 Found 207 hits with Last Name = 'osborn' and Initial = 'r'
Found 207 hits with Last Name = 'osborn' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343152
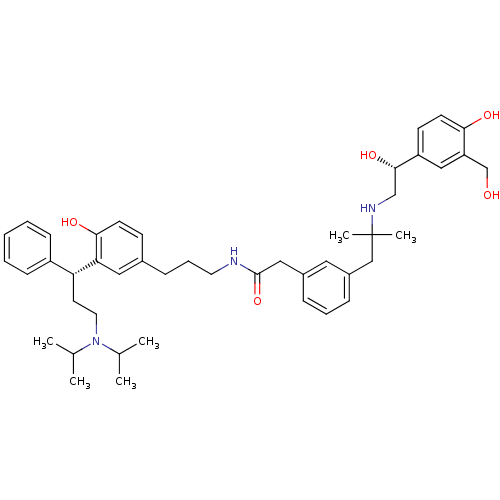
(CHEMBL1773196 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(CO)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H61N3O5/c1-31(2)48(32(3)4)23-21-39(36-15-8-7-9-16-36)40-25-33(17-19-42(40)51)14-11-22-46-44(53)26-34-12-10-13-35(24-34)28-45(5,6)47-29-43(52)37-18-20-41(50)38(27-37)30-49/h7-10,12-13,15-20,24-25,27,31-32,39,43,47,49-52H,11,14,21-23,26,28-30H2,1-6H3,(H,46,53)/t39-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343153
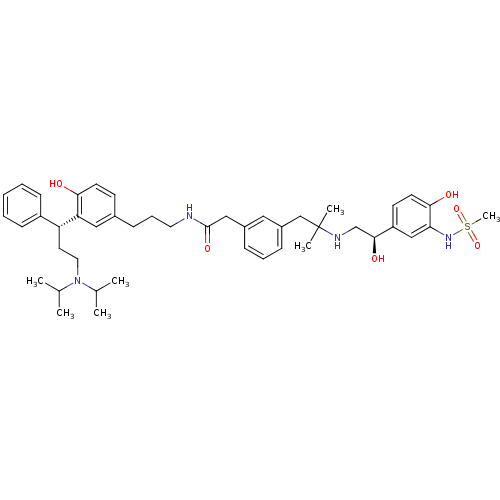
(CHEMBL1773197 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(NS(C)(=O)=O)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H62N4O6S/c1-31(2)49(32(3)4)24-22-38(36-16-9-8-10-17-36)39-26-33(18-20-41(39)50)15-12-23-46-44(53)27-34-13-11-14-35(25-34)29-45(5,6)47-30-43(52)37-19-21-42(51)40(28-37)48-56(7,54)55/h8-11,13-14,16-21,25-26,28,31-32,38,43,47-48,50-52H,12,15,22-24,27,29-30H2,1-7H3,(H,46,53)/t38-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343161
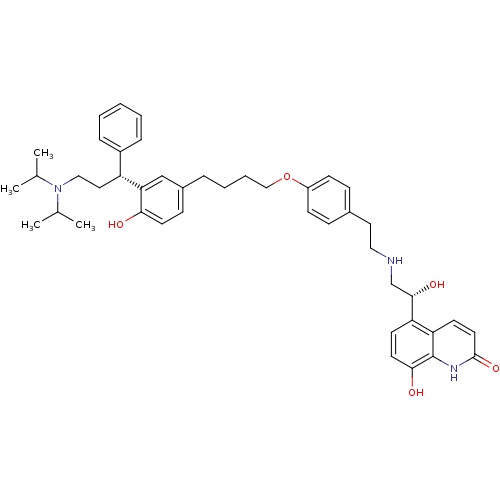
(5-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H55N3O5/c1-30(2)47(31(3)4)26-24-36(34-11-6-5-7-12-34)39-28-33(15-20-40(39)48)10-8-9-27-52-35-16-13-32(14-17-35)23-25-45-29-42(50)37-18-21-41(49)44-38(37)19-22-43(51)46-44/h5-7,11-22,28,30-31,36,42,45,48-50H,8-10,23-27,29H2,1-4H3,(H,46,51)/t36-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343154
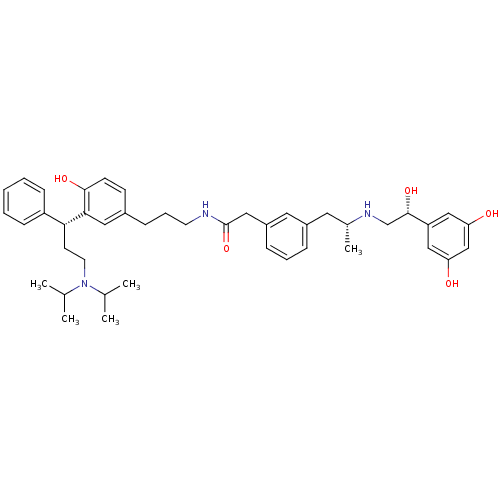
(2-(3-((R)-2-((R)-2-(3,5-dihydroxyphenyl)-2-hydroxy...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(C[C@@H](C)NC[C@H](O)c3cc(O)cc(O)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C43H57N3O5/c1-29(2)46(30(3)4)20-18-39(35-14-7-6-8-15-35)40-23-32(16-17-41(40)49)13-10-19-44-43(51)24-34-12-9-11-33(22-34)21-31(5)45-28-42(50)36-25-37(47)27-38(48)26-36/h6-9,11-12,14-17,22-23,25-27,29-31,39,42,45,47-50H,10,13,18-21,24,28H2,1-5H3,(H,44,51)/t31-,39-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.397 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343157
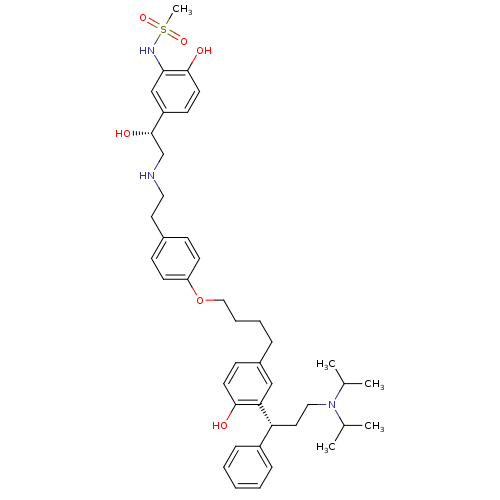
(CHEMBL1773264 | N-(5-((R)-2-(4-(4-(3-((R)-3-(diiso...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(NS(C)(=O)=O)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C42H57N3O6S/c1-30(2)45(31(3)4)25-23-37(34-12-7-6-8-13-34)38-27-33(16-20-40(38)46)11-9-10-26-51-36-18-14-32(15-19-36)22-24-43-29-42(48)35-17-21-41(47)39(28-35)44-52(5,49)50/h6-8,12-21,27-28,30-31,37,42-44,46-48H,9-11,22-26,29H2,1-5H3/t37-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.634 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343158
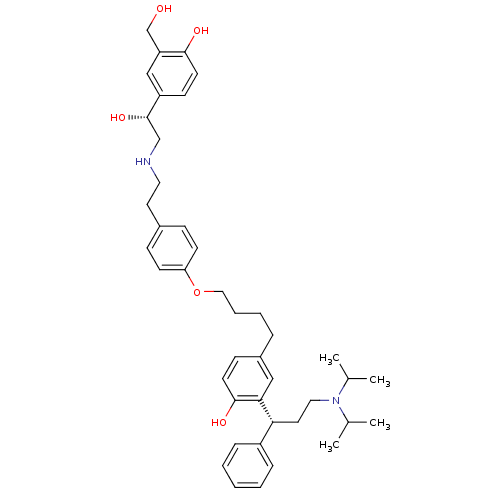
(2-((R)-3-(diisopropylamino)-1-phenylpropyl)-4-(4-(...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(CO)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C42H56N2O5/c1-30(2)44(31(3)4)24-22-38(34-11-6-5-7-12-34)39-26-33(15-19-41(39)47)10-8-9-25-49-37-17-13-32(14-18-37)21-23-43-28-42(48)35-16-20-40(46)36(27-35)29-45/h5-7,11-20,26-27,30-31,38,42-43,45-48H,8-10,21-25,28-29H2,1-4H3/t38-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.765 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343160
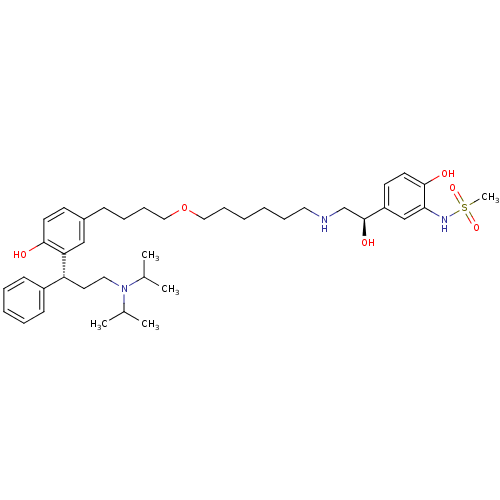
(CHEMBL1773266 | N-(5-((R)-2-(6-(4-(3-((R)-3-(diiso...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOCCCCCCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C40H61N3O6S/c1-30(2)43(31(3)4)24-22-35(33-16-9-8-10-17-33)36-27-32(18-20-38(36)44)15-11-14-26-49-25-13-7-6-12-23-41-29-40(46)34-19-21-39(45)37(28-34)42-50(5,47)48/h8-10,16-21,27-28,30-31,35,40-42,44-46H,6-7,11-15,22-26,29H2,1-5H3/t35-,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343156
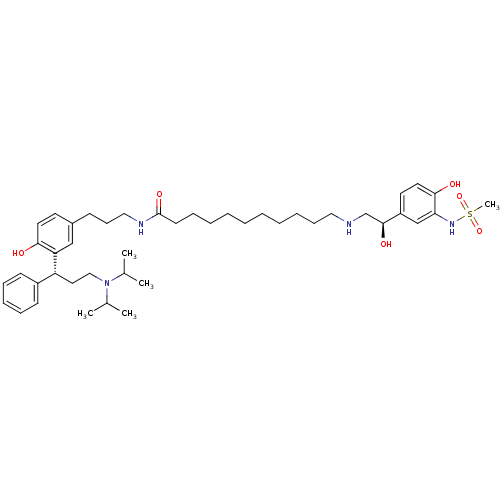
(CHEMBL1773263 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)CCCCCCCCCCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H68N4O6S/c1-33(2)48(34(3)4)29-26-38(36-19-13-12-14-20-36)39-30-35(22-24-41(39)49)18-17-28-46-44(52)21-15-10-8-6-7-9-11-16-27-45-32-43(51)37-23-25-42(50)40(31-37)47-55(5,53)54/h12-14,19-20,22-25,30-31,33-34,38,43,45,47,49-51H,6-11,15-18,21,26-29,32H2,1-5H3,(H,46,52)/t38-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50354566
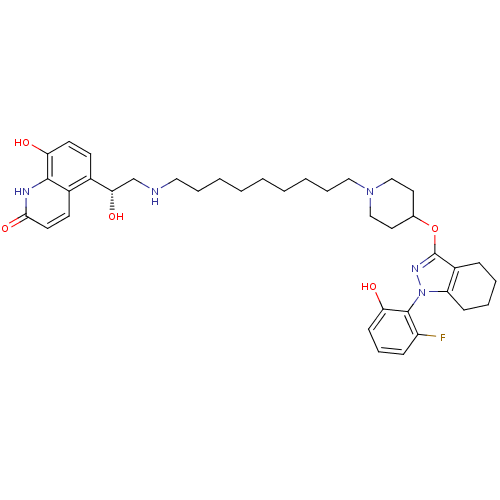
(CHEMBL1836647)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(CC1)Oc1nn(c2CCCCc12)-c1c(O)cccc1F)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,(-3.94,-14.4,;-3.93,-15.94,;-2.6,-16.71,;-1.27,-15.93,;.07,-16.7,;1.4,-15.92,;2.74,-16.69,;4.07,-15.91,;5.41,-16.68,;6.74,-15.9,;8.07,-16.67,;9.4,-15.89,;10.74,-16.66,;12.07,-15.88,;13.4,-16.65,;14.72,-15.88,;14.73,-14.34,;13.39,-13.57,;12.05,-14.35,;16.06,-13.57,;16.06,-12.03,;14.64,-11.42,;14.79,-9.89,;16.3,-9.56,;17.05,-8.23,;18.58,-8.22,;19.36,-9.55,;18.6,-10.88,;17.07,-10.88,;13.45,-9.14,;12.14,-9.94,;12.18,-11.48,;10.79,-9.2,;10.76,-7.66,;12.08,-6.86,;13.43,-7.61,;14.75,-6.82,;-5.26,-16.72,;-6.6,-15.95,;-7.93,-16.72,;-7.93,-18.27,;-9.26,-19.04,;-6.59,-19.03,;-6.59,-20.56,;-5.26,-21.34,;-5.26,-22.88,;-3.93,-20.57,;-3.93,-19.03,;-5.26,-18.27,)| Show InChI InChI=1S/C38H50FN5O5/c39-30-12-10-14-33(46)37(30)44-31-13-7-6-11-29(31)38(42-44)49-26-19-23-43(24-20-26)22-9-5-3-1-2-4-8-21-40-25-34(47)27-15-17-32(45)36-28(27)16-18-35(48)41-36/h10,12,14-18,26,34,40,45-47H,1-9,11,13,19-25H2,(H,41,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human M3 receptor |
J Med Chem 54: 6998-7002 (2011)
Article DOI: 10.1021/jm2007535
BindingDB Entry DOI: 10.7270/Q2SJ1M03 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50354569
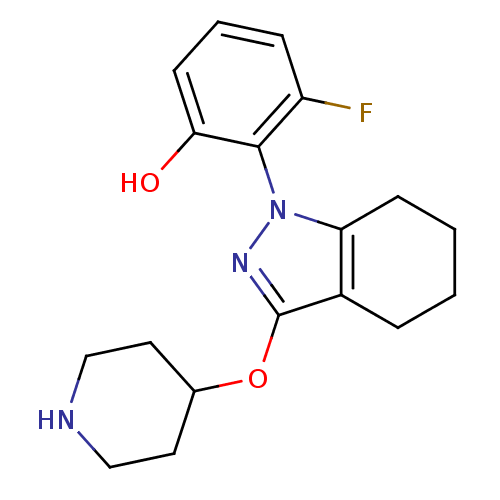
(CHEMBL1836646)Show SMILES Oc1cccc(F)c1-n1nc(OC2CCNCC2)c2CCCCc12 |(36.87,1.24,;36.09,2.57,;36.85,3.91,;36.07,5.24,;34.52,5.22,;33.77,3.88,;32.23,3.86,;34.56,2.56,;33.8,1.22,;34.71,-.02,;33.8,-1.27,;34.59,-2.59,;36.13,-2.57,;36.91,-3.9,;38.44,-3.88,;39.2,-2.54,;38.41,-1.22,;36.87,-1.23,;32.33,-.8,;31,-1.58,;29.67,-.81,;29.67,.74,;31,1.51,;32.33,.75,)| Show InChI InChI=1S/C18H22FN3O2/c19-14-5-3-7-16(23)17(14)22-15-6-2-1-4-13(15)18(21-22)24-12-8-10-20-11-9-12/h3,5,7,12,20,23H,1-2,4,6,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human M3 receptor |
J Med Chem 54: 6998-7002 (2011)
Article DOI: 10.1021/jm2007535
BindingDB Entry DOI: 10.7270/Q2SJ1M03 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human M3 receptor |
J Med Chem 54: 6998-7002 (2011)
Article DOI: 10.1021/jm2007535
BindingDB Entry DOI: 10.7270/Q2SJ1M03 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343159

(4-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(O)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C41H54N2O5/c1-29(2)43(30(3)4)24-22-36(33-11-6-5-7-12-33)37-26-32(15-19-38(37)44)10-8-9-25-48-35-17-13-31(14-18-35)21-23-42-28-41(47)34-16-20-39(45)40(46)27-34/h5-7,11-20,26-27,29-30,36,41-42,44-47H,8-10,21-25,28H2,1-4H3/t36-,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343155
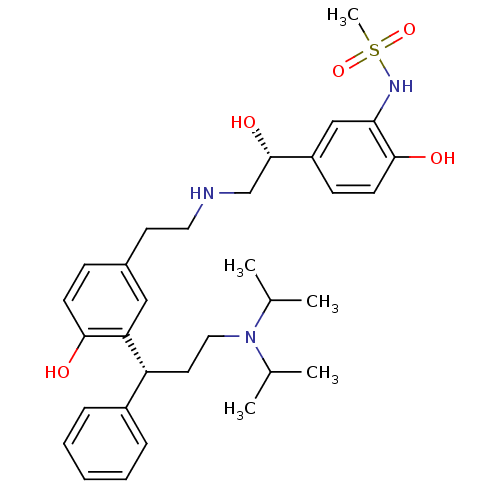
(CHEMBL1773262 | N-(5-((R)-2-(3-((R)-3-(diisopropyl...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C32H45N3O5S/c1-22(2)35(23(3)4)18-16-27(25-9-7-6-8-10-25)28-19-24(11-13-30(28)36)15-17-33-21-32(38)26-12-14-31(37)29(20-26)34-41(5,39)40/h6-14,19-20,22-23,27,32-34,36-38H,15-18,21H2,1-5H3/t27-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50354567
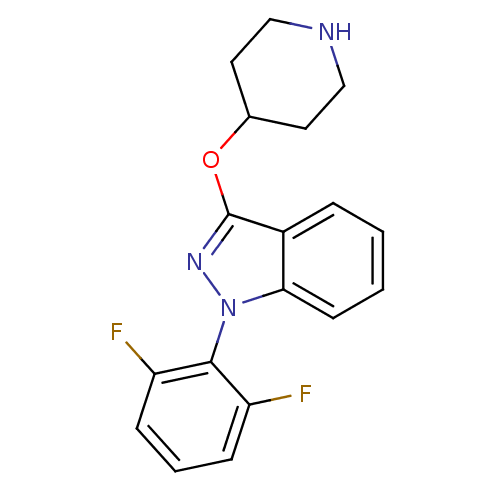
(CHEMBL1836644)Show SMILES Fc1cccc(F)c1-n1nc(OC2CCNCC2)c2ccccc12 |(8.15,4.15,;9.69,4.17,;10.44,5.51,;11.99,5.53,;12.77,4.2,;12.01,2.86,;12.79,1.54,;10.47,2.86,;9.71,1.52,;10.62,.27,;9.72,-.98,;10.51,-2.3,;12.04,-2.28,;12.82,-3.6,;14.36,-3.59,;15.11,-2.25,;14.33,-.92,;12.78,-.94,;8.25,-.5,;6.92,-1.28,;5.58,-.51,;5.59,1.03,;6.91,1.8,;8.25,1.04,)| Show InChI InChI=1S/C18H17F2N3O/c19-14-5-3-6-15(20)17(14)23-16-7-2-1-4-13(16)18(22-23)24-12-8-10-21-11-9-12/h1-7,12,21H,8-11H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human NET receptor |
J Med Chem 54: 6998-7002 (2011)
Article DOI: 10.1021/jm2007535
BindingDB Entry DOI: 10.7270/Q2SJ1M03 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50354568
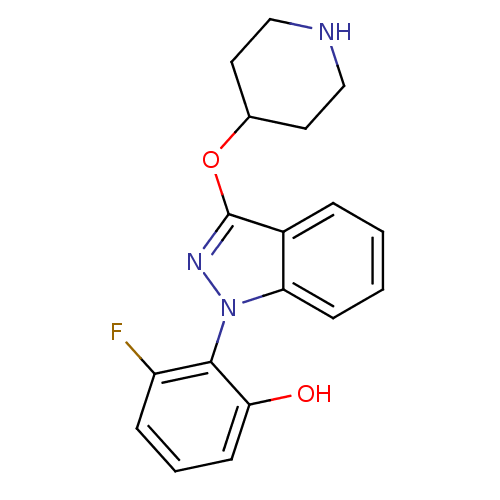
(CHEMBL1836645)Show SMILES Oc1cccc(F)c1-n1nc(OC2CCNCC2)c2ccccc12 |(25.39,1.6,;24.61,2.93,;25.37,4.26,;24.59,5.59,;23.04,5.57,;22.29,4.24,;20.75,4.22,;23.07,2.92,;22.31,1.58,;23.22,.33,;22.32,-.91,;23.11,-2.24,;24.64,-2.21,;25.42,-3.54,;26.96,-3.52,;27.71,-2.18,;26.93,-.86,;25.38,-.87,;20.85,-.44,;19.52,-1.22,;18.18,-.45,;18.19,1.1,;19.51,1.87,;20.85,1.1,)| Show InChI InChI=1S/C18H18FN3O2/c19-14-5-3-7-16(23)17(14)22-15-6-2-1-4-13(15)18(21-22)24-12-8-10-20-11-9-12/h1-7,12,20,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human M3 receptor |
J Med Chem 54: 6998-7002 (2011)
Article DOI: 10.1021/jm2007535
BindingDB Entry DOI: 10.7270/Q2SJ1M03 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357272
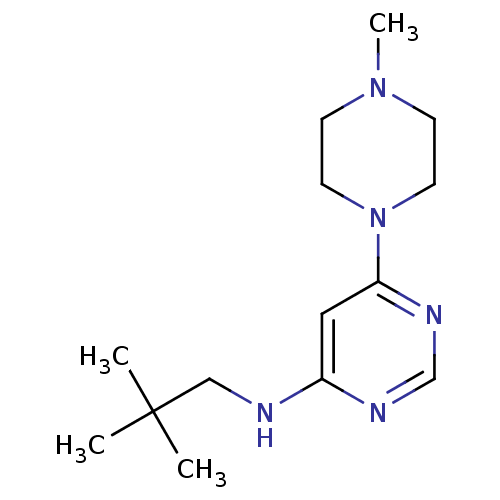
(CHEMBL1916498)Show InChI InChI=1S/C14H25N5/c1-14(2,3)10-15-12-9-13(17-11-16-12)19-7-5-18(4)6-8-19/h9,11H,5-8,10H2,1-4H3,(H,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357277
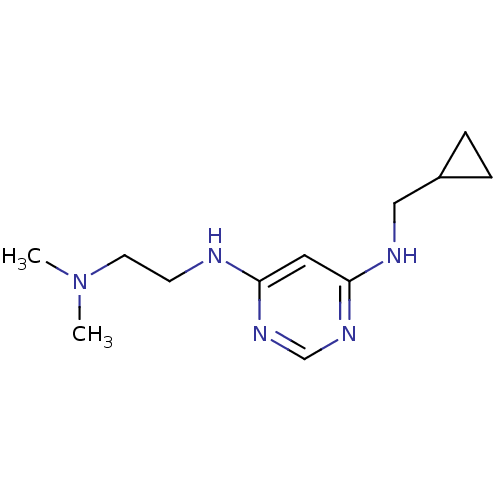
(CHEMBL1916505)Show InChI InChI=1S/C12H21N5/c1-17(2)6-5-13-11-7-12(16-9-15-11)14-8-10-3-4-10/h7,9-10H,3-6,8H2,1-2H3,(H2,13,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 32.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50179335
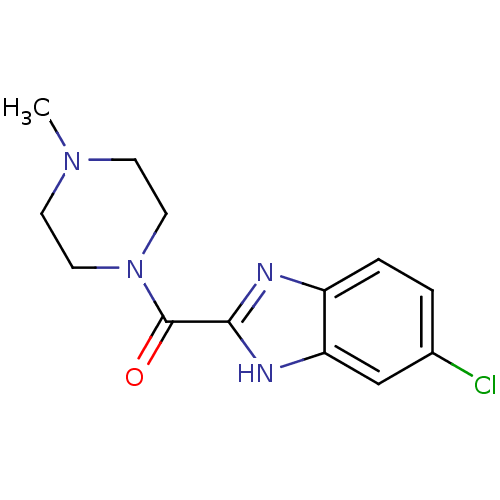
((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...)Show InChI InChI=1S/C13H15ClN4O/c1-17-4-6-18(7-5-17)13(19)12-15-10-3-2-9(14)8-11(10)16-12/h2-3,8H,4-7H2,1H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50179335

((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...)Show InChI InChI=1S/C13H15ClN4O/c1-17-4-6-18(7-5-17)13(19)12-15-10-3-2-9(14)8-11(10)16-12/h2-3,8H,4-7H2,1H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50354567

(CHEMBL1836644)Show SMILES Fc1cccc(F)c1-n1nc(OC2CCNCC2)c2ccccc12 |(8.15,4.15,;9.69,4.17,;10.44,5.51,;11.99,5.53,;12.77,4.2,;12.01,2.86,;12.79,1.54,;10.47,2.86,;9.71,1.52,;10.62,.27,;9.72,-.98,;10.51,-2.3,;12.04,-2.28,;12.82,-3.6,;14.36,-3.59,;15.11,-2.25,;14.33,-.92,;12.78,-.94,;8.25,-.5,;6.92,-1.28,;5.58,-.51,;5.59,1.03,;6.91,1.8,;8.25,1.04,)| Show InChI InChI=1S/C18H17F2N3O/c19-14-5-3-6-15(20)17(14)23-16-7-2-1-4-13(16)18(22-23)24-12-8-10-21-11-9-12/h1-7,12,21H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human M3 receptor |
J Med Chem 54: 6998-7002 (2011)
Article DOI: 10.1021/jm2007535
BindingDB Entry DOI: 10.7270/Q2SJ1M03 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50357273
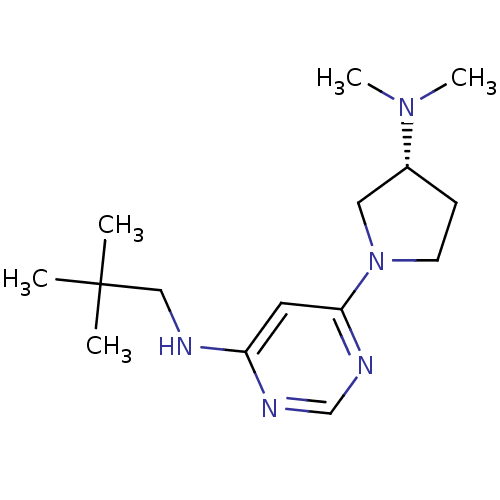
(CHEMBL1916499)Show SMILES CN(C)[C@@H]1CCN(C1)c1cc(NCC(C)(C)C)ncn1 |r| Show InChI InChI=1S/C15H27N5/c1-15(2,3)10-16-13-8-14(18-11-17-13)20-7-6-12(9-20)19(4)5/h8,11-12H,6-7,9-10H2,1-5H3,(H,16,17,18)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357273

(CHEMBL1916499)Show SMILES CN(C)[C@@H]1CCN(C1)c1cc(NCC(C)(C)C)ncn1 |r| Show InChI InChI=1S/C15H27N5/c1-15(2,3)10-16-13-8-14(18-11-17-13)20-7-6-12(9-20)19(4)5/h8,11-12H,6-7,9-10H2,1-5H3,(H,16,17,18)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357274
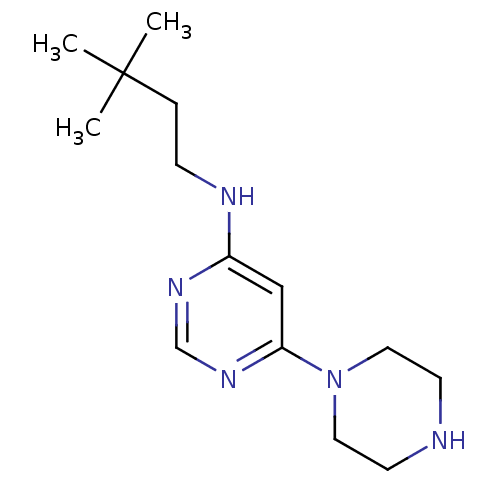
(CHEMBL1916500)Show InChI InChI=1S/C14H25N5/c1-14(2,3)4-5-16-12-10-13(18-11-17-12)19-8-6-15-7-9-19/h10-11,15H,4-9H2,1-3H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50304507
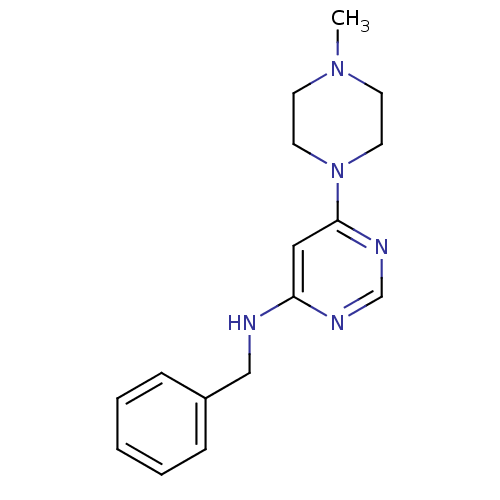
(CHEMBL596562 | N-Benzyl-6-(4-methylpiperazin-1-yl)...)Show InChI InChI=1S/C16H21N5/c1-20-7-9-21(10-8-20)16-11-15(18-13-19-16)17-12-14-5-3-2-4-6-14/h2-6,11,13H,7-10,12H2,1H3,(H,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356890
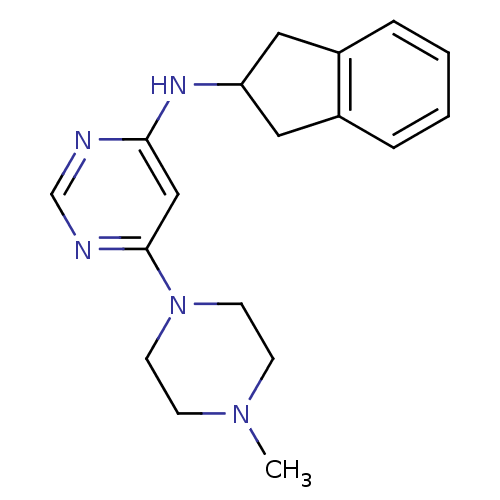
(CHEMBL1915533)Show InChI InChI=1S/C18H23N5/c1-22-6-8-23(9-7-22)18-12-17(19-13-20-18)21-16-10-14-4-2-3-5-15(14)11-16/h2-5,12-13,16H,6-11H2,1H3,(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357275
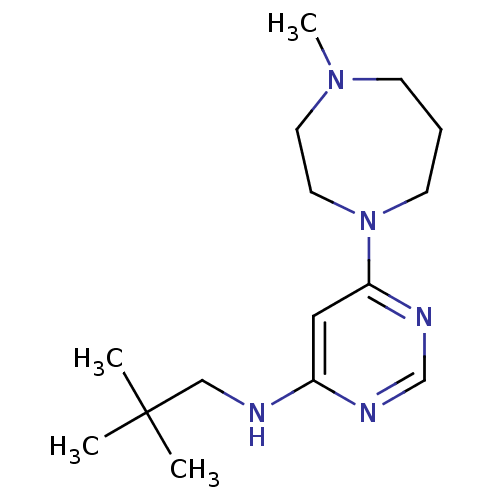
(CHEMBL1916501)Show InChI InChI=1S/C15H27N5/c1-15(2,3)11-16-13-10-14(18-12-17-13)20-7-5-6-19(4)8-9-20/h10,12H,5-9,11H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
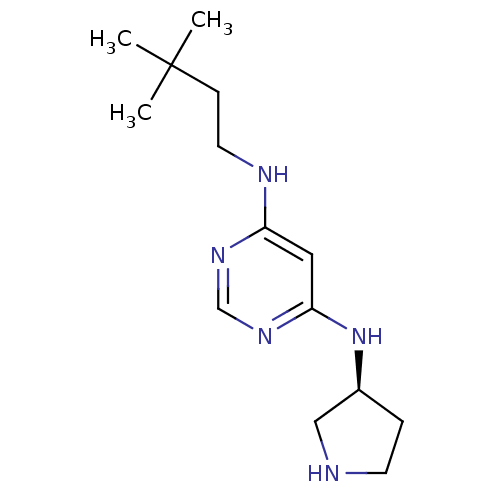
(Homo sapiens (Human)) | BDBM50356878
(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356878

(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
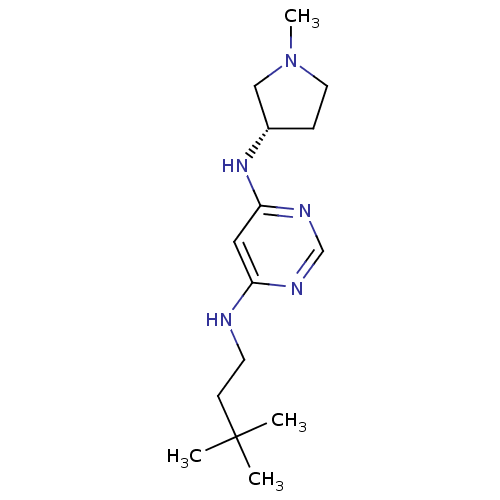
(Homo sapiens (Human)) | BDBM50357271
(CHEMBL1916497)Show InChI InChI=1S/C15H27N5/c1-15(2,3)6-7-16-13-9-14(18-11-17-13)19-12-5-8-20(4)10-12/h9,11-12H,5-8,10H2,1-4H3,(H2,16,17,18,19)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 593 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
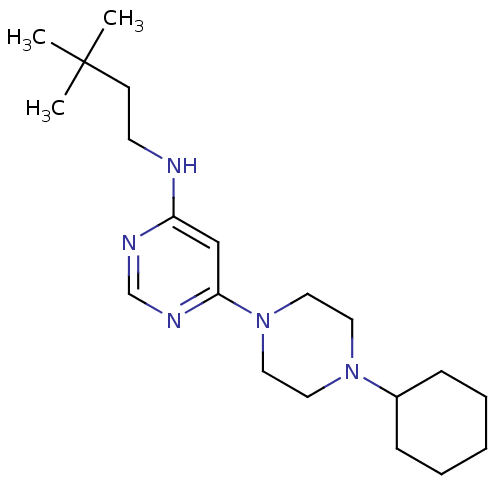
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357276
(CHEMBL1916502)Show InChI InChI=1S/C20H35N5/c1-20(2,3)9-10-21-18-15-19(23-16-22-18)25-13-11-24(12-14-25)17-7-5-4-6-8-17/h15-17H,4-14H2,1-3H3,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 601 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50357272

(CHEMBL1916498)Show InChI InChI=1S/C14H25N5/c1-14(2,3)10-15-12-9-13(17-11-16-12)19-7-5-18(4)6-8-19/h9,11H,5-8,10H2,1-4H3,(H,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 825 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357278
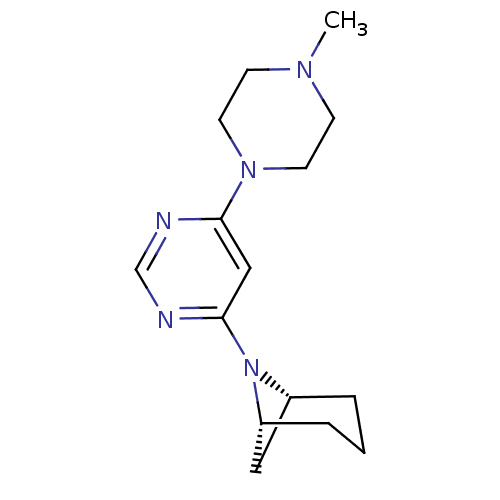
(CHEMBL1916503)Show SMILES CN1CCN(CC1)c1cc(ncn1)N1[C@H]2C[C@H]1CCC2 |r| Show InChI InChI=1S/C15H23N5/c1-18-5-7-19(8-6-18)14-10-15(17-11-16-14)20-12-3-2-4-13(20)9-12/h10-13H,2-9H2,1H3/t12-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357279
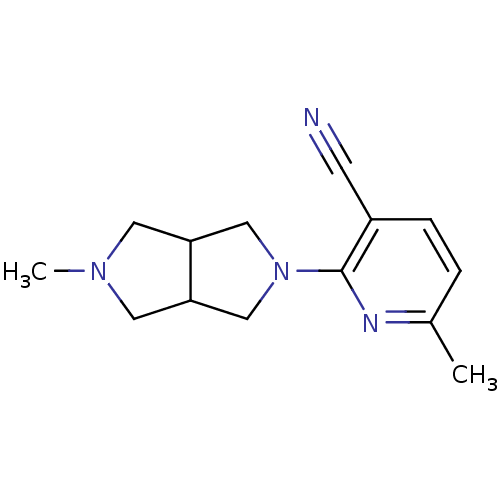
(CHEMBL1916504)Show InChI InChI=1S/C14H18N4/c1-10-3-4-11(5-15)14(16-10)18-8-12-6-17(2)7-13(12)9-18/h3-4,12-13H,6-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357268
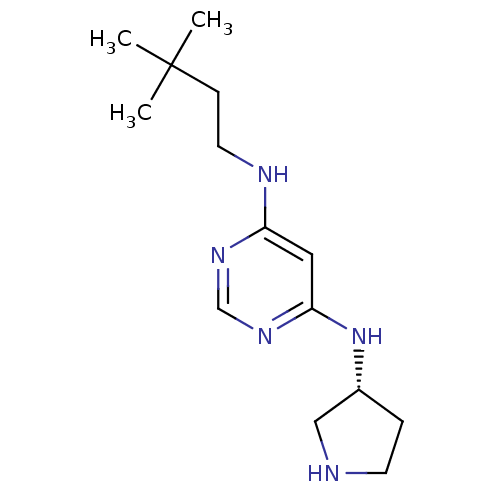
(CHEMBL1916494)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 939 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50060682
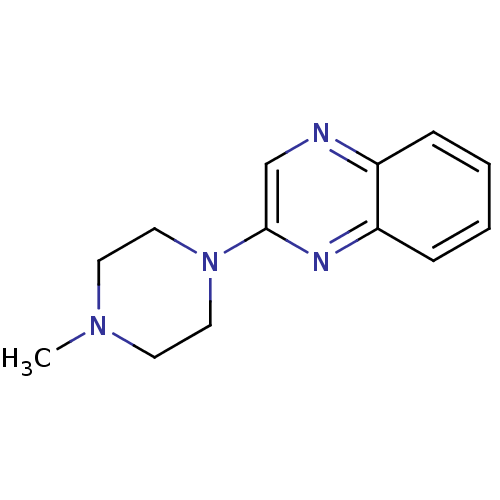
(2-(4-Methyl-piperazin-1-yl)-quinoxaline | CHEMBL12...)Show InChI InChI=1S/C13H16N4/c1-16-6-8-17(9-7-16)13-10-14-11-4-2-3-5-12(11)15-13/h2-5,10H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357267
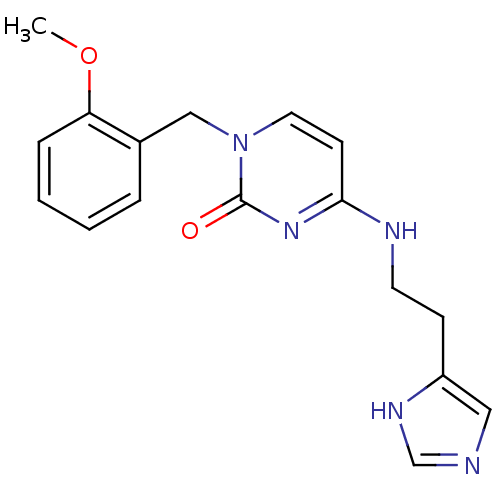
(CHEMBL1916493)Show InChI InChI=1S/C17H19N5O2/c1-24-15-5-3-2-4-13(15)11-22-9-7-16(21-17(22)23)19-8-6-14-10-18-12-20-14/h2-5,7,9-10,12H,6,8,11H2,1H3,(H,18,20)(H,19,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357266
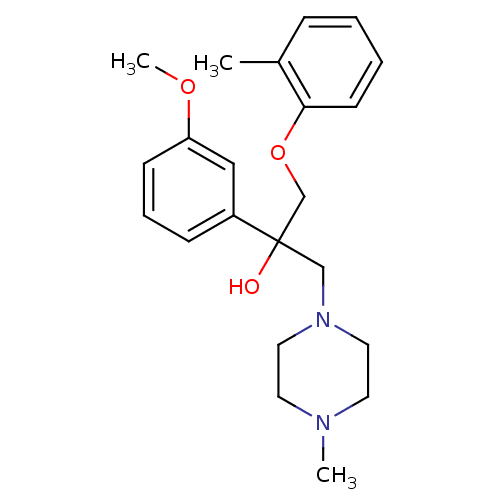
(CHEMBL1916492)Show InChI InChI=1S/C22H30N2O3/c1-18-7-4-5-10-21(18)27-17-22(25,16-24-13-11-23(2)12-14-24)19-8-6-9-20(15-19)26-3/h4-10,15,25H,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357267

(CHEMBL1916493)Show InChI InChI=1S/C17H19N5O2/c1-24-15-5-3-2-4-13(15)11-22-9-7-16(21-17(22)23)19-8-6-14-10-18-12-20-14/h2-5,7,9-10,12H,6,8,11H2,1H3,(H,18,20)(H,19,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356889
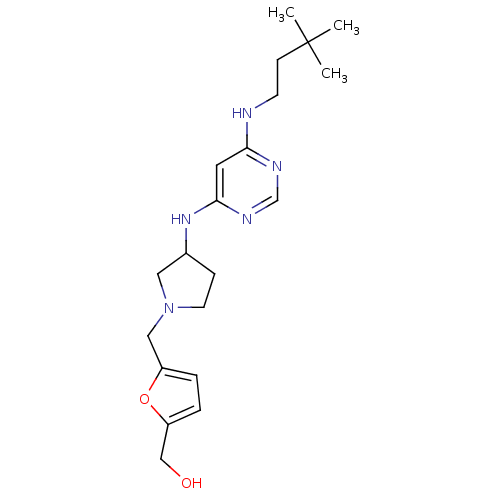
(CHEMBL1915532)Show SMILES CC(C)(C)CCNc1cc(NC2CCN(Cc3ccc(CO)o3)C2)ncn1 Show InChI InChI=1S/C20H31N5O2/c1-20(2,3)7-8-21-18-10-19(23-14-22-18)24-15-6-9-25(11-15)12-16-4-5-17(13-26)27-16/h4-5,10,14-15,26H,6-9,11-13H2,1-3H3,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50357266

(CHEMBL1916492)Show InChI InChI=1S/C22H30N2O3/c1-18-7-4-5-10-21(18)27-17-22(25,16-24-13-11-23(2)12-14-24)19-8-6-9-20(15-19)26-3/h4-10,15,25H,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50354569

(CHEMBL1836646)Show SMILES Oc1cccc(F)c1-n1nc(OC2CCNCC2)c2CCCCc12 |(36.87,1.24,;36.09,2.57,;36.85,3.91,;36.07,5.24,;34.52,5.22,;33.77,3.88,;32.23,3.86,;34.56,2.56,;33.8,1.22,;34.71,-.02,;33.8,-1.27,;34.59,-2.59,;36.13,-2.57,;36.91,-3.9,;38.44,-3.88,;39.2,-2.54,;38.41,-1.22,;36.87,-1.23,;32.33,-.8,;31,-1.58,;29.67,-.81,;29.67,.74,;31,1.51,;32.33,.75,)| Show InChI InChI=1S/C18H22FN3O2/c19-14-5-3-7-16(23)17(14)22-15-6-2-1-4-13(15)18(21-22)24-12-8-10-20-11-9-12/h3,5,7,12,20,23H,1-2,4,6,8-11H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human NET receptor |
J Med Chem 54: 6998-7002 (2011)
Article DOI: 10.1021/jm2007535
BindingDB Entry DOI: 10.7270/Q2SJ1M03 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50357266

(CHEMBL1916492)Show InChI InChI=1S/C22H30N2O3/c1-18-7-4-5-10-21(18)27-17-22(25,16-24-13-11-23(2)12-14-24)19-8-6-9-20(15-19)26-3/h4-10,15,25H,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50356879

(CHEMBL1915535)Show InChI InChI=1S/C15H27N5/c1-15(2,3)5-6-16-13-11-14(18-12-17-13)20-9-7-19(4)8-10-20/h11-12H,5-10H2,1-4H3,(H,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H2 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50356878

(CHEMBL1915534)Show InChI InChI=1S/C14H25N5/c1-14(2,3)5-7-16-12-8-13(18-10-17-12)19-11-4-6-15-9-11/h8,10-11,15H,4-7,9H2,1-3H3,(H2,16,17,18,19)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H3 receptor |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data