

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50447108 (CHEMBL3112881) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013677 (CHEMBL3264512) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013673 (CHEMBL3264509) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013676 (CHEMBL3264511) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013678 (CHEMBL3264513) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013674 (CHEMBL3264510) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384950 (CHEMBL2037386) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013672 (CHEMBL3264508) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013675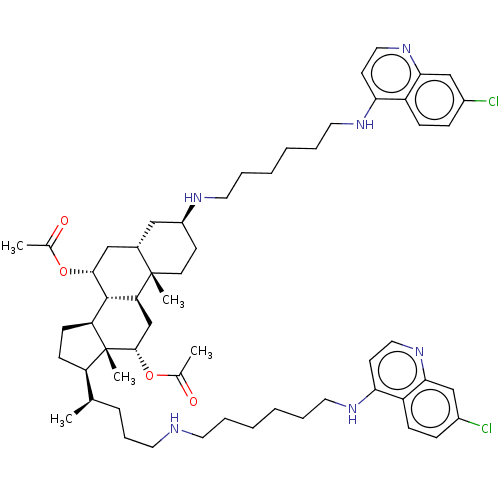 (CHEMBL3259867) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013658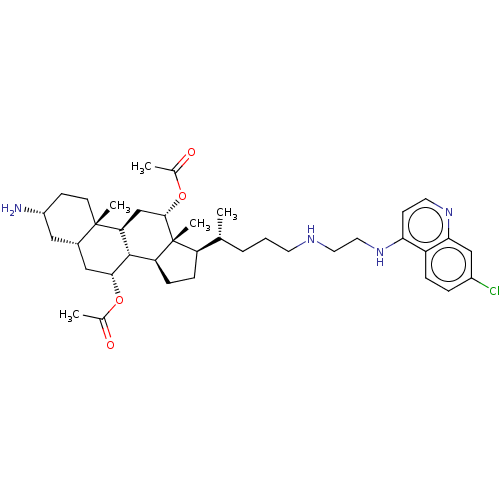 (CHEMBL3264499) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013659 (CHEMBL3264500) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013668 (CHEMBL3264171) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013667 (CHEMBL450398) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013645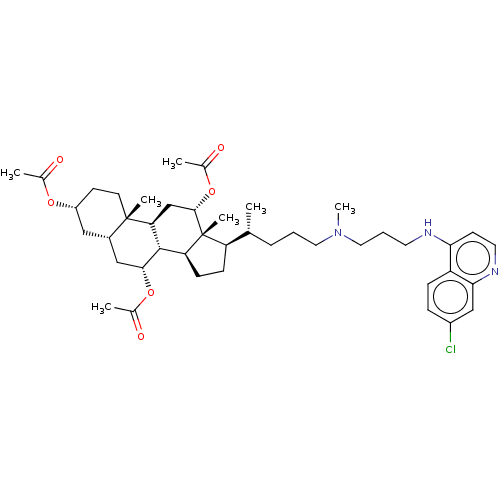 (CHEMBL3264183) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50343187 (CHEMBL1773155 | N,N'-Bis(3-aminopropyl)-3,9-dimeth...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uM | J Med Chem 54: 1157-69 (2011) Article DOI: 10.1021/jm100938u BindingDB Entry DOI: 10.7270/Q26T0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50343188 (CHEMBL1773156 | N,N'-Bis(2-aminoethyl)-3,9-dimethy...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uM | J Med Chem 54: 1157-69 (2011) Article DOI: 10.1021/jm100938u BindingDB Entry DOI: 10.7270/Q26T0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50367328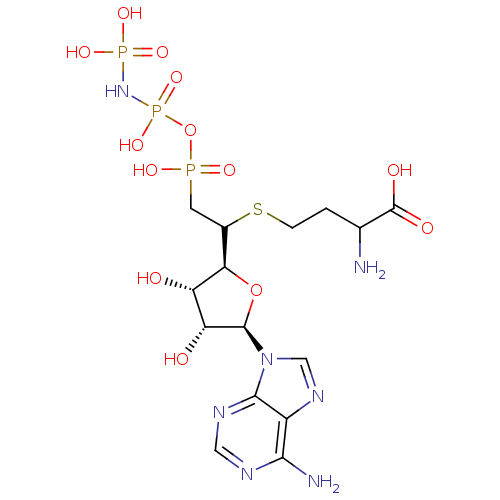 (CHEMBL1791415) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated on novikoff hepatoma (MAT-T) form of rat methionine adenosyltransferase, when ATP was the variable substrate (60 uM... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367328 (CHEMBL1791415) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50367328 (CHEMBL1791415) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney (MAT-2) form of rat methionine adenosyltransferase, when methionine was the variable substrate (2 mM) | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50367329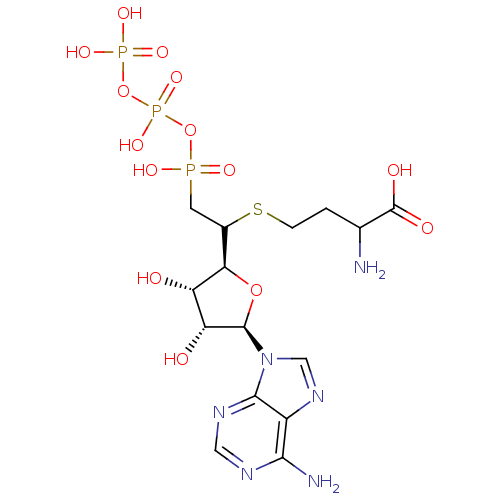 (CHEMBL1791416) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated on novikoff hepatoma (MAT-T) form of rat methionine adenosyltransferase, when ATP was the variable substrate (60 uM... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50367329 (CHEMBL1791416) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated on novikoff hepatoma (MAT-T) form of rat methionine adenosyltransferase, when ATP was the variable substrate (60 uM... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013644 (CHEMBL3264182) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50452293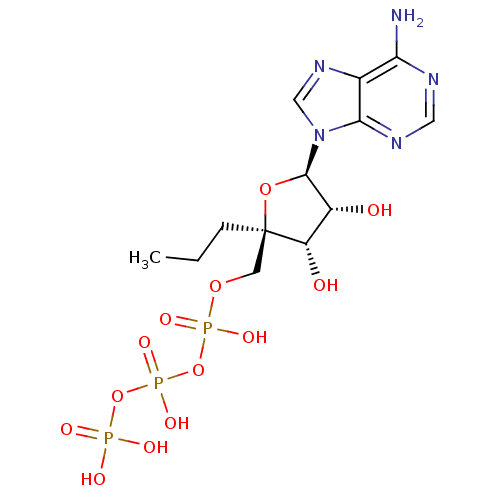 (CHEMBL2092766) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated on novikoff hepatoma (MAT-T) form of rat methionine adenosyltransferase, when ATP was the variable substrate (60 uM... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50452293 (CHEMBL2092766) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated on novikoff hepatoma (MAT-T) form of rat methionine adenosyltransferase, when ATP was the variable substrate (60 uM... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50367328 (CHEMBL1791415) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367329 (CHEMBL1791416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367329 (CHEMBL1791416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50452293 (CHEMBL2092766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50452293 (CHEMBL2092766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50367329 (CHEMBL1791416) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney (MAT-2) form of rat methionine adenosyltransferase, when methionine was the variable substrate (2 mM) | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50367329 (CHEMBL1791416) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney (MAT-2) form of rat methionine adenosyltransferase, when methionine was the variable substrate (2 mM) | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1/type-2 (Rattus norvegicus) | BDBM50367331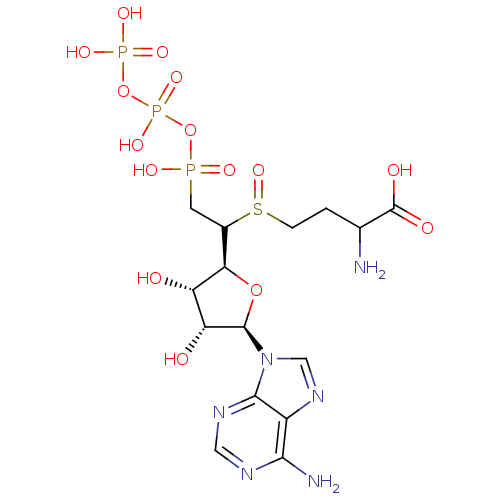 (CHEMBL1791417) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated on novikoff hepatoma (MAT-T) form of rat methionine adenosyltransferase, when ATP was the variable substrate (60 uM... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-2 (Rattus norvegicus) | BDBM50367331 (CHEMBL1791417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated with kidney Methionine adenosyltransferase II form of rat methionine adenosyltransferase, when ATP was the variable... | J Med Chem 29: 1030-8 (1986) BindingDB Entry DOI: 10.7270/Q2CF9QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144555 (US8969568, 108) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144516 (US8969568, 69) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144510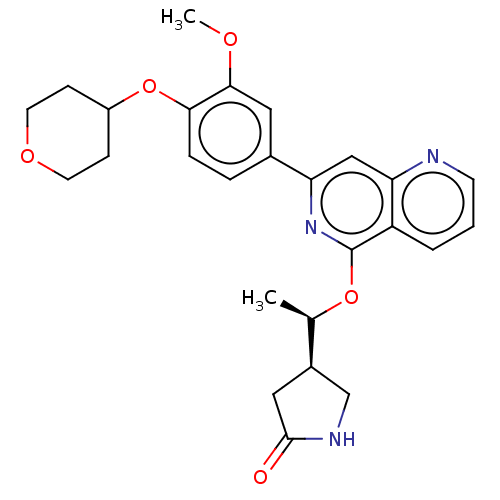 (US8969568, 63) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144512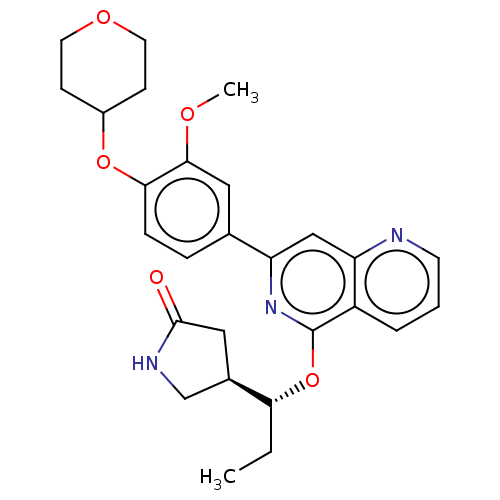 (US8969568, 65) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144541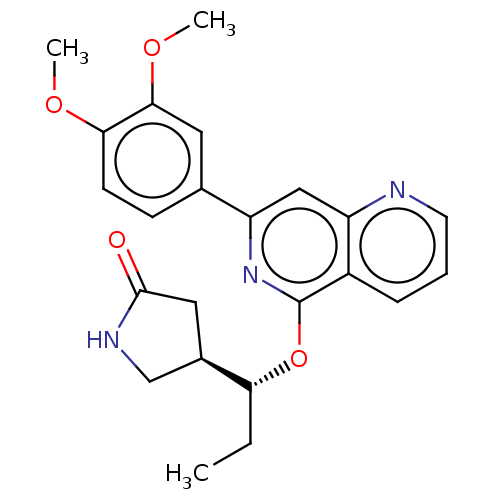 (US8969568, 94) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144532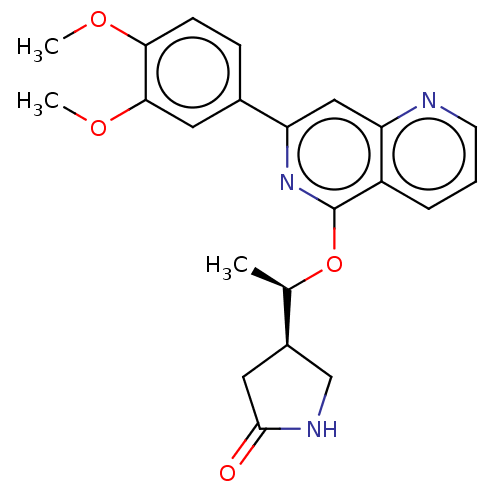 (US8969568, 85) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144560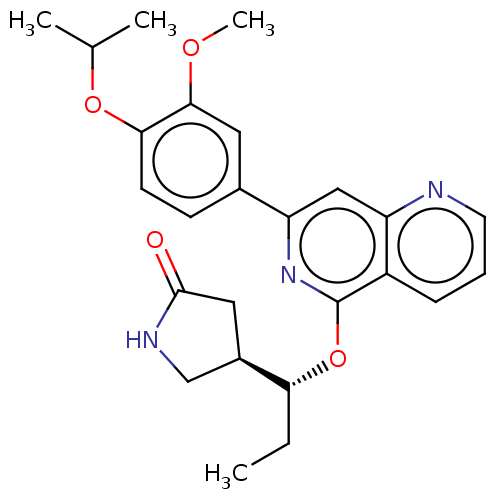 (US8969568, 113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144559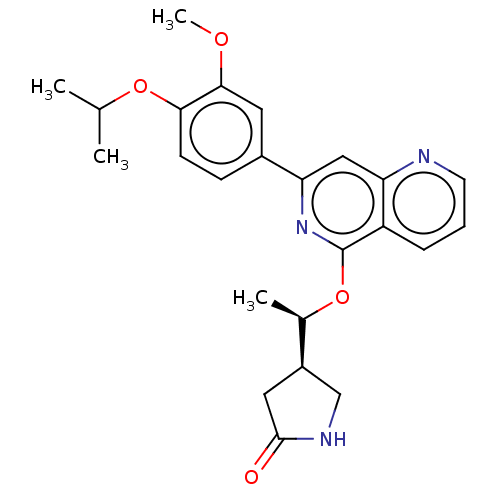 (US8969568, 112) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144493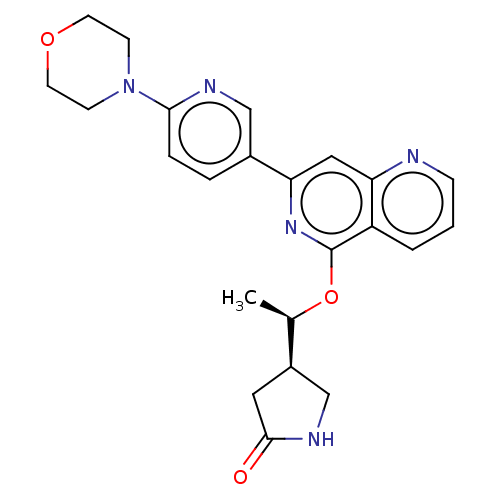 (US8969568, 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144529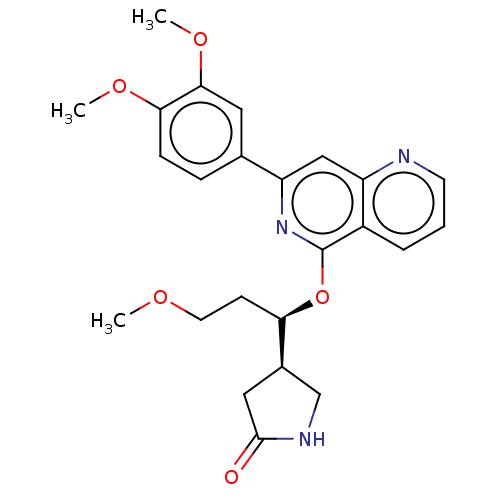 (US8969568, 82) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144518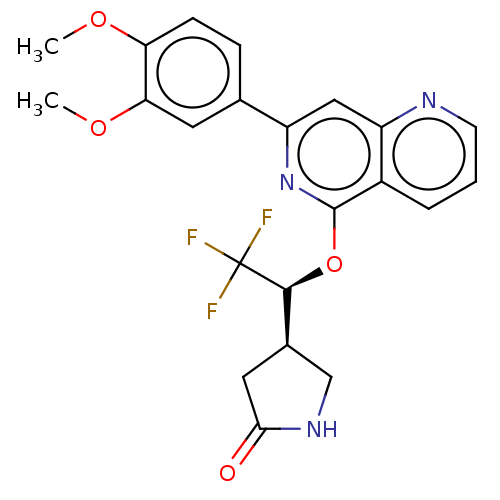 (US8969568, 71) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144483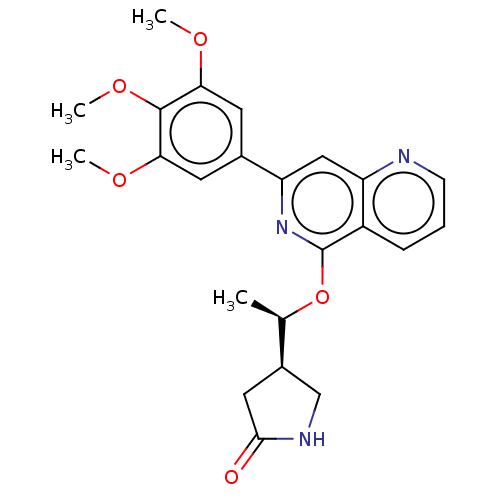 (US8969568, 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144573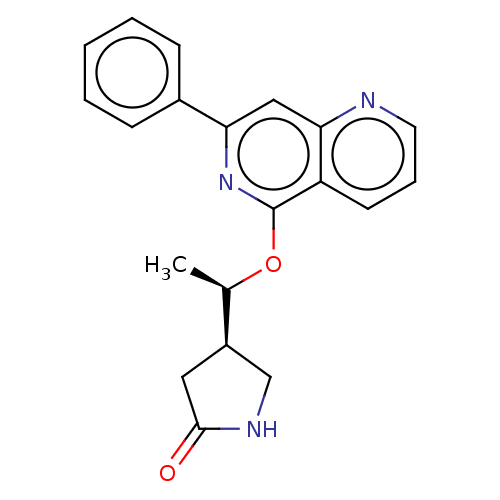 (US8969568, 126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144539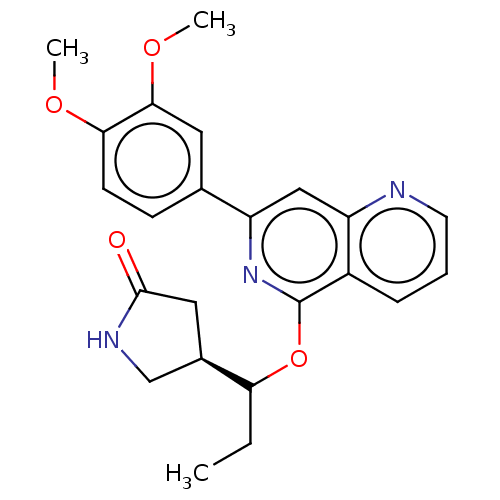 (US8969568, 92) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144519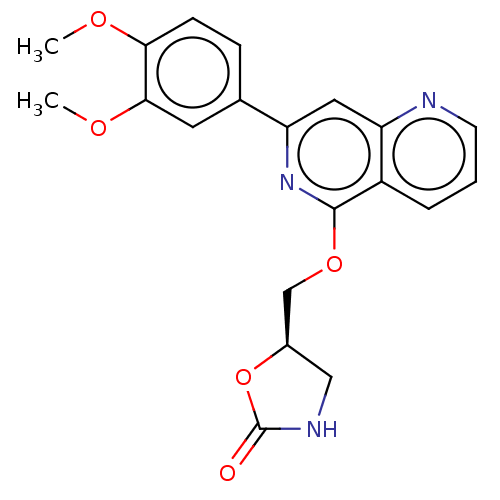 (US8969568, 72) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM144489 (US8969568, 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG US Patent | Assay Description Recombinant human Syk (amino acids 342-635) was expressed as a fusion protein with an N-terminal GST tag, affinity-purified and deep-frozen at a conc... | US Patent US8969568 (2015) BindingDB Entry DOI: 10.7270/Q2154FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 446 total ) | Next | Last >> |