 Found 21 hits with Last Name = 'pélaprat' and Initial = 'd'
Found 21 hits with Last Name = 'pélaprat' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016504

((S)-3-(2-{[(S)-2-(2-{2-[2-tert-Butoxycarbonylamino...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS([O-])(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/p-1/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig brain |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to CCK-8 receptor of guinea pig brain |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM50033676
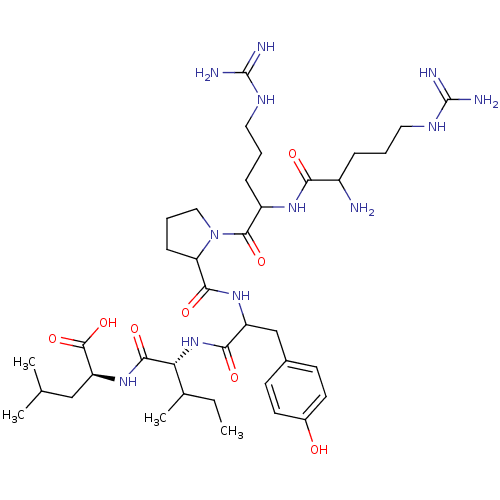
((S)-2-{(R)-2-[2-({1-[2-(2-Amino-5-guanidino-pentan...)Show SMILES CCC(C)[C@@H](NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CCCNC(N)=N)NC(=O)C(N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t22?,25?,26?,27?,28-,29?,30+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig brain |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016504

((S)-3-(2-{[(S)-2-(2-{2-[2-tert-Butoxycarbonylamino...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS([O-])(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/p-1/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig pancreatic membrane |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM50248034
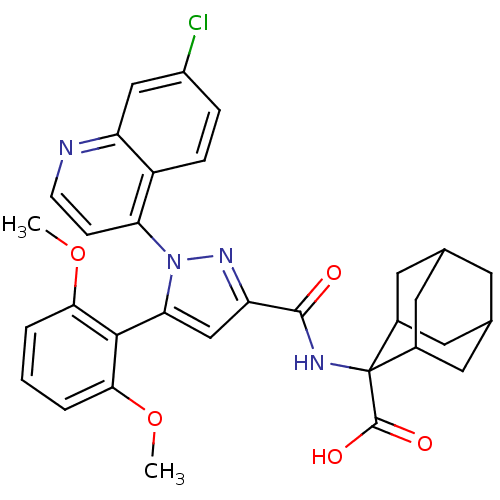
(2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:38:37:35:31.32.33,39:29:35:31.32.33,28:29:31.38.32:36.34.35,THB:38:32:29.37.36:35,39:29:31.38.32:36.34.35,28:29:35:31.32.33,33:32:29:36.34.35,33:34:29:31.38.32,(-7.63,.4,;-6.3,-.36,;-6.29,-1.9,;-7.62,-2.68,;-7.62,-4.22,;-6.29,-4.99,;-4.95,-4.22,;-3.61,-4.99,;-3.61,-6.53,;-4.95,-2.67,;-3.63,-1.9,;-2.22,-2.52,;-1.19,-1.37,;-1.97,-.04,;-3.47,-.36,;-4.62,.66,;-6.08,.17,;-7.22,1.19,;-6.91,2.71,;-5.45,3.19,;-5.14,4.69,;-3.68,5.16,;-3.37,6.67,;-2.53,4.14,;-2.85,2.64,;-4.31,2.16,;.35,-1.53,;.98,-2.93,;1.24,-.27,;2.78,-.43,;3.43,1.2,;4.84,1.23,;6.02,2.07,;5.46,3.5,;3.95,3.52,;2.87,2.58,;3.44,1.99,;4.02,.52,;5.54,.51,;3.42,-1.84,;2.51,-3.09,;4.95,-2,)| Show InChI InChI=1S/C32H31ClN4O5/c1-41-27-4-3-5-28(42-2)29(27)26-16-24(36-37(26)25-8-9-34-23-15-21(33)6-7-22(23)25)30(38)35-32(31(39)40)19-11-17-10-18(13-19)14-20(32)12-17/h3-9,15-20H,10-14H2,1-2H3,(H,35,38)(H,39,40) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016506
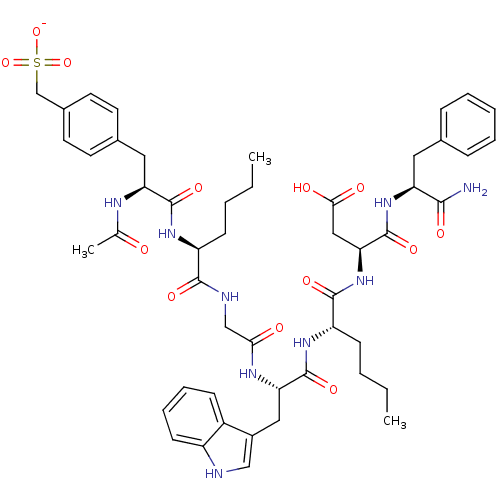
(CHEMBL262687 | Sodium; (4-{2-(2-acetylamino-3-carb...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(CS([O-])(=O)=O)cc1)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H65N9O13S/c1-4-6-16-37(56-48(67)40(54-30(3)60)24-32-19-21-33(22-20-32)29-73(70,71)72)46(65)53-28-43(61)55-41(25-34-27-52-36-18-12-11-15-35(34)36)49(68)57-38(17-7-5-2)47(66)59-42(26-44(62)63)50(69)58-39(45(51)64)23-31-13-9-8-10-14-31/h8-15,18-22,27,37-42,52H,4-7,16-17,23-26,28-29H2,1-3H3,(H2,51,64)(H,53,65)(H,54,60)(H,55,61)(H,56,67)(H,57,68)(H,58,69)(H,59,66)(H,62,63)(H,70,71,72)/p-1/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig pancreatic membrane |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM82417
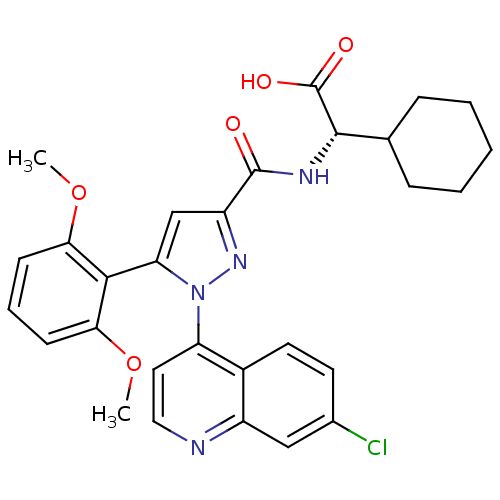
(CHEMBL461604 | SR 48527)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)N[C@@H](C1CCCCC1)C(O)=O |wD:29.32,(4.51,5.44,;5.54,4.29,;5.06,2.83,;3.56,2.51,;3.08,1.04,;4.11,-.1,;5.62,.22,;6.65,-.93,;6.17,-2.39,;6.09,1.68,;7.6,2,;8.08,.54,;9.62,.54,;10.09,2,;8.85,2.91,;8.85,4.45,;7.51,5.22,;7.51,6.76,;8.85,7.53,;10.18,6.76,;11.56,7.58,;12.95,6.79,;14.28,7.57,;12.95,5.19,;11.56,4.39,;10.18,5.22,;10.52,-.71,;12.05,-.55,;9.89,-2.11,;10.8,-3.36,;10.17,-4.77,;8.64,-4.93,;8.01,-6.34,;8.92,-7.58,;10.45,-7.42,;11.08,-6.01,;12.33,-3.2,;13.24,-4.45,;12.96,-1.79,)| Show InChI InChI=1S/C29H29ClN4O5/c1-38-24-9-6-10-25(39-2)26(24)23-16-21(28(35)32-27(29(36)37)17-7-4-3-5-8-17)33-34(23)22-13-14-31-20-15-18(30)11-12-19(20)22/h6,9-17,27H,3-5,7-8H2,1-2H3,(H,32,35)(H,36,37)/t27-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM50248034

(2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:38:37:35:31.32.33,39:29:35:31.32.33,28:29:31.38.32:36.34.35,THB:38:32:29.37.36:35,39:29:31.38.32:36.34.35,28:29:35:31.32.33,33:32:29:36.34.35,33:34:29:31.38.32,(-7.63,.4,;-6.3,-.36,;-6.29,-1.9,;-7.62,-2.68,;-7.62,-4.22,;-6.29,-4.99,;-4.95,-4.22,;-3.61,-4.99,;-3.61,-6.53,;-4.95,-2.67,;-3.63,-1.9,;-2.22,-2.52,;-1.19,-1.37,;-1.97,-.04,;-3.47,-.36,;-4.62,.66,;-6.08,.17,;-7.22,1.19,;-6.91,2.71,;-5.45,3.19,;-5.14,4.69,;-3.68,5.16,;-3.37,6.67,;-2.53,4.14,;-2.85,2.64,;-4.31,2.16,;.35,-1.53,;.98,-2.93,;1.24,-.27,;2.78,-.43,;3.43,1.2,;4.84,1.23,;6.02,2.07,;5.46,3.5,;3.95,3.52,;2.87,2.58,;3.44,1.99,;4.02,.52,;5.54,.51,;3.42,-1.84,;2.51,-3.09,;4.95,-2,)| Show InChI InChI=1S/C32H31ClN4O5/c1-41-27-4-3-5-28(42-2)29(27)26-16-24(36-37(26)25-8-9-34-23-15-21(33)6-7-22(23)25)30(38)35-32(31(39)40)19-11-17-10-18(13-19)14-20(32)12-17/h3-9,15-20H,10-14H2,1-2H3,(H,35,38)(H,39,40) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM50130880
(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016503

(Boc-Asp-D-Tyr(SO3Na)-Nle-Gly-Trp-Nle-Asp-Phe-NH2 |...)Show SMILES CCCC[C@H](NC(=O)[C@@H](Cc1ccc(OS([O-])(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/p-1/t37-,38-,39-,40+,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig brain |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016506

(CHEMBL262687 | Sodium; (4-{2-(2-acetylamino-3-carb...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(CS([O-])(=O)=O)cc1)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H65N9O13S/c1-4-6-16-37(56-48(67)40(54-30(3)60)24-32-19-21-33(22-20-32)29-73(70,71)72)46(65)53-28-43(61)55-41(25-34-27-52-36-18-12-11-15-35(34)36)49(68)57-38(17-7-5-2)47(66)59-42(26-44(62)63)50(69)58-39(45(51)64)23-31-13-9-8-10-14-31/h8-15,18-22,27,37-42,52H,4-7,16-17,23-26,28-29H2,1-3H3,(H2,51,64)(H,53,65)(H,54,60)(H,55,61)(H,56,67)(H,57,68)(H,58,69)(H,59,66)(H,62,63)(H,70,71,72)/p-1/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to CCK-8 receptor of guinea pig pancreatic membrane |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM82417

(CHEMBL461604 | SR 48527)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)N[C@@H](C1CCCCC1)C(O)=O |wD:29.32,(4.51,5.44,;5.54,4.29,;5.06,2.83,;3.56,2.51,;3.08,1.04,;4.11,-.1,;5.62,.22,;6.65,-.93,;6.17,-2.39,;6.09,1.68,;7.6,2,;8.08,.54,;9.62,.54,;10.09,2,;8.85,2.91,;8.85,4.45,;7.51,5.22,;7.51,6.76,;8.85,7.53,;10.18,6.76,;11.56,7.58,;12.95,6.79,;14.28,7.57,;12.95,5.19,;11.56,4.39,;10.18,5.22,;10.52,-.71,;12.05,-.55,;9.89,-2.11,;10.8,-3.36,;10.17,-4.77,;8.64,-4.93,;8.01,-6.34,;8.92,-7.58,;10.45,-7.42,;11.08,-6.01,;12.33,-3.2,;13.24,-4.45,;12.96,-1.79,)| Show InChI InChI=1S/C29H29ClN4O5/c1-38-24-9-6-10-25(39-2)26(24)23-16-21(28(35)32-27(29(36)37)17-7-4-3-5-8-17)33-34(23)22-13-14-31-20-15-18(30)11-12-19(20)22/h6,9-17,27H,3-5,7-8H2,1-2H3,(H,32,35)(H,36,37)/t27-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016505
(CHEMBL217767 | Sodium; (4-{2-(2-acetylamino-3-carb...)Show SMILES CCCC[C@H](NC(=O)[C@@H](Cc1ccc(CS([O-])(=O)=O)cc1)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H65N9O13S/c1-4-6-16-37(56-48(67)40(54-30(3)60)24-32-19-21-33(22-20-32)29-73(70,71)72)46(65)53-28-43(61)55-41(25-34-27-52-36-18-12-11-15-35(34)36)49(68)57-38(17-7-5-2)47(66)59-42(26-44(62)63)50(69)58-39(45(51)64)23-31-13-9-8-10-14-31/h8-15,18-22,27,37-42,52H,4-7,16-17,23-26,28-29H2,1-3H3,(H2,51,64)(H,53,65)(H,54,60)(H,55,61)(H,56,67)(H,57,68)(H,58,69)(H,59,66)(H,62,63)(H,70,71,72)/p-1/t37-,38-,39-,40+,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig pancreatic membrane |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM50033676

((S)-2-{(R)-2-[2-({1-[2-(2-Amino-5-guanidino-pentan...)Show SMILES CCC(C)[C@@H](NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CCCNC(N)=N)NC(=O)C(N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t22?,25?,26?,27?,28-,29?,30+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016505

(CHEMBL217767 | Sodium; (4-{2-(2-acetylamino-3-carb...)Show SMILES CCCC[C@H](NC(=O)[C@@H](Cc1ccc(CS([O-])(=O)=O)cc1)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H65N9O13S/c1-4-6-16-37(56-48(67)40(54-30(3)60)24-32-19-21-33(22-20-32)29-73(70,71)72)46(65)53-28-43(61)55-41(25-34-27-52-36-18-12-11-15-35(34)36)49(68)57-38(17-7-5-2)47(66)59-42(26-44(62)63)50(69)58-39(45(51)64)23-31-13-9-8-10-14-31/h8-15,18-22,27,37-42,52H,4-7,16-17,23-26,28-29H2,1-3H3,(H2,51,64)(H,53,65)(H,54,60)(H,55,61)(H,56,67)(H,57,68)(H,58,69)(H,59,66)(H,62,63)(H,70,71,72)/p-1/t37-,38-,39-,40+,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig brain |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016503

(Boc-Asp-D-Tyr(SO3Na)-Nle-Gly-Trp-Nle-Asp-Phe-NH2 |...)Show SMILES CCCC[C@H](NC(=O)[C@@H](Cc1ccc(OS([O-])(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/p-1/t37-,38-,39-,40+,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig pancreatic membrane |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM82418

(CAS_3025353 | NSC_3025353 | Neuromedin N)Show SMILES CCC(C)C(N)C(=O)C(C)(CC(=O)C(N)CCCCN)C(C(=O)C(N)Cc1ccc(O)cc1)(C(=O)C1CCCN1)C(N)(C(O)=O)C(=O)C(N)C(C)CC Show InChI InChI=1S/C38H63N7O8/c1-6-21(3)29(42)33(50)36(5,20-28(47)25(40)11-8-9-17-39)37(32(49)27-12-10-18-45-27,31(48)26(41)19-23-13-15-24(46)16-14-23)38(44,35(52)53)34(51)30(43)22(4)7-2/h13-16,21-22,25-27,29-30,45-46H,6-12,17-20,39-44H2,1-5H3,(H,52,53) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM82419

(SR 49711)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)N[C@H](C1CCCCC1)C(O)=O |wU:29.32,(4.51,5.44,;5.54,4.29,;5.06,2.83,;3.56,2.51,;3.08,1.04,;4.11,-.1,;5.62,.22,;6.65,-.93,;6.17,-2.39,;6.09,1.68,;7.6,2,;8.08,.54,;9.62,.54,;10.09,2,;8.85,2.91,;8.85,4.45,;7.51,5.22,;7.51,6.76,;8.85,7.53,;10.18,6.76,;11.56,7.58,;12.95,6.79,;14.28,7.57,;12.95,5.19,;11.56,4.39,;10.18,5.22,;10.52,-.71,;12.05,-.55,;9.89,-2.11,;10.8,-3.36,;10.17,-4.77,;8.64,-4.93,;8.01,-6.34,;8.92,-7.58,;10.45,-7.42,;11.08,-6.01,;12.33,-3.2,;13.24,-4.45,;12.96,-1.79,)| Show InChI InChI=1S/C29H29ClN4O5/c1-38-24-9-6-10-25(39-2)26(24)23-16-21(28(35)32-27(29(36)37)17-7-4-3-5-8-17)33-34(23)22-13-14-31-20-15-18(30)11-12-19(20)22/h6,9-17,27H,3-5,7-8H2,1-2H3,(H,32,35)(H,36,37)/t27-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM82419

(SR 49711)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)N[C@H](C1CCCCC1)C(O)=O |wU:29.32,(4.51,5.44,;5.54,4.29,;5.06,2.83,;3.56,2.51,;3.08,1.04,;4.11,-.1,;5.62,.22,;6.65,-.93,;6.17,-2.39,;6.09,1.68,;7.6,2,;8.08,.54,;9.62,.54,;10.09,2,;8.85,2.91,;8.85,4.45,;7.51,5.22,;7.51,6.76,;8.85,7.53,;10.18,6.76,;11.56,7.58,;12.95,6.79,;14.28,7.57,;12.95,5.19,;11.56,4.39,;10.18,5.22,;10.52,-.71,;12.05,-.55,;9.89,-2.11,;10.8,-3.36,;10.17,-4.77,;8.64,-4.93,;8.01,-6.34,;8.92,-7.58,;10.45,-7.42,;11.08,-6.01,;12.33,-3.2,;13.24,-4.45,;12.96,-1.79,)| Show InChI InChI=1S/C29H29ClN4O5/c1-38-24-9-6-10-25(39-2)26(24)23-16-21(28(35)32-27(29(36)37)17-7-4-3-5-8-17)33-34(23)22-13-14-31-20-15-18(30)11-12-19(20)22/h6,9-17,27H,3-5,7-8H2,1-2H3,(H,32,35)(H,36,37)/t27-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hôpital Saint-Antoine
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1450-8 (1995)
BindingDB Entry DOI: 10.7270/Q2GB22J5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data