

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039635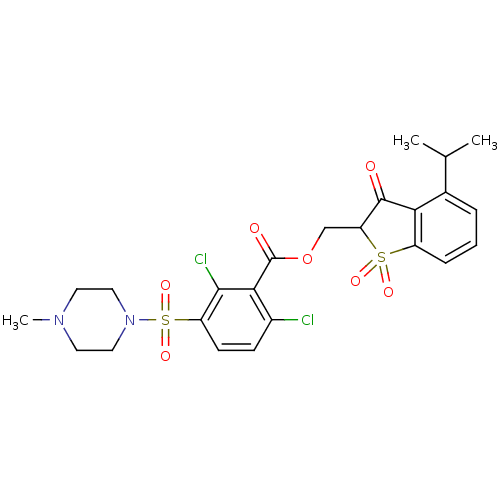 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036480 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039646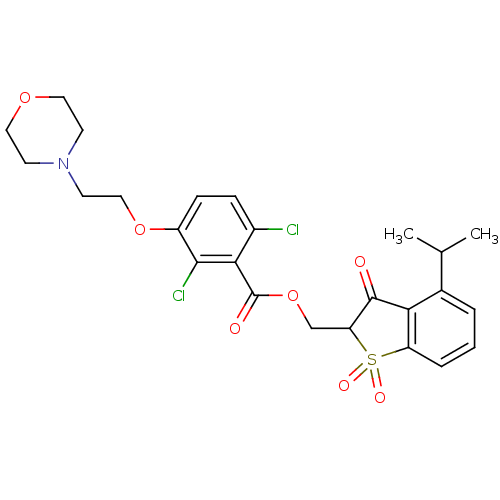 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039637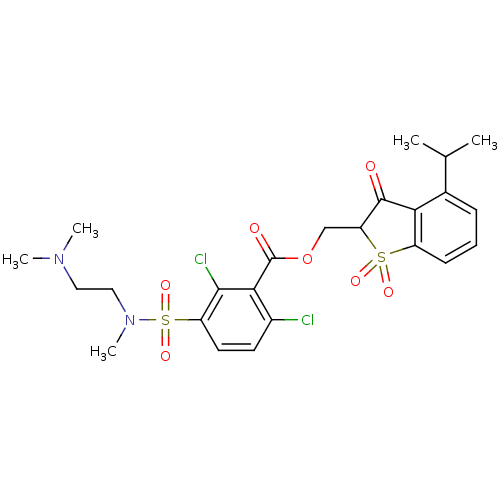 (2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036478 (3-Carboxymethoxy-2,6-dichloro-benzoic acid 4-isopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036476 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036481 (2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036477 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036475 (2,6-Dichloro-3-(2-pyrrolidin-1-yl-ethoxy)-benzoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0210 | -63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285289 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699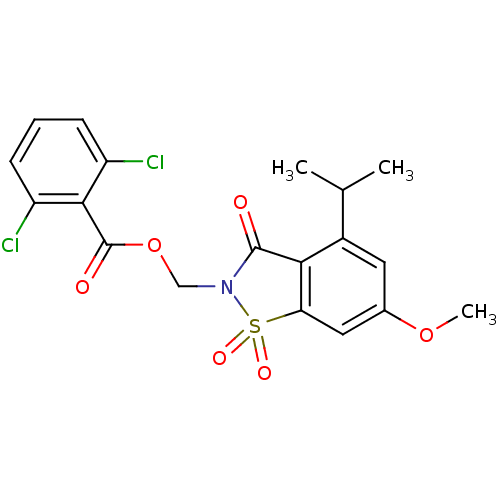 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631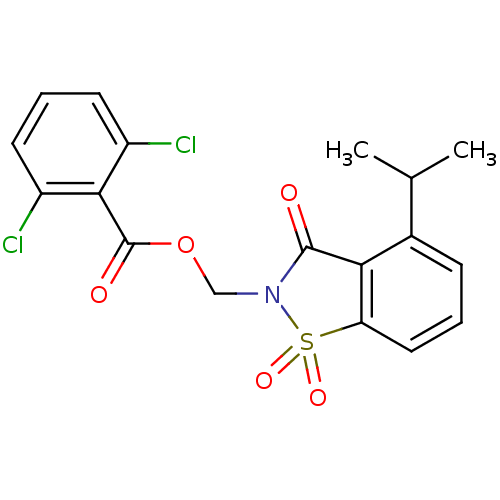 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286326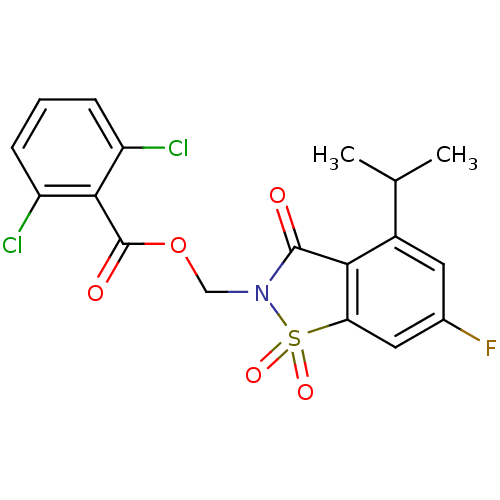 (2,6-Dichloro-benzoic acid 6-fluoro-4-isopropyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50034671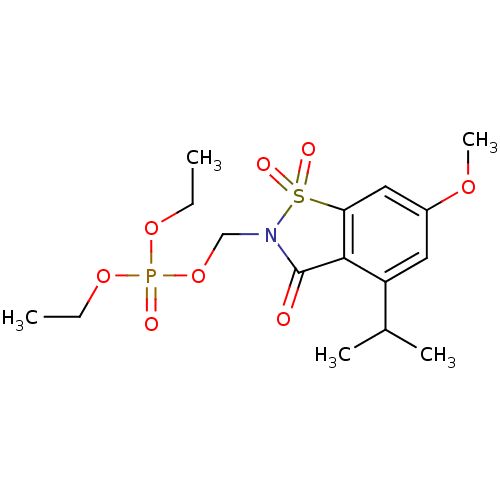 (CHEMBL41327 | Phosphoric acid diethyl ester 4-isop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description In vitro inhibitory activity against Human leukocyte elastase | J Med Chem 38: 1571-4 (1995) BindingDB Entry DOI: 10.7270/Q2M32TS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433530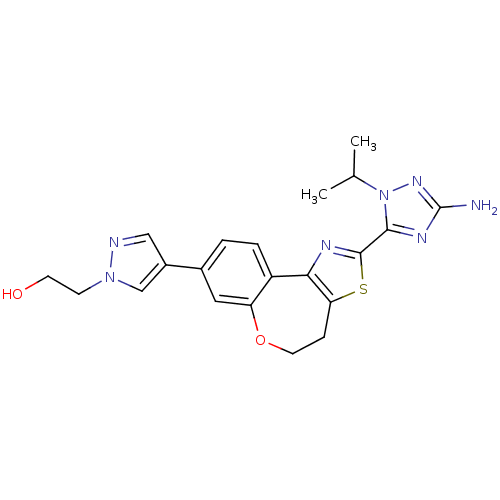 (CHEMBL2381382) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285280 (2,6-Dichloro-benzoic acid 4-ethoxy-6-methoxy-1,1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433533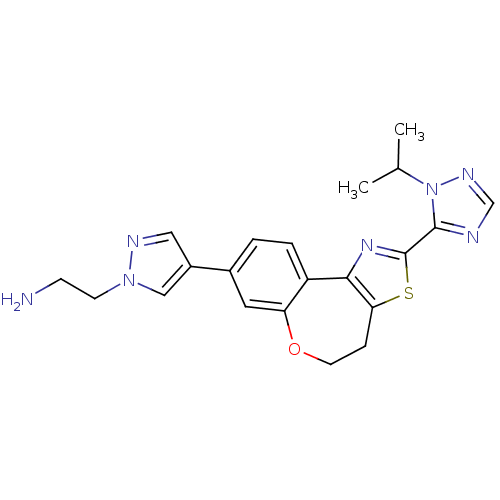 (CHEMBL2381376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039641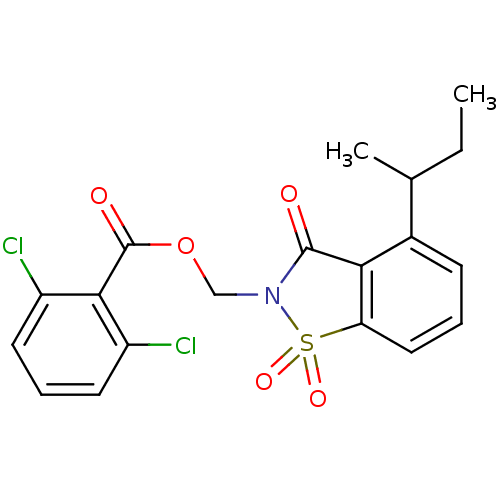 (2,6-Dichloro-benzoic acid 4-sec-butyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433534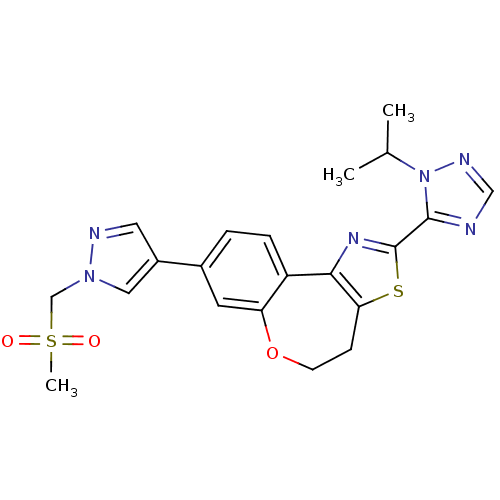 (CHEMBL2381375) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50034676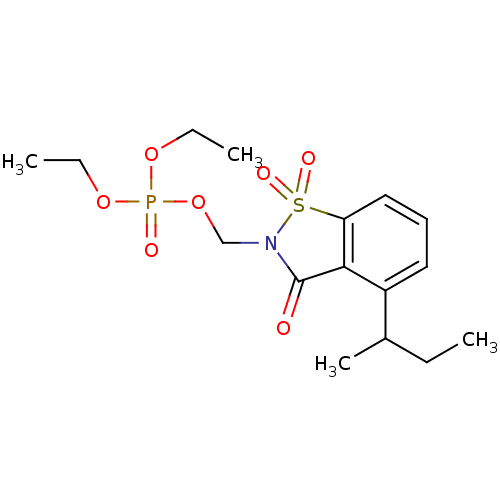 (CHEMBL41881 | Phosphoric acid 4-sec-butyl-1,1,3-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description In vitro inhibitory activity against Human leukocyte elastase | J Med Chem 38: 1571-4 (1995) BindingDB Entry DOI: 10.7270/Q2M32TS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285286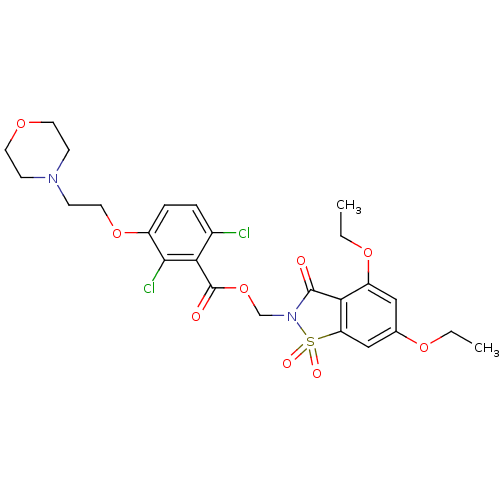 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285284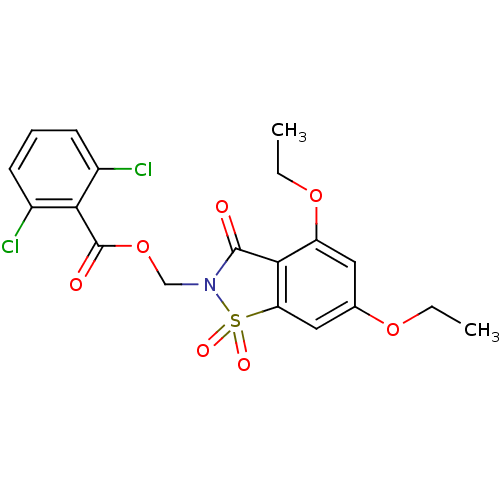 (2,6-Dichloro-benzoic acid 4,6-diethoxy-1,1,3-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285283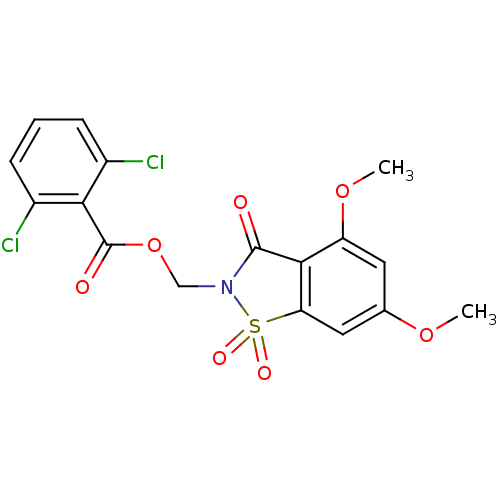 (2,6-Dichloro-benzoic acid 4,6-dimethoxy-1,1,3-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233601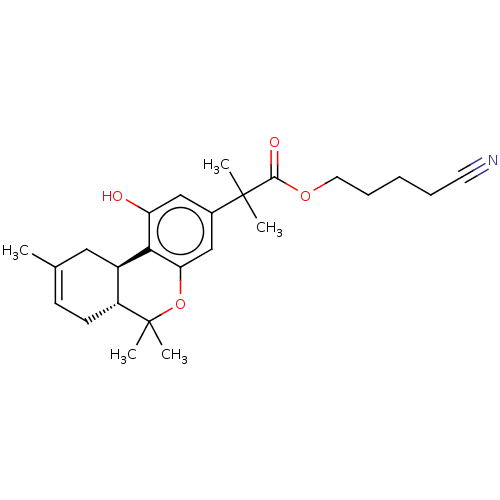 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50034677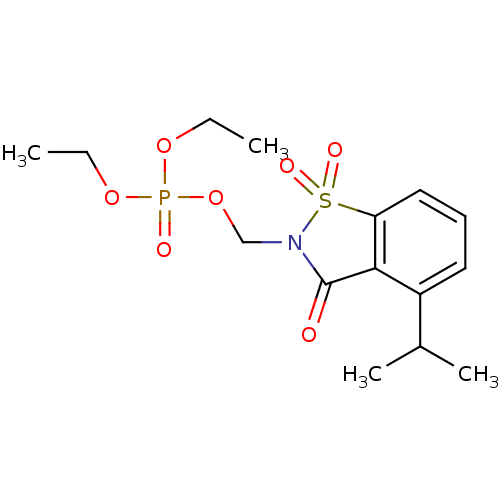 (CHEMBL288810 | Phosphoric acid diethyl ester 4-iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description In vitro inhibitory activity against Human leukocyte elastase | J Med Chem 38: 1571-4 (1995) BindingDB Entry DOI: 10.7270/Q2M32TS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433532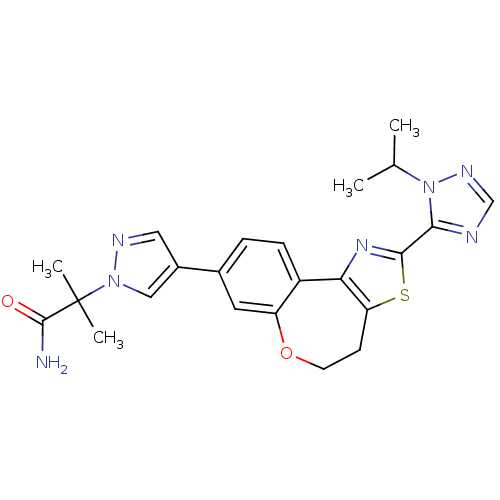 (CHEMBL2381377) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285287 (2,6-Dichloro-3-(2-pyrrolidin-1-yl-ethoxy)-benzoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433529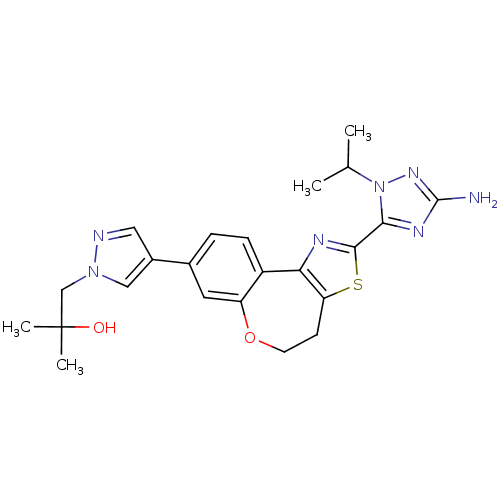 (CHEMBL2381380) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433527 (CHEMBL2381379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (Homo sapiens (Human)) | BDBM85484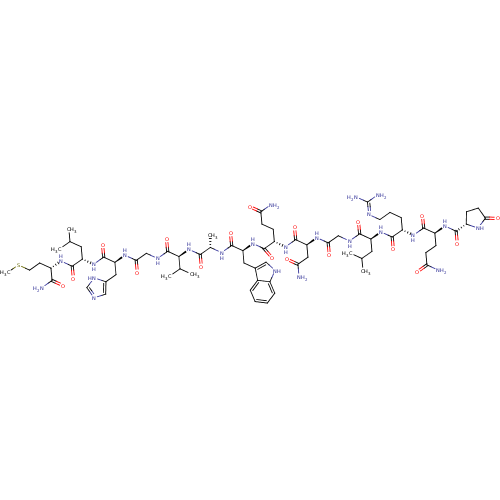 (Bombesin) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against [125 I][4Tyr]-bombesin labeled cloned human GRP(gastrin releasing peptide) receptors stably expressed in CHO cells | Bioorg Med Chem Lett 6: 2617-2622 (1996) Article DOI: 10.1016/0960-894X(96)00481-7 BindingDB Entry DOI: 10.7270/Q2NC61QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433535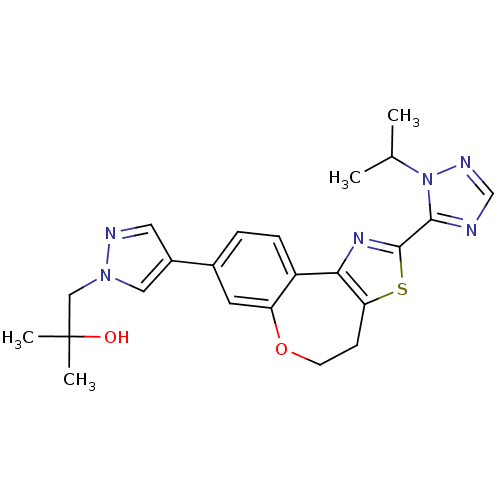 (CHEMBL2381374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039642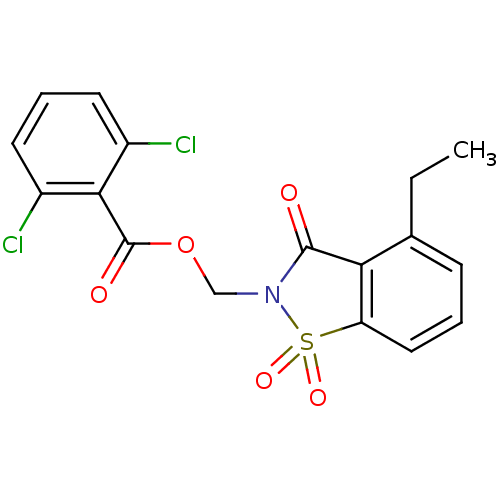 (2,6-Dichloro-benzoic acid 4-ethyl-1,1,3-trioxo-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285290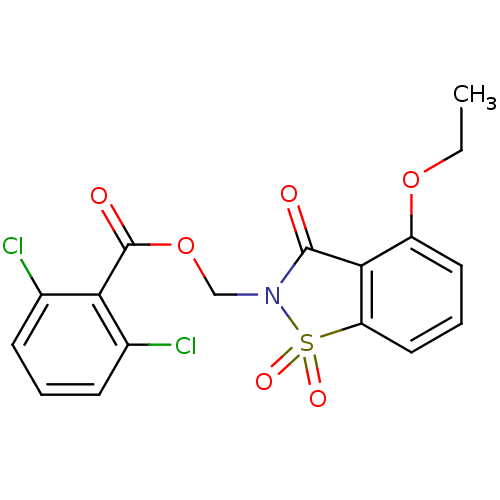 (2,6-Dichloro-benzoic acid 4-ethoxy-1,1,3-trioxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433531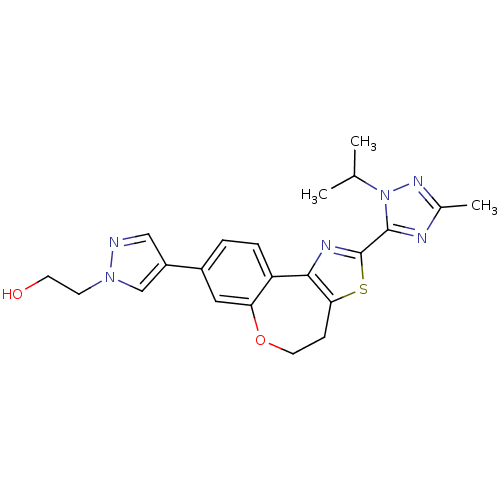 (CHEMBL2381378) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433528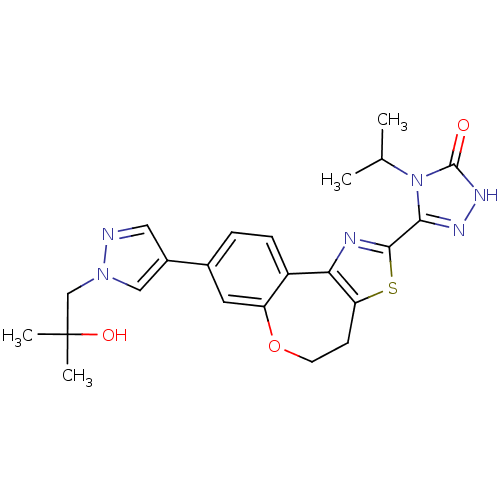 (CHEMBL2381381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,I543V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -57.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036482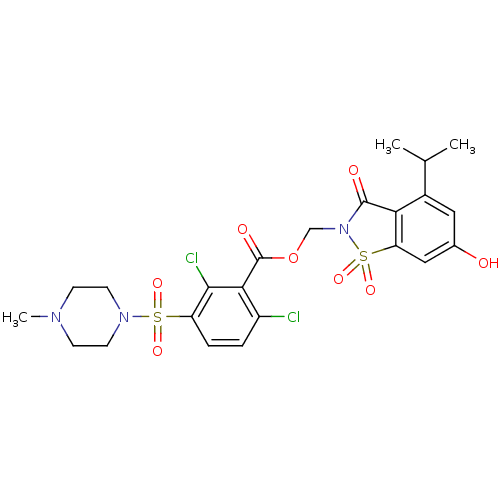 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286333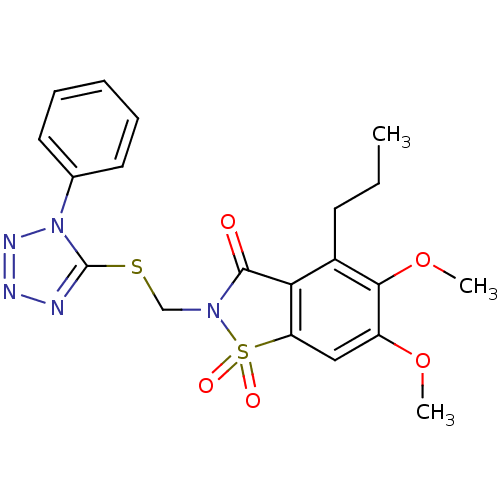 (5,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285282 (2,6-Dichloro-benzoic acid 4-isopropoxy-1,1,3-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029703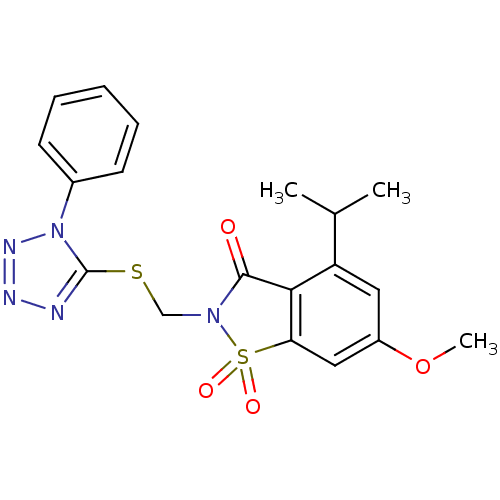 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(1-phenyl-1H-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50433536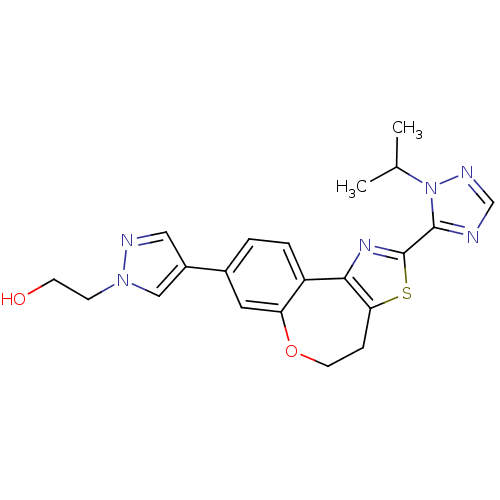 (CHEMBL2381373) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... | Bioorg Med Chem Lett 23: 2606-13 (2013) Article DOI: 10.1016/j.bmcl.2013.02.102 BindingDB Entry DOI: 10.7270/Q2W95BKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160917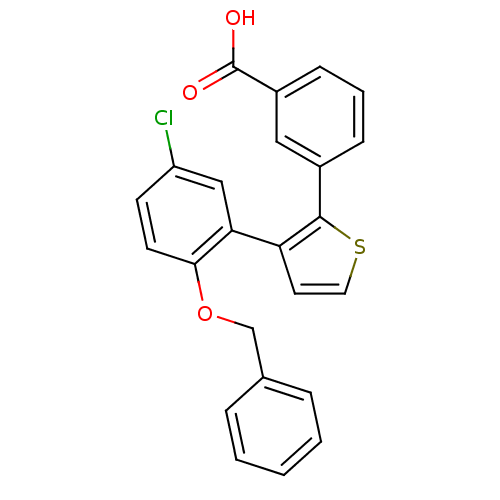 (3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPR | Bioorg Med Chem Lett 16: 2666-71 (2006) Article DOI: 10.1016/j.bmcl.2006.02.014 BindingDB Entry DOI: 10.7270/Q2J102RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599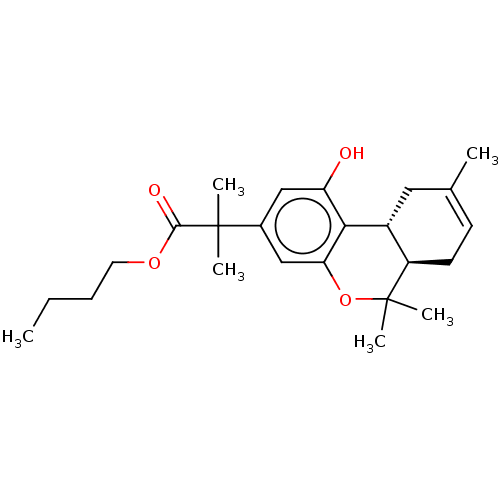 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain by scintillation counting analysis | J Med Chem 56: 10142-57 (2013) Article DOI: 10.1021/jm4016075 BindingDB Entry DOI: 10.7270/Q241711Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282874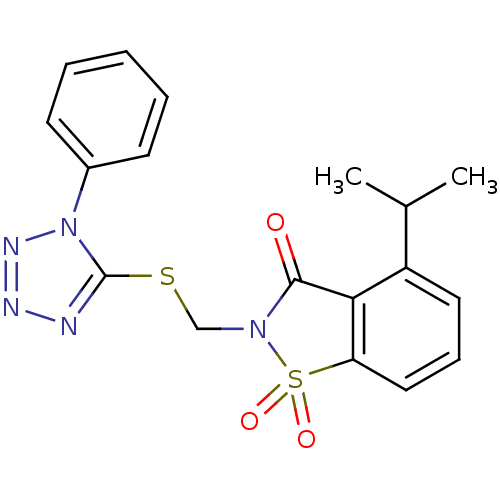 (4-Isopropyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233619 (CHEMBL4085404 | US10882838, Example 1.18) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8876 total ) | Next | Last >> |