 Found 293 hits with Last Name = 'pacquet' and Initial = 'f'
Found 293 hits with Last Name = 'pacquet' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433404

(CHEMBL2380641)Show SMILES CS(=O)(=O)N1CCN(CC1)c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(2.53,-27.78,;3.31,-29.1,;3.3,-30.64,;1.97,-29.87,;4.85,-29.11,;5.62,-30.43,;7.15,-30.43,;7.92,-29.1,;7.15,-27.77,;5.61,-27.78,;9.45,-29.1,;10.22,-30.44,;11.76,-30.44,;12.53,-29.1,;11.75,-27.76,;10.22,-27.77,;14.07,-29.1,;14.84,-30.44,;16.37,-30.44,;17.15,-29.11,;18.69,-29.12,;19.46,-30.45,;19.61,-27.88,;21.15,-27.91,;22.34,-26.63,;22.33,-25.15,;23.68,-24.67,;22.64,-25.9,;22.65,-27.49,;24.05,-28.05,;25.07,-26.78,;26.61,-26.78,;25.08,-25.25,;23.67,-27.12,;16.38,-27.78,;17.16,-26.45,;16.4,-25.1,;14.84,-25.1,;14.07,-26.43,;14.84,-27.77,)| Show InChI InChI=1S/C28H37N7O4S/c1-40(38,39)33-8-6-32(7-9-33)22-17-29-26(30-18-22)34-10-11-35(24-5-3-2-4-23(24)34)27(36)31-25-20-12-19-13-21(25)16-28(37,14-19)15-20/h2-5,17-21,25,37H,6-16H2,1H3,(H,31,36)/t19?,20?,21?,25-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433406
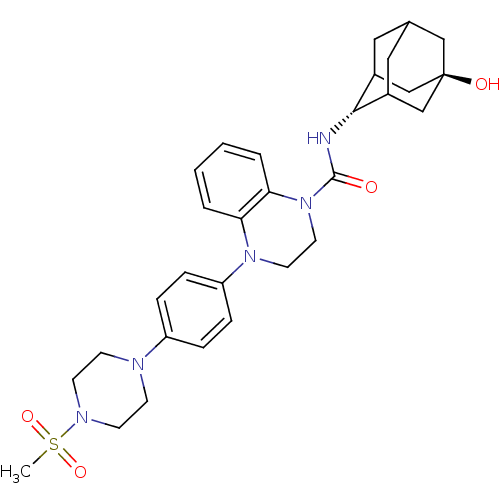
(CHEMBL2380643)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(1.07,-35.62,;1.84,-36.95,;1.84,-38.49,;.51,-37.71,;3.39,-36.95,;4.15,-38.27,;5.69,-38.27,;6.46,-36.95,;5.69,-35.62,;4.15,-35.62,;7.99,-36.94,;8.76,-38.28,;10.3,-38.28,;11.07,-36.95,;10.29,-35.61,;8.76,-35.61,;12.61,-36.94,;13.38,-38.28,;14.91,-38.29,;15.69,-36.95,;17.23,-36.96,;18,-38.29,;18.15,-35.73,;19.69,-35.75,;20.88,-34.48,;20.87,-32.99,;22.22,-32.51,;21.18,-33.75,;21.19,-35.33,;22.59,-35.9,;23.61,-34.62,;25.15,-34.62,;23.62,-33.09,;22.21,-34.97,;14.92,-35.62,;15.7,-34.29,;14.94,-32.95,;13.38,-32.94,;12.61,-34.28,;13.37,-35.61,)| Show InChI InChI=1S/C30H39N5O4S/c1-40(38,39)33-12-10-32(11-13-33)24-6-8-25(9-7-24)34-14-15-35(27-5-3-2-4-26(27)34)29(36)31-28-22-16-21-17-23(28)20-30(37,18-21)19-22/h2-9,21-23,28,37H,10-20H2,1H3,(H,31,36)/t21?,22?,23?,28-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411047
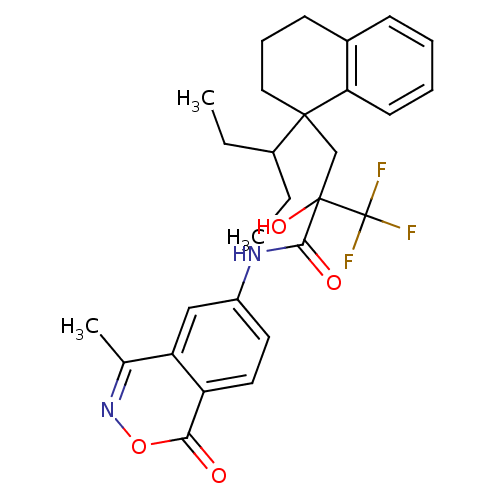
(CHEMBL211441)Show SMILES CCC(CC)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C28H31F3N2O4/c1-4-19(5-2)26(14-8-10-18-9-6-7-11-23(18)26)16-27(36,28(29,30)31)25(35)32-20-12-13-21-22(15-20)17(3)33-37-24(21)34/h6-7,9,11-13,15,19,36H,4-5,8,10,14,16H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411024

(CHEMBL208996)Show SMILES Cc1noc(=O)c2ccc(NC(=O)[C@](O)(C[C@]3(CCCc4ccccc34)C3CCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C28H29F3N2O4/c1-17-22-15-20(12-13-21(22)24(34)37-33-17)32-25(35)27(36,28(29,30)31)16-26(19-9-3-4-10-19)14-6-8-18-7-2-5-11-23(18)26/h2,5,7,11-13,15,19,36H,3-4,6,8-10,14,16H2,1H3,(H,32,35)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433405

(CHEMBL2380642)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(25.74,-29.77,;26.51,-31.1,;26.5,-32.64,;25.17,-31.86,;28.05,-31.1,;28.82,-32.42,;30.36,-32.43,;31.12,-31.1,;30.35,-29.77,;28.81,-29.77,;32.66,-31.09,;33.42,-32.43,;34.96,-32.43,;35.73,-31.1,;34.96,-29.76,;33.42,-29.76,;37.28,-31.1,;38.05,-32.43,;39.58,-32.44,;40.35,-31.11,;41.89,-31.11,;42.66,-32.45,;42.82,-29.88,;44.35,-29.9,;45.55,-28.63,;45.54,-27.14,;46.89,-26.66,;45.85,-27.9,;45.85,-29.48,;47.26,-30.05,;48.27,-28.77,;49.81,-28.77,;48.28,-27.24,;46.88,-29.12,;39.59,-29.77,;40.37,-28.44,;39.6,-27.1,;38.05,-27.09,;37.28,-28.43,;38.04,-29.76,)| Show InChI InChI=1S/C29H38N6O4S/c1-40(38,39)33-10-8-32(9-11-33)23-6-7-26(30-19-23)34-12-13-35(25-5-3-2-4-24(25)34)28(36)31-27-21-14-20-15-22(27)18-29(37,16-20)17-21/h2-7,19-22,27,37H,8-18H2,1H3,(H,31,36)/t20?,21?,22?,27-,29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411026
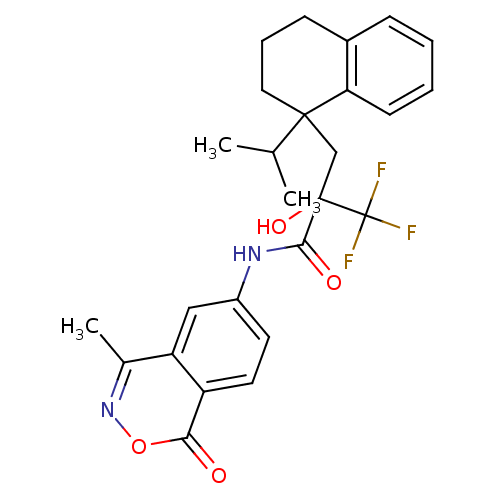
(CHEMBL214336)Show SMILES CC(C)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-15(2)24(12-6-8-17-7-4-5-9-21(17)24)14-25(34,26(27,28)29)23(33)30-18-10-11-19-20(13-18)16(3)31-35-22(19)32/h4-5,7,9-11,13,15,34H,6,8,12,14H2,1-3H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50499970
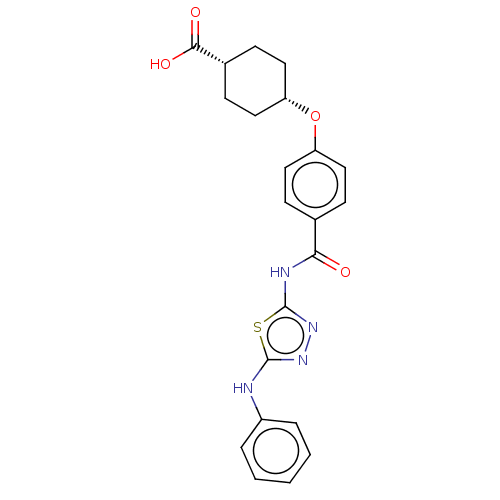
(CHEMBL3741505)Show SMILES OC(=O)[C@@H]1CC[C@@H](CC1)Oc1ccc(cc1)C(=O)Nc1nnc(Nc2ccccc2)s1 |r,wU:6.9,3.2,(-9.07,-.95,;-8,-1.56,;-7.99,-2.79,;-6.67,-.78,;-5.33,-1.55,;-4,-.77,;-4,.77,;-5.34,1.53,;-6.67,.76,;-2.67,1.54,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;2.67,-1.54,;3.73,-.93,;2.66,-3.08,;4,-3.86,;4.14,-5.38,;5.64,-5.7,;6.41,-4.36,;7.94,-4.2,;8.57,-2.79,;10.1,-2.63,;10.72,-1.22,;9.81,.03,;8.28,-.14,;7.66,-1.55,;5.38,-3.22,)| Show InChI InChI=1S/C22H22N4O4S/c27-19(24-22-26-25-21(31-22)23-16-4-2-1-3-5-16)14-6-10-17(11-7-14)30-18-12-8-15(9-13-18)20(28)29/h1-7,10-11,15,18H,8-9,12-13H2,(H,23,25)(H,28,29)(H,24,26,27)/t15-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of DGAT-1 in human Chang cells assessed as lipid level after 6 hrs in presence of substrate [14C]glycerol by HPLC analysis |
Bioorg Med Chem Lett 26: 25-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.046
BindingDB Entry DOI: 10.7270/Q2Z89GDN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411039

(CHEMBL385450)Show SMILES CCCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C27H29F3N2O4/c1-3-4-13-25(14-7-9-18-8-5-6-10-22(18)25)16-26(35,27(28,29)30)24(34)31-19-11-12-20-21(15-19)17(2)32-36-23(20)33/h5-6,8,10-12,15,35H,3-4,7,9,13-14,16H2,1-2H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411034
(CHEMBL378785)Show SMILES Cc1cccc2C(CC(O)(C(=O)Nc3ccc4c(c3)c(C)noc4=O)C(F)(F)F)CCCc12 Show InChI InChI=1S/C24H23F3N2O4/c1-13-5-3-8-18-15(6-4-7-17(13)18)12-23(32,24(25,26)27)22(31)28-16-9-10-19-20(11-16)14(2)29-33-21(19)30/h3,5,8-11,15,32H,4,6-7,12H2,1-2H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
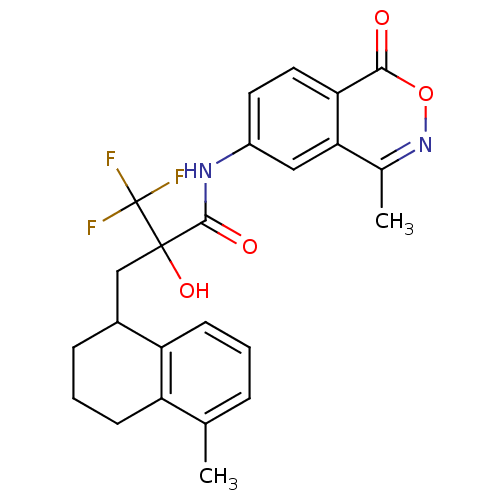
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411034

(CHEMBL378785)Show SMILES Cc1cccc2C(CC(O)(C(=O)Nc3ccc4c(c3)c(C)noc4=O)C(F)(F)F)CCCc12 Show InChI InChI=1S/C24H23F3N2O4/c1-13-5-3-8-18-15(6-4-7-17(13)18)12-23(32,24(25,26)27)22(31)28-16-9-10-19-20(11-16)14(2)29-33-21(19)30/h3,5,8-11,15,32H,4,6-7,12H2,1-2H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411036
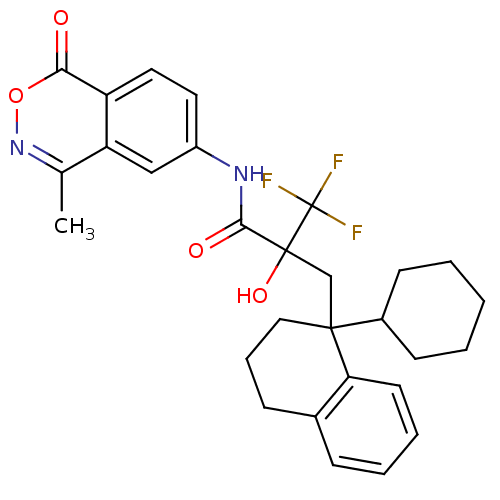
(CHEMBL383890)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCCc4ccccc34)C3CCCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C29H31F3N2O4/c1-18-23-16-21(13-14-22(23)25(35)38-34-18)33-26(36)28(37,29(30,31)32)17-27(20-10-3-2-4-11-20)15-7-9-19-8-5-6-12-24(19)27/h5-6,8,12-14,16,20,37H,2-4,7,9-11,15,17H2,1H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411020

(CHEMBL211266)Show SMILES CC(C)CC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C27H29F3N2O4/c1-16(2)14-25(12-6-8-18-7-4-5-9-22(18)25)15-26(35,27(28,29)30)24(34)31-19-10-11-20-21(13-19)17(3)32-36-23(20)33/h4-5,7,9-11,13,16,35H,6,8,12,14-15H2,1-3H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340438
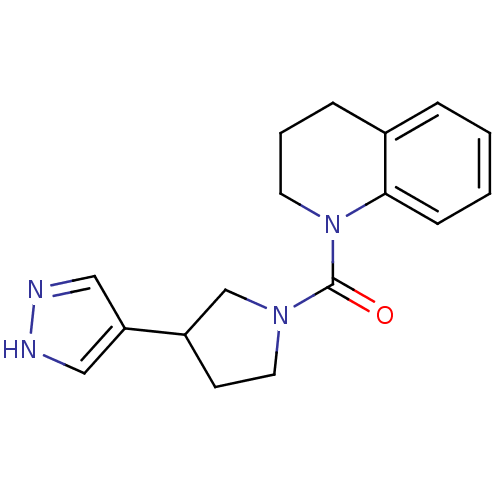
((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(3,4-dihydroqu...)Show InChI InChI=1S/C17H20N4O/c22-17(20-9-7-14(12-20)15-10-18-19-11-15)21-8-3-5-13-4-1-2-6-16(13)21/h1-2,4,6,10-11,14H,3,5,7-9,12H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433411
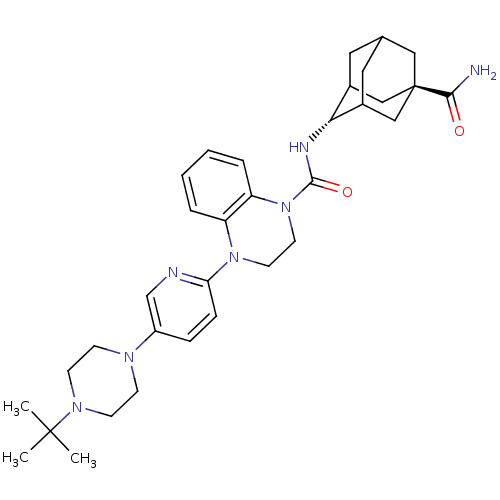
(CHEMBL2380648)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(27.21,-52.3,;27.98,-53.63,;27.21,-54.97,;26.44,-53.63,;29.52,-53.63,;30.29,-54.95,;31.82,-54.95,;32.59,-53.63,;31.82,-52.3,;30.28,-52.3,;34.12,-53.62,;34.89,-52.29,;36.42,-52.29,;37.2,-53.63,;36.43,-54.96,;34.89,-54.96,;38.74,-53.62,;39.51,-54.96,;41.04,-54.97,;41.82,-53.63,;43.36,-53.64,;44.13,-54.97,;44.28,-52.41,;45.81,-52.44,;47.01,-51.16,;47,-49.68,;48.35,-49.2,;47.31,-50.43,;47.31,-52.02,;48.72,-52.58,;49.73,-51.3,;49.74,-49.78,;48.34,-51.65,;51.27,-51.3,;52.04,-52.64,;52.04,-49.97,;41.05,-52.3,;41.83,-50.97,;41.07,-49.63,;39.51,-49.62,;38.74,-50.96,;39.51,-52.29,)| Show InChI InChI=1S/C33H45N7O2/c1-32(2,3)38-12-10-37(11-13-38)25-8-9-28(35-21-25)39-14-15-40(27-7-5-4-6-26(27)39)31(42)36-29-23-16-22-17-24(29)20-33(18-22,19-23)30(34)41/h4-9,21-24,29H,10-20H2,1-3H3,(H2,34,41)(H,36,42)/t22?,23?,24?,29-,33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433412
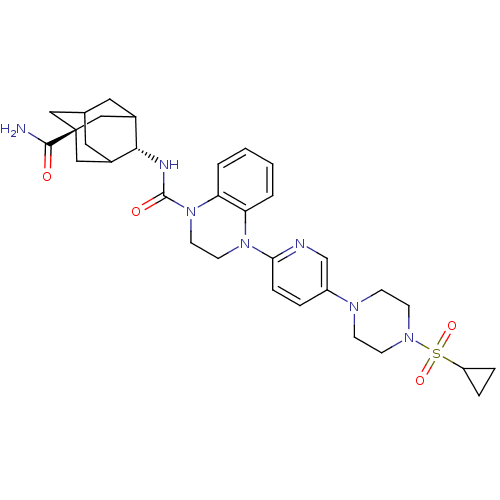
(CHEMBL2380649)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)N1CCN(c4ccc(cn4)N4CCN(CC4)S(=O)(=O)C4CC4)c4ccccc14)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:1:3:9.41.42:6,10:9:3.8.43:42.5.6,10:9:6:3.8.4,THB:43:3:9.41.42:6,43:41:6:3.8.4,4:3:9:42.5.6,4:5:9:3.8.43,(26.96,-60.09,;26.19,-58.76,;26.96,-57.43,;24.65,-58.76,;24.66,-57.23,;23.27,-56.65,;21.92,-57.13,;21.93,-58.62,;23.26,-59.11,;20.73,-59.89,;19.19,-59.87,;18.27,-61.1,;19.04,-62.44,;16.73,-61.1,;15.96,-62.43,;14.42,-62.43,;13.65,-61.09,;12.11,-61.09,;11.33,-59.75,;9.8,-59.75,;9.03,-61.08,;9.8,-62.42,;11.34,-62.42,;7.5,-61.09,;6.73,-62.42,;5.2,-62.41,;4.43,-61.09,;5.19,-59.76,;6.73,-59.76,;2.89,-61.09,;2.88,-62.63,;1.55,-61.85,;2.12,-59.77,;2.1,-58.24,;.78,-59.01,;14.42,-59.75,;13.65,-58.42,;14.43,-57.08,;15.98,-57.09,;16.75,-58.43,;15.97,-59.77,;22.23,-59.47,;22.23,-57.89,;23.64,-60.04,)| Show InChI InChI=1S/C32H41N7O4S/c33-30(40)32-17-21-15-22(18-32)29(23(16-21)19-32)35-31(41)39-14-13-38(26-3-1-2-4-27(26)39)28-8-5-24(20-34-28)36-9-11-37(12-10-36)44(42,43)25-6-7-25/h1-5,8,20-23,25,29H,6-7,9-19H2,(H2,33,40)(H,35,41)/t21?,22?,23?,29-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50499970

(CHEMBL3741505)Show SMILES OC(=O)[C@@H]1CC[C@@H](CC1)Oc1ccc(cc1)C(=O)Nc1nnc(Nc2ccccc2)s1 |r,wU:6.9,3.2,(-9.07,-.95,;-8,-1.56,;-7.99,-2.79,;-6.67,-.78,;-5.33,-1.55,;-4,-.77,;-4,.77,;-5.34,1.53,;-6.67,.76,;-2.67,1.54,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;2.67,-1.54,;3.73,-.93,;2.66,-3.08,;4,-3.86,;4.14,-5.38,;5.64,-5.7,;6.41,-4.36,;7.94,-4.2,;8.57,-2.79,;10.1,-2.63,;10.72,-1.22,;9.81,.03,;8.28,-.14,;7.66,-1.55,;5.38,-3.22,)| Show InChI InChI=1S/C22H22N4O4S/c27-19(24-22-26-25-21(31-22)23-16-4-2-1-3-5-16)14-6-10-17(11-7-14)30-18-12-8-15(9-13-18)20(28)29/h1-7,10-11,15,18H,8-9,12-13H2,(H,23,25)(H,28,29)(H,24,26,27)/t15-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DGAT-1 using 1,2,di(cis-9-octadecenoyl)-sn-glycerol as substrate by Microbeta counter |
Bioorg Med Chem Lett 26: 25-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.046
BindingDB Entry DOI: 10.7270/Q2Z89GDN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411048
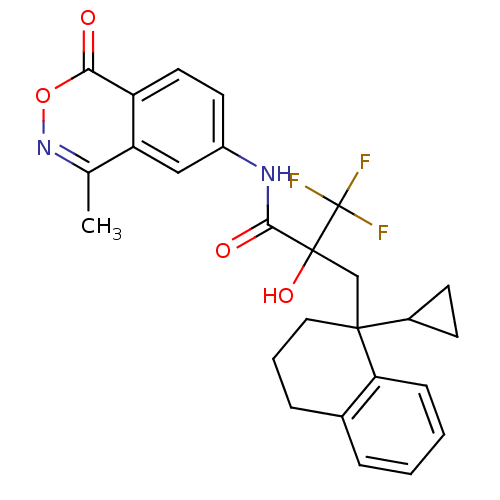
(CHEMBL386448)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCCc4ccccc34)C3CC3)C(F)(F)F)cc12 Show InChI InChI=1S/C26H25F3N2O4/c1-15-20-13-18(10-11-19(20)22(32)35-31-15)30-23(33)25(34,26(27,28)29)14-24(17-8-9-17)12-4-6-16-5-2-3-7-21(16)24/h2-3,5,7,10-11,13,17,34H,4,6,8-9,12,14H2,1H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Activity at GR assessed as ability to antagonize dexamethasone-induced MMTV luciferase reporter gene transactivation in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411037

(CHEMBL213453)Show SMILES CCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-3-12-24(13-6-8-17-7-4-5-9-21(17)24)15-25(34,26(27,28)29)23(33)30-18-10-11-19-20(14-18)16(2)31-35-22(19)32/h4-5,7,9-11,14,34H,3,6,8,12-13,15H2,1-2H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411026

(CHEMBL214336)Show SMILES CC(C)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-15(2)24(12-6-8-17-7-4-5-9-21(17)24)14-25(34,26(27,28)29)23(33)30-18-10-11-19-20(13-18)16(3)31-35-22(19)32/h4-5,7,9-11,13,15,34H,6,8,12,14H2,1-3H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411035
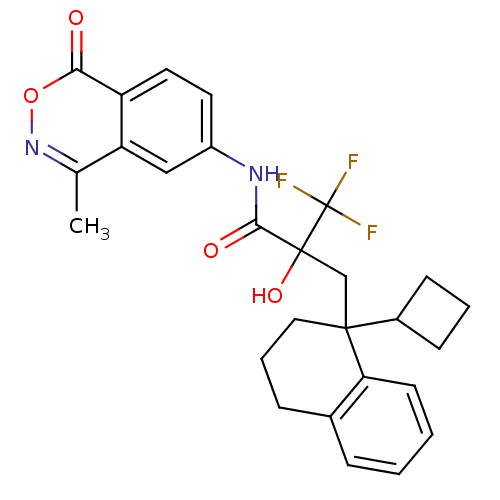
(CHEMBL210185)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCCc4ccccc34)C3CCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C27H27F3N2O4/c1-16-21-14-19(11-12-20(21)23(33)36-32-16)31-24(34)26(35,27(28,29)30)15-25(18-8-4-9-18)13-5-7-17-6-2-3-10-22(17)25/h2-3,6,10-12,14,18,35H,4-5,7-9,13,15H2,1H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411030

(CHEMBL210077)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C25H25F3N2O4/c1-3-23(12-6-8-16-7-4-5-9-20(16)23)14-24(33,25(26,27)28)22(32)29-17-10-11-18-19(13-17)15(2)30-34-21(18)31/h4-5,7,9-11,13,33H,3,6,8,12,14H2,1-2H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411033
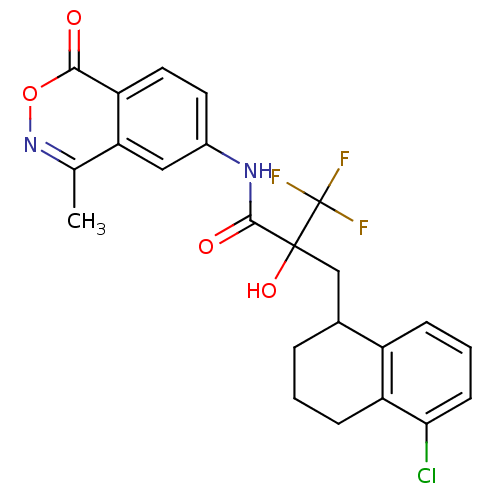
(CHEMBL211387)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3CCCc4c(Cl)cccc34)C(F)(F)F)cc12 Show InChI InChI=1S/C23H20ClF3N2O4/c1-12-18-10-14(8-9-17(18)20(30)33-29-12)28-21(31)22(32,23(25,26)27)11-13-4-2-6-16-15(13)5-3-7-19(16)24/h3,5,7-10,13,32H,2,4,6,11H2,1H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411023
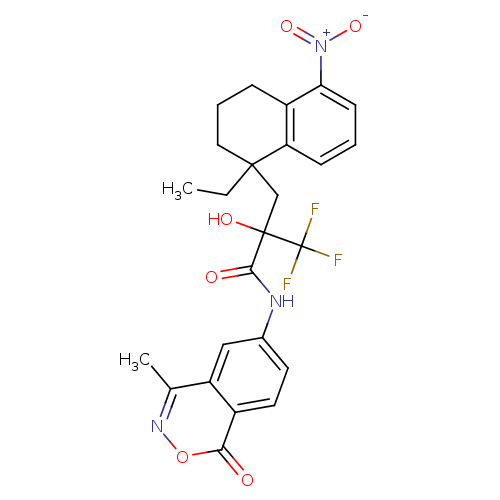
(CHEMBL208779)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2c(cccc12)[N+]([O-])=O Show InChI InChI=1S/C25H24F3N3O6/c1-3-23(11-5-6-17-19(23)7-4-8-20(17)31(35)36)13-24(34,25(26,27)28)22(33)29-15-9-10-16-18(12-15)14(2)30-37-21(16)32/h4,7-10,12,34H,3,5-6,11,13H2,1-2H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50422693
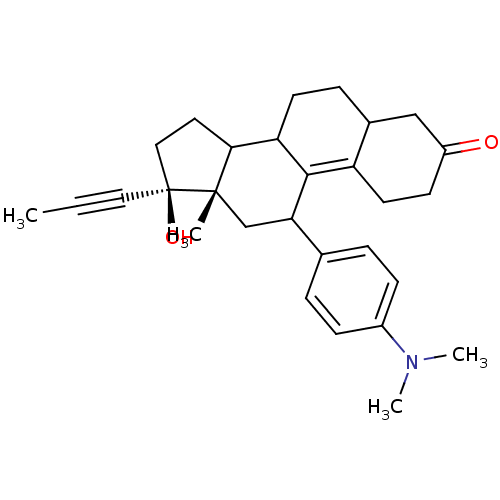
(CHEMBL433964 | RU-846)Show SMILES CC#C[C@]1(O)CCC2C3CCC4CC(=O)CCC4=C3C(C[C@]12C)c1ccc(cc1)N(C)C |c:18| Show InChI InChI=1S/C29H37NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,20,24-26,32H,8,11-14,16-18H2,1-4H3/t20?,24?,25?,26?,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity for Glucocorticoid receptor in MMTV transactivation assay in human A549 lung epithelial cells at 10 uM |
J Med Chem 48: 4507-10 (2005)
Article DOI: 10.1021/jm050345y
BindingDB Entry DOI: 10.7270/Q2T43VCV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433400
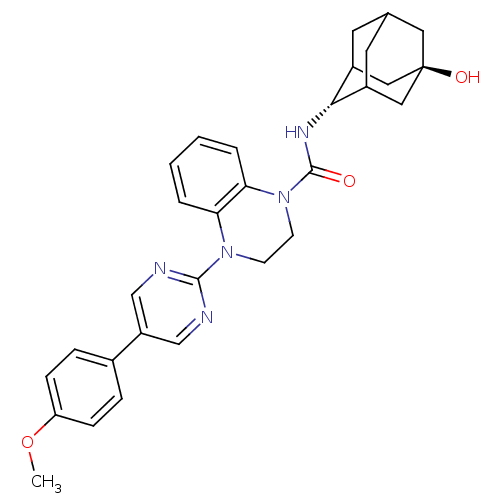
(CHEMBL2380637)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:21.22,wD:28.31,TLB:20:21:31.27.28:25.24.23,20:21:23:31.28.30,29:28:21.26.25:23,THB:27:26:23:31.28.30,27:28:21.26.25:23,29:28:21:25.24.23,30:28:21:25.24.23,30:24:21:31.27.28,(.2,-12.39,;.98,-13.73,;2.52,-13.72,;3.29,-15.05,;4.83,-15.05,;5.59,-13.72,;4.83,-12.39,;3.28,-12.39,;7.13,-13.71,;7.9,-15.05,;9.44,-15.05,;10.21,-13.72,;9.43,-12.38,;7.9,-12.38,;11.76,-13.72,;12.53,-15.06,;14.06,-15.06,;14.84,-13.73,;16.38,-13.73,;17.15,-15.07,;17.3,-12.49,;18.84,-12.52,;20.04,-11.24,;20.03,-9.76,;21.38,-9.28,;20.34,-10.51,;20.35,-12.1,;21.75,-12.67,;22.77,-11.39,;24.31,-11.33,;22.78,-9.86,;21.37,-11.73,;14.07,-12.39,;14.85,-11.06,;14.09,-9.71,;12.53,-9.71,;11.76,-11.04,;12.52,-12.38,)| Show InChI InChI=1S/C30H33N5O3/c1-38-24-8-6-20(7-9-24)23-17-31-28(32-18-23)34-10-11-35(26-5-3-2-4-25(26)34)29(36)33-27-21-12-19-13-22(27)16-30(37,14-19)15-21/h2-9,17-19,21-22,27,37H,10-16H2,1H3,(H,33,36)/t19?,21?,22?,27-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433403
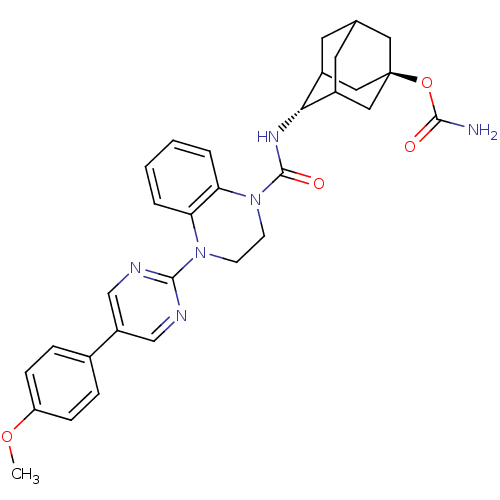
(CHEMBL2380640)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)OC(N)=O)c2ccccc12 |r,wU:21.22,wD:28.35,TLB:31:28:21.26.25:23,20:21:28.30.27:25.24.23,20:21:23:28.30.29,THB:27:28:21.26.25:23,27:26:23:28.30.29,29:28:21:25.24.23,29:24:21:28.30.27,(27.37,-22.08,;28.15,-23.41,;29.69,-23.41,;30.45,-24.73,;31.99,-24.73,;32.76,-23.4,;31.99,-22.07,;30.45,-22.08,;34.29,-23.4,;35.06,-24.74,;36.6,-24.74,;37.37,-23.4,;36.59,-22.06,;35.06,-22.07,;38.91,-23.4,;39.68,-24.74,;41.22,-24.74,;41.99,-23.41,;43.53,-23.42,;44.3,-24.75,;44.45,-22.18,;45.99,-22.21,;47.19,-20.93,;47.18,-19.45,;48.53,-18.97,;47.49,-20.2,;47.49,-21.79,;48.9,-22.35,;49.91,-21.08,;49.92,-19.55,;48.52,-21.42,;51.45,-21.08,;52.22,-22.41,;53.76,-22.41,;51.45,-23.74,;41.22,-22.08,;42.01,-20.75,;41.24,-19.4,;39.68,-19.4,;38.91,-20.73,;39.68,-22.07,)| Show InChI InChI=1S/C31H34N6O4/c1-40-24-8-6-20(7-9-24)23-17-33-29(34-18-23)36-10-11-37(26-5-3-2-4-25(26)36)30(39)35-27-21-12-19-13-22(27)16-31(14-19,15-21)41-28(32)38/h2-9,17-19,21-22,27H,10-16H2,1H3,(H2,32,38)(H,35,39)/t19?,21?,22?,27-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433407
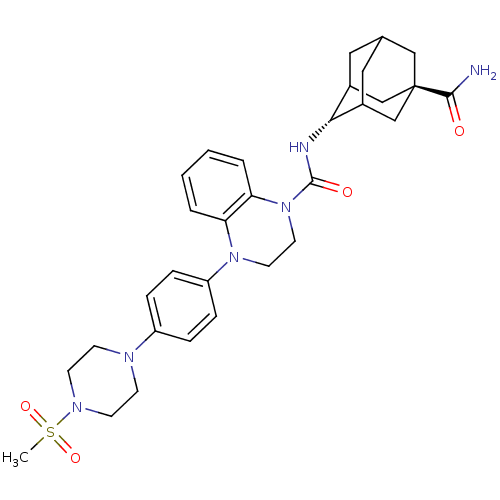
(CHEMBL2380644)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(28.99,-37.06,;29.76,-38.38,;29.75,-39.92,;28.42,-39.15,;31.3,-38.39,;32.07,-39.71,;33.6,-39.71,;34.37,-38.38,;33.6,-37.05,;32.06,-37.06,;35.9,-38.38,;36.67,-39.72,;38.21,-39.72,;38.98,-38.38,;38.21,-37.04,;36.67,-37.05,;40.52,-38.38,;41.29,-39.72,;42.83,-39.72,;43.6,-38.39,;45.14,-38.4,;45.91,-39.73,;46.06,-37.16,;47.59,-37.19,;48.79,-35.92,;48.78,-34.43,;50.13,-33.95,;49.09,-35.19,;49.1,-36.77,;50.5,-37.34,;51.51,-36.06,;51.53,-34.53,;50.12,-36.41,;53.05,-36.06,;53.82,-34.73,;53.82,-37.39,;42.84,-37.06,;43.62,-35.73,;42.85,-34.38,;41.3,-34.38,;40.52,-35.71,;41.29,-37.05,)| Show InChI InChI=1S/C31H40N6O4S/c1-42(40,41)35-12-10-34(11-13-35)24-6-8-25(9-7-24)36-14-15-37(27-5-3-2-4-26(27)36)30(39)33-28-22-16-21-17-23(28)20-31(18-21,19-22)29(32)38/h2-9,21-23,28H,10-20H2,1H3,(H2,32,38)(H,33,39)/t21?,22?,23?,28-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411047

(CHEMBL211441)Show SMILES CCC(CC)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C28H31F3N2O4/c1-4-19(5-2)26(14-8-10-18-9-6-7-11-23(18)26)16-27(36,28(29,30)31)25(35)32-20-12-13-21-22(15-20)17(3)33-37-24(21)34/h6-7,9,11-13,15,19,36H,4-5,8,10,14,16H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411037

(CHEMBL213453)Show SMILES CCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-3-12-24(13-6-8-17-7-4-5-9-21(17)24)15-25(34,26(27,28)29)23(33)30-18-10-11-19-20(14-18)16(2)31-35-22(19)32/h4-5,7,9-11,14,34H,3,6,8,12-13,15H2,1-2H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50433407

(CHEMBL2380644)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(28.99,-37.06,;29.76,-38.38,;29.75,-39.92,;28.42,-39.15,;31.3,-38.39,;32.07,-39.71,;33.6,-39.71,;34.37,-38.38,;33.6,-37.05,;32.06,-37.06,;35.9,-38.38,;36.67,-39.72,;38.21,-39.72,;38.98,-38.38,;38.21,-37.04,;36.67,-37.05,;40.52,-38.38,;41.29,-39.72,;42.83,-39.72,;43.6,-38.39,;45.14,-38.4,;45.91,-39.73,;46.06,-37.16,;47.59,-37.19,;48.79,-35.92,;48.78,-34.43,;50.13,-33.95,;49.09,-35.19,;49.1,-36.77,;50.5,-37.34,;51.51,-36.06,;51.53,-34.53,;50.12,-36.41,;53.05,-36.06,;53.82,-34.73,;53.82,-37.39,;42.84,-37.06,;43.62,-35.73,;42.85,-34.38,;41.3,-34.38,;40.52,-35.71,;41.29,-37.05,)| Show InChI InChI=1S/C31H40N6O4S/c1-42(40,41)35-12-10-34(11-13-35)24-6-8-25(9-7-24)36-14-15-37(27-5-3-2-4-26(27)36)30(39)33-28-22-16-21-17-23(28)20-31(18-21,19-22)29(32)38/h2-9,21-23,28H,10-20H2,1H3,(H2,32,38)(H,33,39)/t21?,22?,23?,28-,31- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433411

(CHEMBL2380648)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(27.21,-52.3,;27.98,-53.63,;27.21,-54.97,;26.44,-53.63,;29.52,-53.63,;30.29,-54.95,;31.82,-54.95,;32.59,-53.63,;31.82,-52.3,;30.28,-52.3,;34.12,-53.62,;34.89,-52.29,;36.42,-52.29,;37.2,-53.63,;36.43,-54.96,;34.89,-54.96,;38.74,-53.62,;39.51,-54.96,;41.04,-54.97,;41.82,-53.63,;43.36,-53.64,;44.13,-54.97,;44.28,-52.41,;45.81,-52.44,;47.01,-51.16,;47,-49.68,;48.35,-49.2,;47.31,-50.43,;47.31,-52.02,;48.72,-52.58,;49.73,-51.3,;49.74,-49.78,;48.34,-51.65,;51.27,-51.3,;52.04,-52.64,;52.04,-49.97,;41.05,-52.3,;41.83,-50.97,;41.07,-49.63,;39.51,-49.62,;38.74,-50.96,;39.51,-52.29,)| Show InChI InChI=1S/C33H45N7O2/c1-32(2,3)38-12-10-37(11-13-38)25-8-9-28(35-21-25)39-14-15-40(27-7-5-4-6-26(27)39)31(42)36-29-23-16-22-17-24(29)20-33(18-22,19-23)30(34)41/h4-9,21-24,29H,10-20H2,1-3H3,(H2,34,41)(H,36,42)/t22?,23?,24?,29-,33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411030

(CHEMBL210077)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C25H25F3N2O4/c1-3-23(12-6-8-16-7-4-5-9-20(16)23)14-24(33,25(26,27)28)22(32)29-17-10-11-18-19(13-17)15(2)30-34-21(18)31/h4-5,7,9-11,13,33H,3,6,8,12,14H2,1-2H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411041
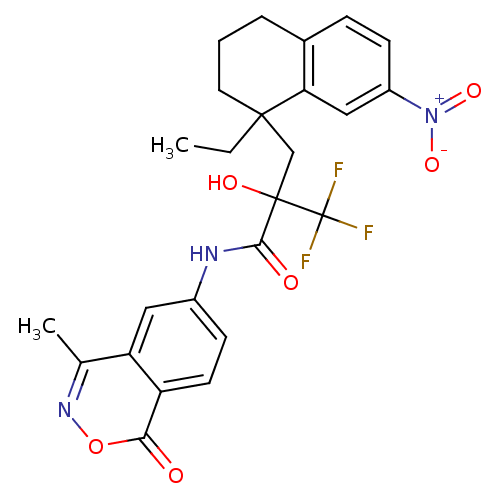
(CHEMBL213621)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccc(cc12)[N+]([O-])=O Show InChI InChI=1S/C25H24F3N3O6/c1-3-23(10-4-5-15-6-8-17(31(35)36)12-20(15)23)13-24(34,25(26,27)28)22(33)29-16-7-9-18-19(11-16)14(2)30-37-21(18)32/h6-9,11-12,34H,3-5,10,13H2,1-2H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411039

(CHEMBL385450)Show SMILES CCCCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C27H29F3N2O4/c1-3-4-13-25(14-7-9-18-8-5-6-10-22(18)25)16-26(35,27(28,29)30)24(34)31-19-11-12-20-21(15-19)17(2)32-36-23(20)33/h5-6,8,10-12,15,35H,3-4,7,9,13-14,16H2,1-2H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50340439

((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(4,4-dimethyl-...)Show SMILES CC1(C)CCN(C(=O)N2CCC(C2)c2cn[nH]c2)c2ccccc12 Show InChI InChI=1S/C19H24N4O/c1-19(2)8-10-23(17-6-4-3-5-16(17)19)18(24)22-9-7-14(13-22)15-11-20-21-12-15/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340442

((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(3,4-dihydroqu...)Show InChI InChI=1S/C16H19N5O/c22-16(20-7-5-12(11-20)13-9-18-19-10-13)21-8-6-17-14-3-1-2-4-15(14)21/h1-4,9-10,12,17H,5-8,11H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50433411

(CHEMBL2380648)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)c2ccccc12 |r,wU:23.24,wD:30.37,TLB:33:30:23.28.27:25,22:23:30.32.29:27.26.25,22:23:25:30.32.31,THB:29:30:23.28.27:25,29:28:25:30.32.31,31:30:23:27.26.25,31:26:23:30.32.29,(27.21,-52.3,;27.98,-53.63,;27.21,-54.97,;26.44,-53.63,;29.52,-53.63,;30.29,-54.95,;31.82,-54.95,;32.59,-53.63,;31.82,-52.3,;30.28,-52.3,;34.12,-53.62,;34.89,-52.29,;36.42,-52.29,;37.2,-53.63,;36.43,-54.96,;34.89,-54.96,;38.74,-53.62,;39.51,-54.96,;41.04,-54.97,;41.82,-53.63,;43.36,-53.64,;44.13,-54.97,;44.28,-52.41,;45.81,-52.44,;47.01,-51.16,;47,-49.68,;48.35,-49.2,;47.31,-50.43,;47.31,-52.02,;48.72,-52.58,;49.73,-51.3,;49.74,-49.78,;48.34,-51.65,;51.27,-51.3,;52.04,-52.64,;52.04,-49.97,;41.05,-52.3,;41.83,-50.97,;41.07,-49.63,;39.51,-49.62,;38.74,-50.96,;39.51,-52.29,)| Show InChI InChI=1S/C33H45N7O2/c1-32(2,3)38-12-10-37(11-13-38)25-8-9-28(35-21-25)39-14-15-40(27-7-5-4-6-26(27)39)31(42)36-29-23-16-22-17-24(29)20-33(18-22,19-23)30(34)41/h4-9,21-24,29H,10-20H2,1-3H3,(H2,34,41)(H,36,42)/t22?,23?,24?,29-,33- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411049

(CHEMBL190838)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3CCCc4ccccc34)C(F)(F)F)cc12 Show InChI InChI=1S/C23H21F3N2O4/c1-13-19-11-16(9-10-18(19)20(29)32-28-13)27-21(30)22(31,23(24,25)26)12-15-7-4-6-14-5-2-3-8-17(14)15/h2-3,5,8-11,15,31H,4,6-7,12H2,1H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411032

(CHEMBL209259)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2cc(ccc12)[N+]([O-])=O Show InChI InChI=1S/C25H24F3N3O6/c1-3-23(10-4-5-15-11-17(31(35)36)7-9-20(15)23)13-24(34,25(26,27)28)22(33)29-16-6-8-18-19(12-16)14(2)30-37-21(18)32/h6-9,11-12,34H,3-5,10,13H2,1-2H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411045

(CHEMBL208840)Show SMILES CCC1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCCc2ccccc12 Show InChI InChI=1S/C26H27F3N2O4/c1-3-24(13-7-6-9-17-8-4-5-10-21(17)24)15-25(34,26(27,28)29)23(33)30-18-11-12-19-20(14-18)16(2)31-35-22(19)32/h4-5,8,10-12,14,34H,3,6-7,9,13,15H2,1-2H3,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50340439

((3-(1H-pyrazol-4-yl)pyrrolidin-1-yl)(4,4-dimethyl-...)Show SMILES CC1(C)CCN(C(=O)N2CCC(C2)c2cn[nH]c2)c2ccccc12 Show InChI InChI=1S/C19H24N4O/c1-19(2)8-10-23(17-6-4-3-5-16(17)19)18(24)22-9-7-14(13-22)15-11-20-21-12-15/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D,
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 11beta-HSD1 assessed as conversion of radiolabelled-cortisone to cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2244-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.111
BindingDB Entry DOI: 10.7270/Q26H4HQS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433408
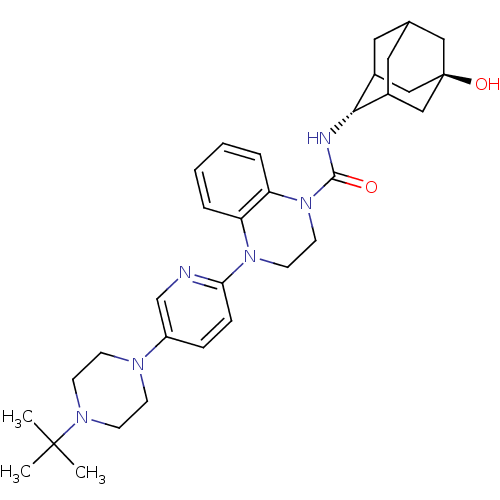
(CHEMBL2380645)Show SMILES CC(C)(C)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(1.87,-44.23,;2.65,-45.56,;1.88,-46.9,;1.1,-45.55,;4.19,-45.56,;4.95,-46.88,;6.49,-46.88,;7.26,-45.55,;6.49,-44.22,;4.95,-44.23,;8.79,-45.55,;9.56,-44.22,;11.09,-44.21,;11.87,-45.55,;11.1,-46.89,;9.56,-46.89,;13.41,-45.55,;14.18,-46.89,;15.71,-46.89,;16.49,-45.56,;18.03,-45.57,;18.8,-46.9,;18.95,-44.33,;20.49,-44.36,;21.68,-43.08,;21.67,-41.6,;23.02,-41.12,;21.98,-42.35,;21.99,-43.94,;23.39,-44.5,;24.41,-43.23,;25.95,-43.23,;24.42,-41.7,;23.01,-43.57,;15.72,-44.23,;16.5,-42.9,;15.74,-41.55,;14.18,-41.55,;13.41,-42.88,;14.17,-44.22,)| Show InChI InChI=1S/C32H44N6O2/c1-31(2,3)36-12-10-35(11-13-36)25-8-9-28(33-21-25)37-14-15-38(27-7-5-4-6-26(27)37)30(39)34-29-23-16-22-17-24(29)20-32(40,18-22)19-23/h4-9,21-24,29,40H,10-20H2,1-3H3,(H,34,39)/t22?,23?,24?,29-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433412

(CHEMBL2380649)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](NC(=O)N1CCN(c4ccc(cn4)N4CCN(CC4)S(=O)(=O)C4CC4)c4ccccc14)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:1:3:9.41.42:6,10:9:3.8.43:42.5.6,10:9:6:3.8.4,THB:43:3:9.41.42:6,43:41:6:3.8.4,4:3:9:42.5.6,4:5:9:3.8.43,(26.96,-60.09,;26.19,-58.76,;26.96,-57.43,;24.65,-58.76,;24.66,-57.23,;23.27,-56.65,;21.92,-57.13,;21.93,-58.62,;23.26,-59.11,;20.73,-59.89,;19.19,-59.87,;18.27,-61.1,;19.04,-62.44,;16.73,-61.1,;15.96,-62.43,;14.42,-62.43,;13.65,-61.09,;12.11,-61.09,;11.33,-59.75,;9.8,-59.75,;9.03,-61.08,;9.8,-62.42,;11.34,-62.42,;7.5,-61.09,;6.73,-62.42,;5.2,-62.41,;4.43,-61.09,;5.19,-59.76,;6.73,-59.76,;2.89,-61.09,;2.88,-62.63,;1.55,-61.85,;2.12,-59.77,;2.1,-58.24,;.78,-59.01,;14.42,-59.75,;13.65,-58.42,;14.43,-57.08,;15.98,-57.09,;16.75,-58.43,;15.97,-59.77,;22.23,-59.47,;22.23,-57.89,;23.64,-60.04,)| Show InChI InChI=1S/C32H41N7O4S/c33-30(40)32-17-21-15-22(18-32)29(23(16-21)19-32)35-31(41)39-14-13-38(26-3-1-2-4-27(26)39)28-8-5-24(20-34-28)36-9-11-37(12-10-36)44(42,43)25-6-7-25/h1-5,8,20-23,25,29H,6-7,9-19H2,(H2,33,40)(H,35,41)/t21?,22?,23?,29-,32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411024

(CHEMBL208996)Show SMILES Cc1noc(=O)c2ccc(NC(=O)[C@](O)(C[C@]3(CCCc4ccccc34)C3CCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C28H29F3N2O4/c1-17-22-15-20(12-13-21(22)24(34)37-33-17)32-25(35)27(36,28(29,30)31)16-26(19-9-3-4-10-19)14-6-8-18-7-2-5-11-23(18)26/h2,5,7,11-13,15,19,36H,3-4,6,8-10,14,16H2,1H3,(H,32,35)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411036

(CHEMBL383890)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCCc4ccccc34)C3CCCCC3)C(F)(F)F)cc12 Show InChI InChI=1S/C29H31F3N2O4/c1-18-23-16-21(13-14-22(23)25(35)38-34-18)33-26(36)28(37,29(30,31)32)17-27(20-10-3-2-4-11-20)15-7-9-19-8-5-6-12-24(19)27/h5-6,8,12-14,16,20,37H,2-4,7,9-11,15,17H2,1H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of fluorescent-labeled Dexamethasone binding to GR |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19190
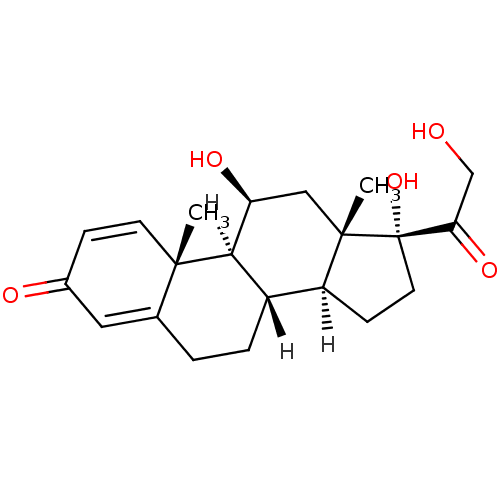
((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...)Show SMILES [H][C@@]12CC[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |r,c:27,t:23| Show InChI InChI=1S/C21H28O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-16,18,22,24,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,16-,18+,19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data