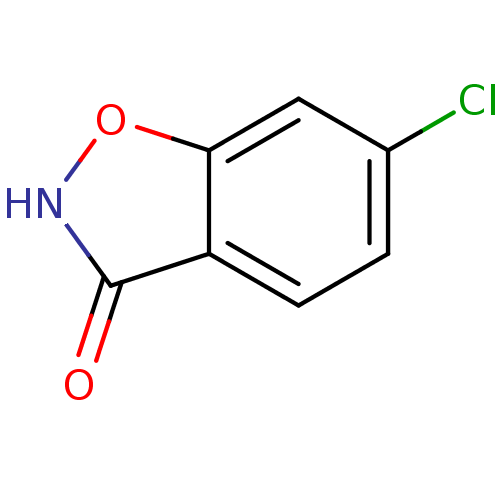 (6-chloro-1,2-benzoxazol-3-ol | 6-chlorobenzo[d]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAAO expressed in Escherichia coli assessed as H2O2 production from D-serine degradation after 30 mins by fluorescenc... | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31147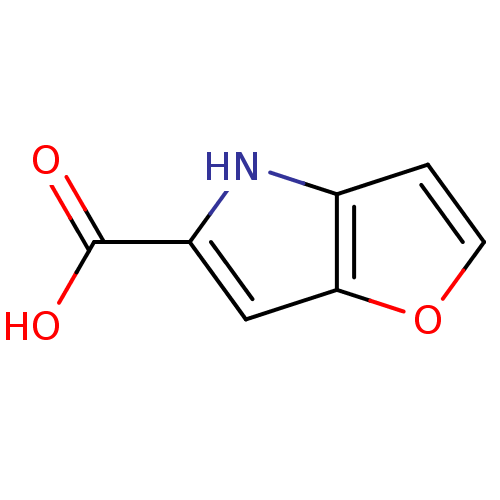 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAAO expressed in Escherichia coli assessed as H2O2 production from D-serine degradation after 30 mins by fluorescenc... | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410855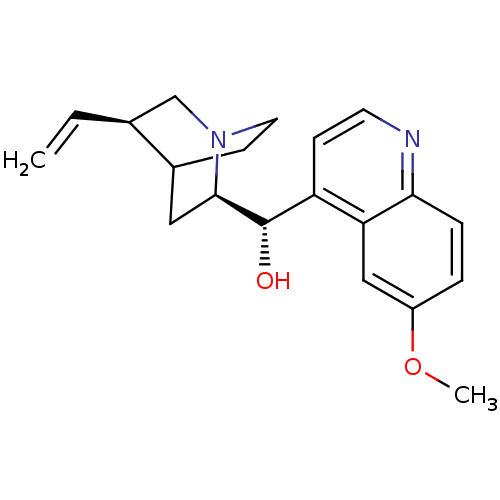 (CIN-QUIN | QUINIDEX | QUINIDINE SULFATE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185249 (BS 7840 | CHEMBL380748) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260725 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human DAAO | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185254 (BS 7581 | CHEMBL444541) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185248 (CHEMBL203446 | GBR 12530) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185257 (BS 9106 | CHEMBL206647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50211362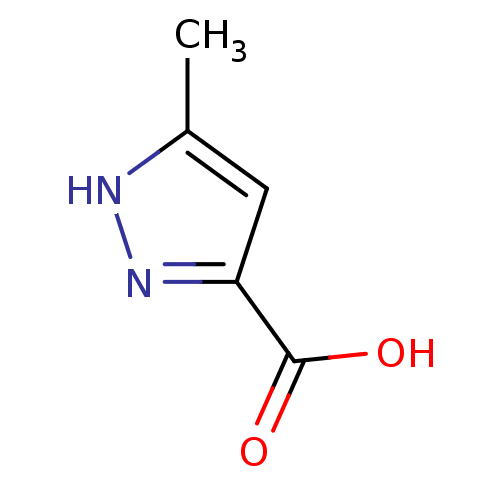 (5-methyl-1H-pyrazole-3-carboxylic acid | 5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 473 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAAO expressed in Escherichia coli assessed as H2O2 production from D-serine degradation after 30 mins by fluorescenc... | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50211362 (5-methyl-1H-pyrazole-3-carboxylic acid | 5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human DAAO | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50116806 (1-{2-[Bis-(4-fluoro-phenyl)-methoxy]-ethyl}-4-(3-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50367247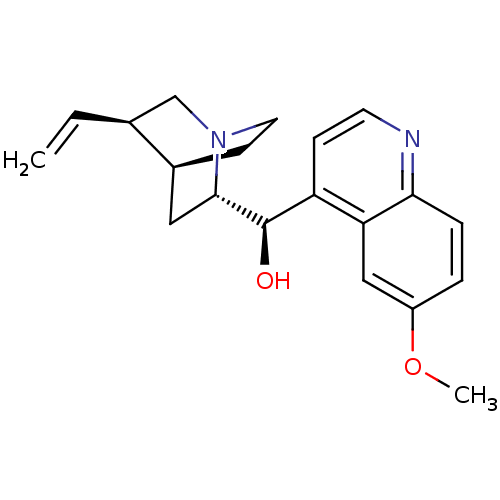 (QUININE | Quinamm | Quinsan | cid_3034034) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50366613 (DEXTROMETHORPHAN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185250 (3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM31172 (3-hydroxyquinolin-2(1H)-one, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells by whole cell voltage patch clamp technique | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells by whole cell voltage patch clamp technique | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50260725 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells by whole cell voltage patch clamp technique | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM31147 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells by whole cell voltage patch clamp technique | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM23174 (6-chloro-1,2-benzoxazol-3-ol | 6-chlorobenzo[d]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells by whole cell voltage patch clamp technique | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50211362 (5-methyl-1H-pyrazole-3-carboxylic acid | 5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells by whole cell voltage patch clamp technique | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM50260725 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM31147 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM23174 (6-chloro-1,2-benzoxazol-3-ol | 6-chlorobenzo[d]iso...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM50211362 (5-methyl-1H-pyrazole-3-carboxylic acid | 5-methyl-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM31172 (3-hydroxyquinolin-2(1H)-one, 26) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185251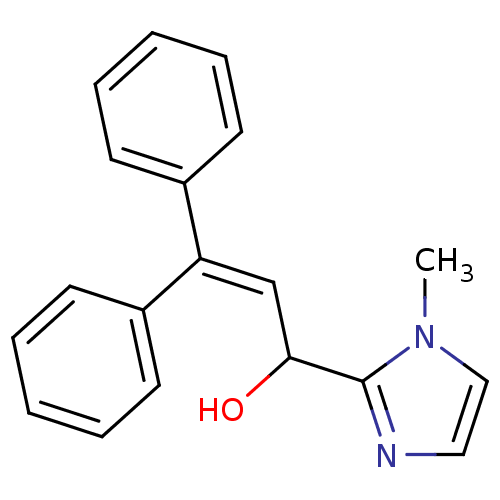 (2-(1-hydroxy-3,3-diphenyl-allyl)-3-methyl-3H-imida...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185255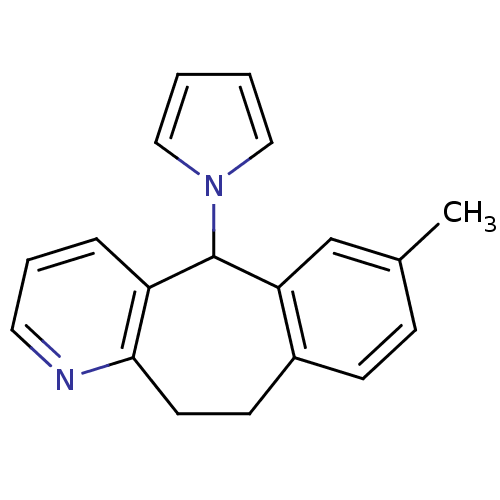 (7-methyl-5-pyrrol-1-yl-10,11-dihydro-5H-benzo[4,5]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370708 (SPARTEINE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185253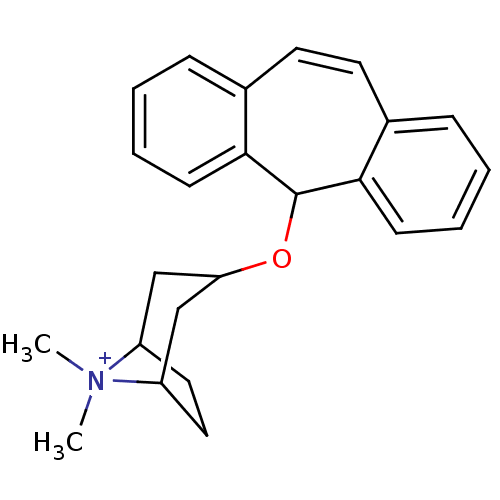 (CHEMBL206345 | GBR 13036) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185256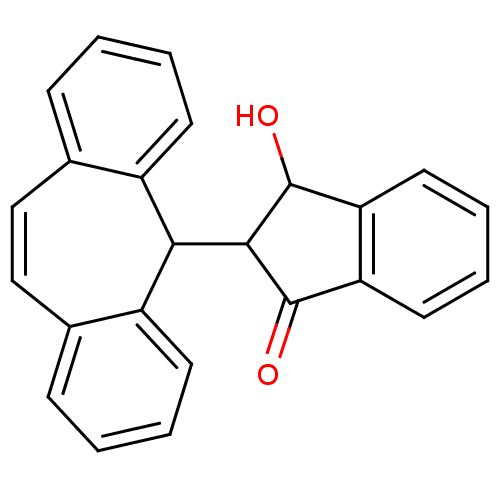 (2-(5H-dibenzo[a,d]cyclohepten-5-yl)-3-hydroxy-inda...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185258 (2-bromo-2-(2-ethylphenyl)-2H-indene-1,3-dione | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185252 (2-(2-aminophenyl)quinazolin-4-amine | CHEMBL206398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185243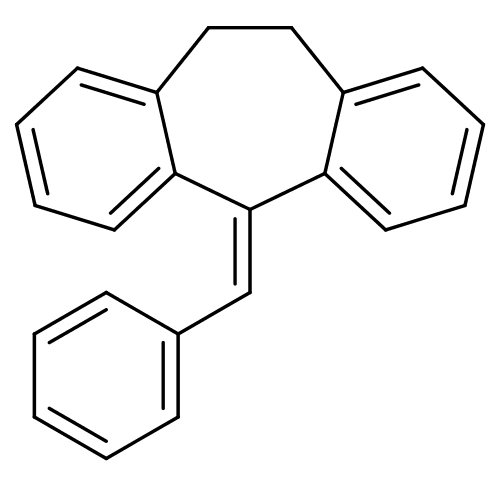 (5-benzylidene-10,11-dihydro-5H-dibenzo[a,d]cyclohe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185242 (10,11-dihydro-5H-benzo[4,5]cyclohepta[1,2-b]pyridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM768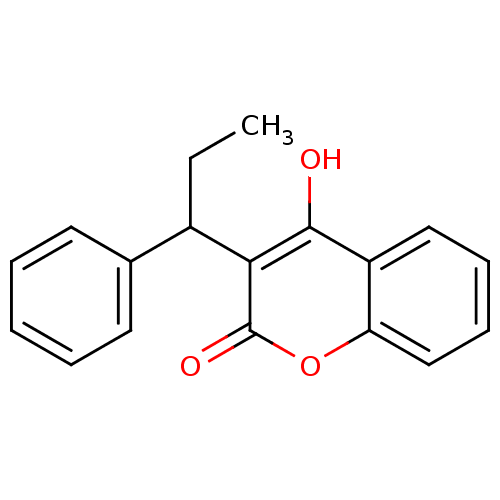 (4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50185241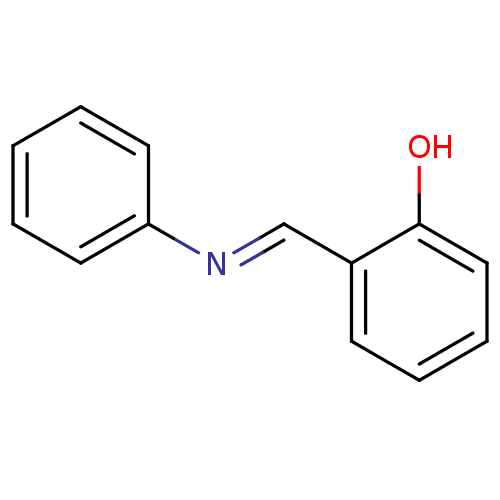 (2-((phenylimino)methyl)phenol | CHEMBL205927 | GBR...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli JM109 | J Med Chem 49: 2417-30 (2006) Article DOI: 10.1021/jm0508538 BindingDB Entry DOI: 10.7270/Q2930TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31172 (3-hydroxyquinolin-2(1H)-one, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by steady state study scintillation proximity assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by steady state study scintillation proximity assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260725 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by steady state study scintillation proximity assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31147 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by steady state study scintillation proximity assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM23174 (6-chloro-1,2-benzoxazol-3-ol | 6-chlorobenzo[d]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by steady state study scintillation proximity assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50260725 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by kinetic study scintillation proximity assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31147 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by kinetic study scintillation proximity assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31172 (3-hydroxyquinolin-2(1H)-one, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by isothermal titration calorimeter analysis | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50211362 (5-methyl-1H-pyrazole-3-carboxylic acid | 5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 617 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by isothermal titration calorimeter analysis | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM23174 (6-chloro-1,2-benzoxazol-3-ol | 6-chlorobenzo[d]iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Binding affinity to human recombinant DAAO by isothermal titration calorimeter analysis | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |