 Found 213 hits with Last Name = 'paderes' and Initial = 'g'
Found 213 hits with Last Name = 'paderes' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380320
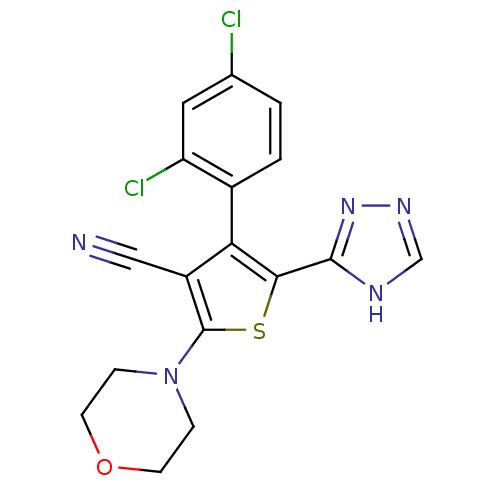
(CHEMBL2017653)Show SMILES Clc1ccc(-c2c(sc(N3CCOCC3)c2C#N)-c2nnc[nH]2)c(Cl)c1 |(13.97,-25.89,;13.2,-24.55,;13.97,-23.21,;13.2,-21.89,;11.67,-21.88,;10.91,-20.55,;11.39,-19.08,;10.14,-18.17,;8.88,-19.08,;7.54,-18.32,;7.53,-16.78,;6.2,-16.02,;4.87,-16.79,;4.88,-18.33,;6.21,-19.1,;9.36,-20.55,;8.46,-21.8,;7.56,-23.05,;12.84,-18.6,;14.09,-19.51,;15.33,-18.61,;14.86,-17.14,;13.32,-17.14,;10.89,-23.21,;9.36,-23.21,;11.66,-24.55,)| Show InChI InChI=1S/C17H13Cl2N5OS/c18-10-1-2-11(13(19)7-10)14-12(8-20)17(24-3-5-25-6-4-24)26-15(14)16-21-9-22-23-16/h1-2,7,9H,3-6H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50187673
(5-(3,3,6-trimethyl-indan-5-yloxy)-furan-2-carboxyl...)Show SMILES COc1nc(NCCCN2CCOCC2)nc(OC)c1NC(=O)c1ccc(Oc2cc3c(CCC3(C)C)cc2C)o1 Show InChI InChI=1S/C30H39N5O6/c1-19-17-20-9-10-30(2,3)21(20)18-23(19)41-24-8-7-22(40-24)26(36)32-25-27(37-4)33-29(34-28(25)38-5)31-11-6-12-35-13-15-39-16-14-35/h7-8,17-18H,6,9-16H2,1-5H3,(H,32,36)(H,31,33,34) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to rat pituitary GnRH receptor |
J Med Chem 49: 3362-7 (2006)
Article DOI: 10.1021/jm060012g
BindingDB Entry DOI: 10.7270/Q2F76C54 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50187673

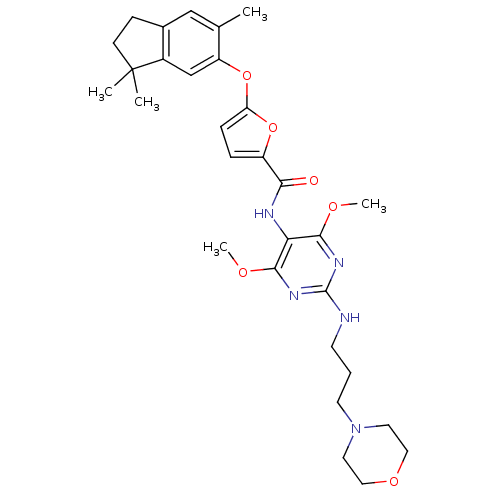
(5-(3,3,6-trimethyl-indan-5-yloxy)-furan-2-carboxyl...)Show SMILES COc1nc(NCCCN2CCOCC2)nc(OC)c1NC(=O)c1ccc(Oc2cc3c(CCC3(C)C)cc2C)o1 Show InChI InChI=1S/C30H39N5O6/c1-19-17-20-9-10-30(2,3)21(20)18-23(19)41-24-8-7-22(40-24)26(36)32-25-27(37-4)33-29(34-28(25)38-5)31-11-6-12-35-13-15-39-16-14-35/h7-8,17-18H,6,9-16H2,1-5H3,(H,32,36)(H,31,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant GnRH receptor |
J Med Chem 49: 3362-7 (2006)
Article DOI: 10.1021/jm060012g
BindingDB Entry DOI: 10.7270/Q2F76C54 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380313
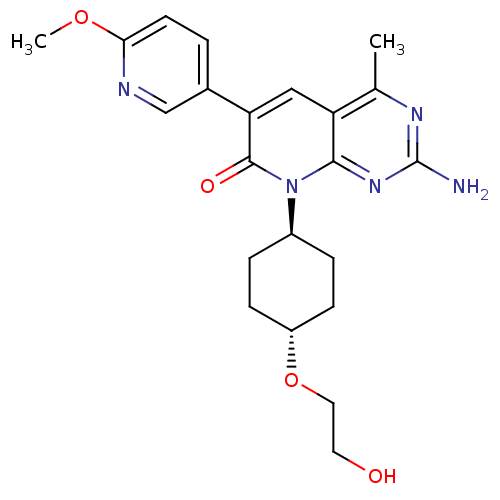
(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380321

(CHEMBL2017654)Show SMILES Fc1cc(ccc1-c1c(sc(N2CCOCC2)c1C#N)-c1nnc[nH]1)C#N |(23.39,-23.59,;24.93,-23.6,;25.69,-24.93,;27.23,-24.94,;28,-23.6,;27.24,-22.27,;25.7,-22.27,;24.94,-20.94,;25.42,-19.47,;24.17,-18.56,;22.91,-19.47,;21.57,-18.7,;21.56,-17.16,;20.23,-16.4,;18.89,-17.18,;18.9,-18.72,;20.24,-19.48,;23.39,-20.94,;22.49,-22.19,;21.58,-23.43,;26.88,-18.99,;28.12,-19.89,;29.37,-18.99,;28.89,-17.52,;27.35,-17.52,;28,-26.27,;28.77,-27.6,)| Show InChI InChI=1S/C18H13FN6OS/c19-14-7-11(8-20)1-2-12(14)15-13(9-21)18(25-3-5-26-6-4-25)27-16(15)17-22-10-23-24-17/h1-2,7,10H,3-6H2,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317223
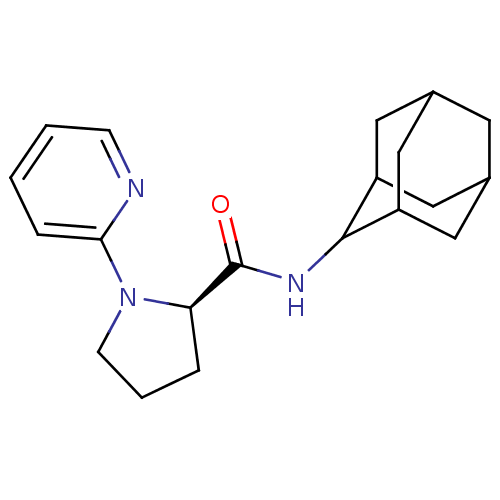
((2R)-N-(adamantan-2-yl)-1-(pyridin-2-yl)pyrrolidin...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1c1ccccn1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(6.04,2.3,;4.69,1.55,;4.66,.01,;3.32,-.73,;3.31,-2.26,;1.91,-2.62,;.57,-2.12,;-.63,-3.4,;.88,-2.98,;2.29,-3.54,;.87,-1.39,;1.92,-.15,;.56,-.63,;3.18,2.06,;1.97,1.09,;.73,1.99,;1.2,3.45,;2.72,3.47,;4.03,4.27,;4,5.82,;5.31,6.62,;6.67,5.87,;6.7,4.35,;5.39,3.54,)| Show InChI InChI=1S/C20H27N3O/c24-20(17-4-3-7-23(17)18-5-1-2-6-21-18)22-19-15-9-13-8-14(11-15)12-16(19)10-13/h1-2,5-6,13-17,19H,3-4,7-12H2,(H,22,24)/t13?,14?,15?,16?,17-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317213
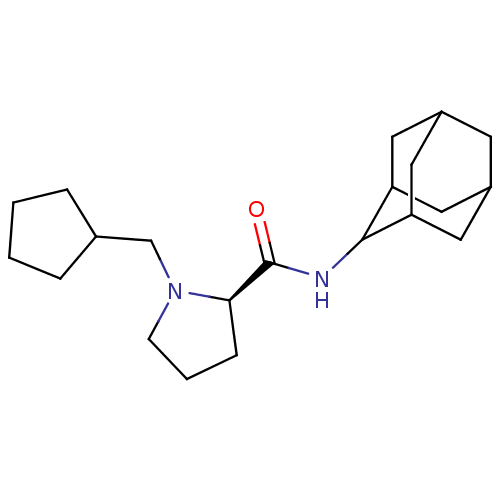
((2R)-N-(adamantan-2-yl)-1-(cyclopentylmethyl)pyrro...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(25.66,-12.64,;24.31,-13.39,;24.29,-14.93,;22.94,-15.67,;22.93,-17.2,;21.53,-17.56,;20.2,-17.06,;19,-18.34,;20.5,-17.92,;21.92,-18.49,;20.5,-16.33,;21.54,-15.09,;20.18,-15.58,;22.8,-12.88,;21.59,-13.85,;20.36,-12.95,;20.82,-11.49,;22.35,-11.47,;23.25,-10.22,;22.62,-8.82,;23.38,-7.49,;22.34,-6.35,;20.94,-6.98,;21.1,-8.51,)| Show InChI InChI=1S/C21H34N2O/c24-21(19-6-3-7-23(19)13-14-4-1-2-5-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317211
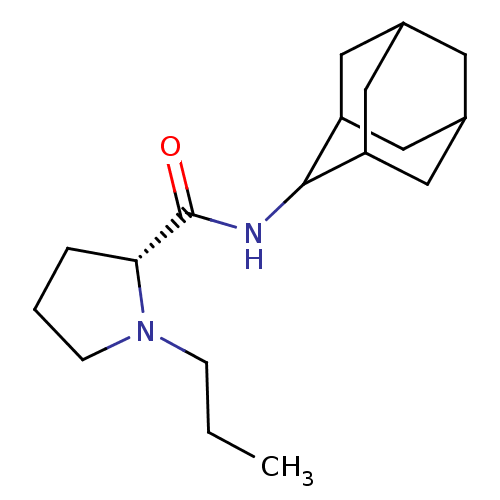
((2R)-N-(adamantan-2-yl)-1-propylpyrrolidine-2-carb...)Show SMILES CCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(3.7,-6.03,;2.79,-7.28,;3.42,-8.69,;2.52,-9.94,;.99,-9.95,;.52,-11.41,;1.76,-12.31,;2.97,-11.34,;4.48,-11.85,;5.83,-11.1,;4.45,-13.39,;3.11,-14.13,;3.1,-15.67,;1.7,-16.02,;.36,-15.52,;-.84,-16.81,;.67,-16.38,;2.08,-16.95,;.66,-14.8,;1.71,-13.55,;.35,-14.04,)| Show InChI InChI=1S/C18H30N2O/c1-2-5-20-6-3-4-16(20)18(21)19-17-14-8-12-7-13(10-14)11-15(17)9-12/h12-17H,2-11H2,1H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317224
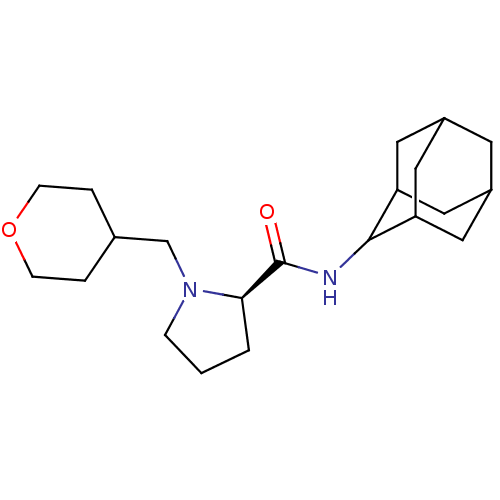
((2R)-N-(adamantan-2-yl)-1-(oxan-4-ylmethyl)pyrroli...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCOCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(16.19,2.36,;14.84,1.61,;14.82,.07,;13.47,-.68,;13.46,-2.21,;12.06,-2.57,;10.72,-2.07,;9.51,-3.36,;11.02,-2.93,;12.44,-3.5,;11.01,-1.34,;12.06,-.09,;10.7,-.58,;13.33,2.12,;12.11,1.14,;10.87,2.05,;11.33,3.52,;12.87,3.53,;13.64,4.87,;15.19,4.87,;15.96,3.52,;17.5,3.52,;18.28,4.86,;17.51,6.2,;15.96,6.21,)| Show InChI InChI=1S/C21H34N2O2/c24-21(19-2-1-5-23(19)13-14-3-6-25-7-4-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317221
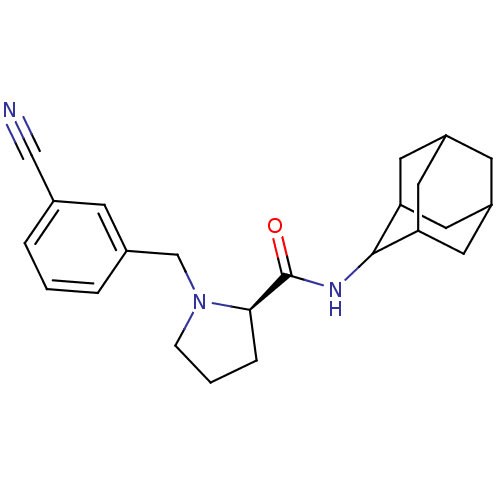
((2R)-N-(adamantan-2-yl)-1-[(3-cyanophenyl)methyl]p...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1Cc1cccc(c1)C#N |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(29.81,-42.16,;28.46,-42.91,;28.44,-44.45,;27.09,-45.2,;27.08,-46.73,;25.68,-47.08,;24.34,-46.59,;23.15,-47.87,;24.65,-47.44,;26.06,-48.01,;24.64,-45.86,;25.69,-44.62,;24.33,-45.1,;26.95,-42.4,;25.74,-43.38,;24.5,-42.47,;24.97,-41.02,;26.5,-41,;27.4,-39.75,;26.63,-38.41,;25.1,-38.41,;24.33,-37.08,;25.11,-35.74,;26.65,-35.75,;27.41,-37.09,;27.43,-34.42,;28.2,-33.09,)| Show InChI InChI=1S/C23H29N3O/c24-13-15-3-1-4-16(7-15)14-26-6-2-5-21(26)23(27)25-22-19-9-17-8-18(11-19)12-20(22)10-17/h1,3-4,7,17-22H,2,5-6,8-12,14H2,(H,25,27)/t17?,18?,19?,20?,21-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317218
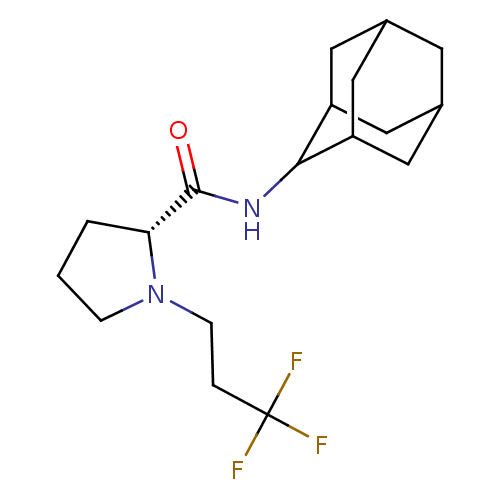
((2R)-N-(adamantan-2-yl)-1-(3,3,3-trifluoropropyl)p...)Show SMILES FC(F)(F)CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(-5.42,-32.99,;-4.79,-34.4,;-3.25,-34.55,;-4.03,-33.05,;-5.69,-35.65,;-5.05,-37.05,;-5.95,-38.3,;-7.48,-38.31,;-7.95,-39.77,;-6.71,-40.68,;-5.5,-39.7,;-3.99,-40.21,;-2.65,-39.46,;-4.02,-41.75,;-5.36,-42.5,;-5.37,-44.03,;-6.77,-44.38,;-8.11,-43.89,;-9.31,-45.17,;-7.8,-44.74,;-6.39,-45.31,;-7.81,-43.16,;-6.76,-41.92,;-8.12,-42.4,)| Show InChI InChI=1S/C18H27F3N2O/c19-18(20,21)3-5-23-4-1-2-15(23)17(24)22-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,1-10H2,(H,22,24)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317217
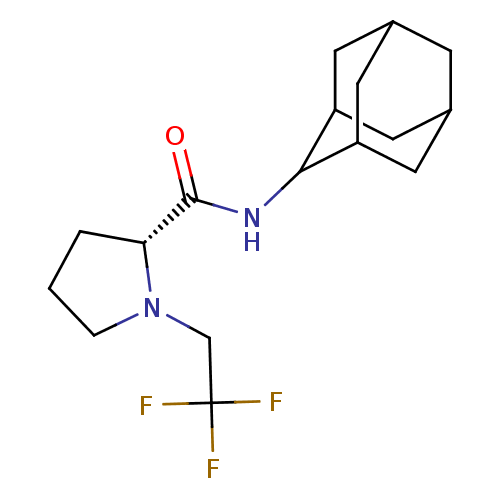
((2R)-N-(adamantan-2-yl)-1-(2,2,2-trifluoroethyl)py...)Show SMILES FC(F)(F)CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:9.10,TLB:19:18:22:15.14.13,19:14:17.18.20:22,THB:12:13:17.18.20:22,13:14:17:20.21.22,13:21:17:15.19.14,(27.97,-20.63,;27.2,-21.96,;25.66,-21.96,;26.42,-20.62,;27.97,-23.29,;27.07,-24.54,;25.53,-24.56,;25.07,-26.02,;26.31,-26.92,;27.52,-25.95,;29.03,-26.46,;30.37,-25.71,;29,-28,;27.66,-28.74,;27.65,-30.27,;26.25,-30.63,;24.91,-30.13,;23.71,-31.41,;25.22,-30.99,;26.63,-31.56,;25.21,-29.4,;26.26,-28.16,;24.9,-28.65,)| Show InChI InChI=1S/C17H25F3N2O/c18-17(19,20)9-22-3-1-2-14(22)16(23)21-15-12-5-10-4-11(7-12)8-13(15)6-10/h10-15H,1-9H2,(H,21,23)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317209
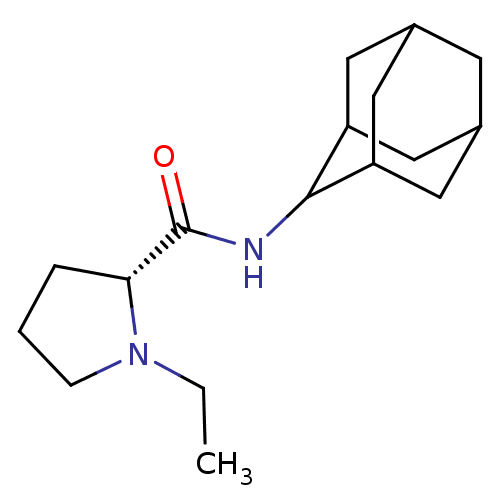
((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317211

((2R)-N-(adamantan-2-yl)-1-propylpyrrolidine-2-carb...)Show SMILES CCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(3.7,-6.03,;2.79,-7.28,;3.42,-8.69,;2.52,-9.94,;.99,-9.95,;.52,-11.41,;1.76,-12.31,;2.97,-11.34,;4.48,-11.85,;5.83,-11.1,;4.45,-13.39,;3.11,-14.13,;3.1,-15.67,;1.7,-16.02,;.36,-15.52,;-.84,-16.81,;.67,-16.38,;2.08,-16.95,;.66,-14.8,;1.71,-13.55,;.35,-14.04,)| Show InChI InChI=1S/C18H30N2O/c1-2-5-20-6-3-4-16(20)18(21)19-17-14-8-12-7-13(10-14)11-15(17)9-12/h12-17H,2-11H2,1H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121472
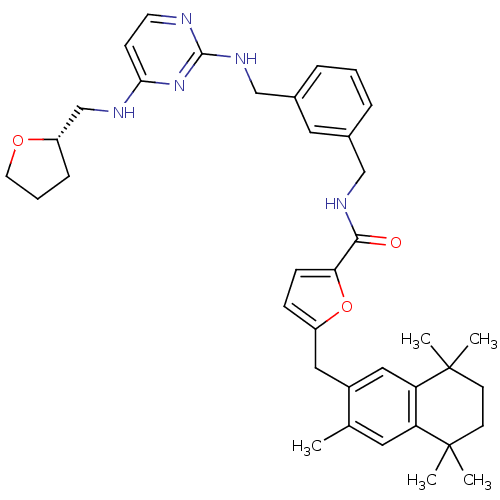
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3nccc(NC[C@@H]4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-22-26-8-6-9-27(19-26)23-42-36-39-16-13-34(43-36)40-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43)/t30-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317212
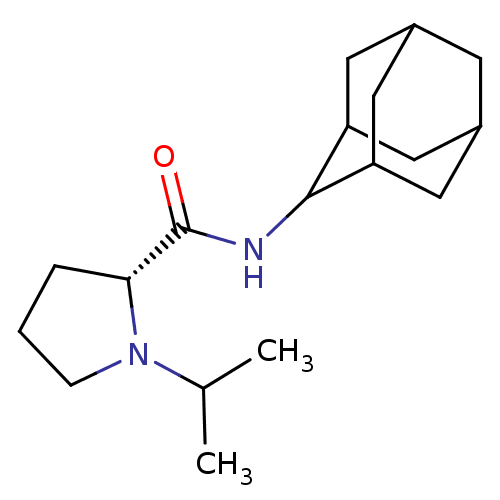
((2R)-N-(adamantan-2-yl)-1-(propan-2-yl)pyrrolidine...)Show SMILES CC(C)N1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(12.99,-6.92,;13.62,-8.33,;15.15,-8.49,;12.71,-9.58,;11.18,-9.59,;10.72,-11.05,;11.95,-11.95,;13.17,-10.98,;14.67,-11.49,;16.02,-10.74,;14.65,-13.03,;13.3,-13.78,;13.3,-15.31,;11.9,-15.66,;10.56,-15.17,;9.36,-16.45,;10.87,-16.02,;12.28,-16.59,;10.86,-14.44,;11.9,-13.19,;10.55,-13.68,)| Show InChI InChI=1S/C18H30N2O/c1-11(2)20-5-3-4-16(20)18(21)19-17-14-7-12-6-13(9-14)10-15(17)8-12/h11-17H,3-10H2,1-2H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380314
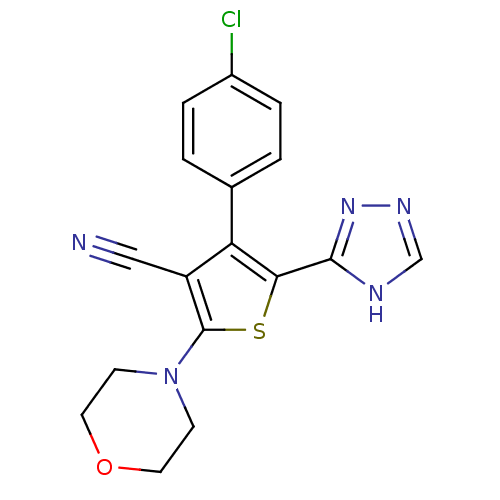
(CHEMBL2017648)Show SMILES Clc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1nnc[nH]1 Show InChI InChI=1S/C17H14ClN5OS/c18-12-3-1-11(2-4-12)14-13(9-19)17(23-5-7-24-8-6-23)25-15(14)16-20-10-21-22-16/h1-4,10H,5-8H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380315
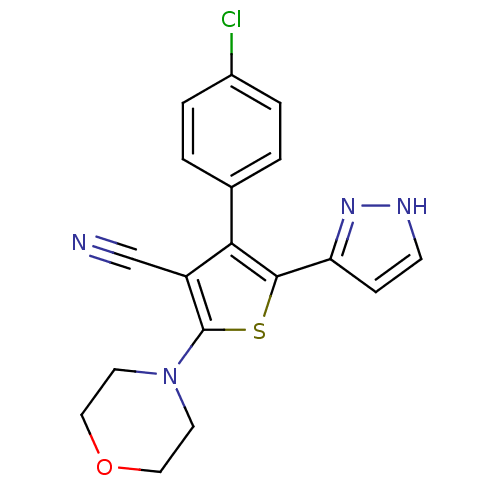
(CHEMBL2017649)Show SMILES Clc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1cc[nH]n1 Show InChI InChI=1S/C18H15ClN4OS/c19-13-3-1-12(2-4-13)16-14(11-20)18(23-7-9-24-10-8-23)25-17(16)15-5-6-21-22-15/h1-6H,7-10H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317219
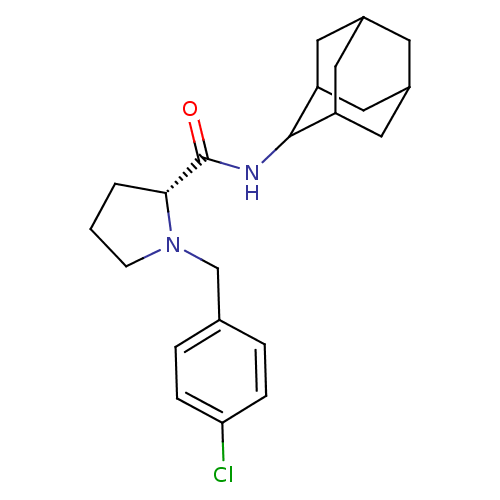
((2R)-N-(adamantan-2-yl)-1-[(4-chlorophenyl)methyl]...)Show SMILES Clc1ccc(CN2CCC[C@@H]2C(=O)NC2C3CC4CC(C3)CC2C4)cc1 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(2.87,-33.68,;3.64,-35.01,;5.18,-35.02,;5.94,-36.36,;5.16,-37.69,;5.93,-39.03,;5.02,-40.28,;3.49,-40.29,;3.03,-41.75,;4.27,-42.66,;5.48,-41.68,;6.99,-42.19,;8.34,-41.44,;6.96,-43.73,;5.62,-44.48,;5.61,-46.01,;4.21,-46.36,;2.87,-45.87,;1.67,-47.15,;3.18,-46.72,;4.59,-47.29,;3.17,-45.14,;4.22,-43.9,;2.86,-44.38,;3.63,-37.69,;2.86,-36.35,)| Show InChI InChI=1S/C22H29ClN2O/c23-19-5-3-14(4-6-19)13-25-7-1-2-20(25)22(26)24-21-17-9-15-8-16(11-17)12-18(21)10-15/h3-6,15-18,20-21H,1-2,7-13H2,(H,24,26)/t15?,16?,17?,18?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317210
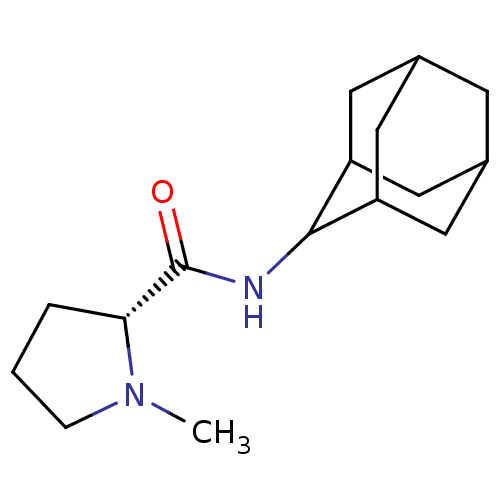
((2R)-N-(adamantan-2-yl)-1-methylpyrrolidine-2-carb...)Show SMILES CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:5.6,TLB:15:14:18:11.10.9,15:10:13.14.16:18,THB:8:9:13.14.16:18,9:10:13:16.17.18,9:17:13:11.15.10,(-4.95,-6.94,;-5.86,-8.19,;-7.4,-8.21,;-7.87,-9.67,;-6.63,-10.58,;-5.41,-9.6,;-3.89,-10.12,;-2.54,-9.36,;-3.91,-11.66,;-5.26,-12.4,;-5.27,-13.94,;-6.68,-14.3,;-8.02,-13.8,;-9.23,-15.09,;-7.71,-14.66,;-6.29,-15.23,;-7.72,-13.07,;-6.67,-11.82,;-8.03,-12.3,)| Show InChI InChI=1S/C16H26N2O/c1-18-4-2-3-14(18)16(19)17-15-12-6-10-5-11(8-12)9-13(15)7-10/h10-15H,2-9H2,1H3,(H,17,19)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317216
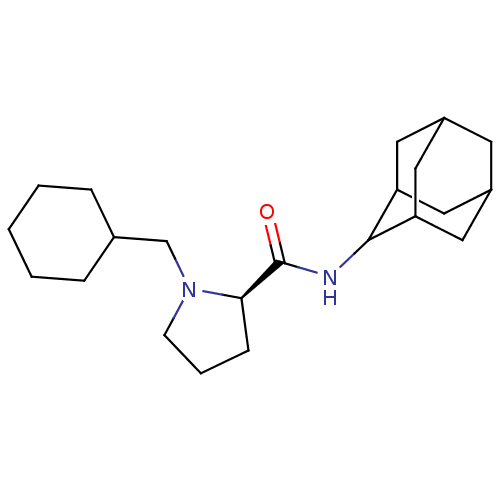
((2R)-N-(adamantan-2-yl)-1-(cyclohexylmethyl)pyrrol...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(18.88,-25.64,;17.53,-26.39,;17.51,-27.93,;16.16,-28.68,;16.15,-30.21,;14.75,-30.56,;13.41,-30.07,;12.22,-31.35,;13.72,-30.92,;15.13,-31.49,;13.71,-29.34,;14.76,-28.1,;13.4,-28.58,;16.02,-25.88,;14.81,-26.86,;13.57,-25.95,;14.04,-24.49,;15.57,-24.48,;16.47,-23.23,;15.7,-21.89,;16.48,-20.57,;15.71,-19.24,;14.17,-19.23,;13.4,-20.56,;14.17,-21.9,)| Show InChI InChI=1S/C22H36N2O/c25-22(20-7-4-8-24(20)14-15-5-2-1-3-6-15)23-21-18-10-16-9-17(12-18)13-19(21)11-16/h15-21H,1-14H2,(H,23,25)/t16?,17?,18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121475
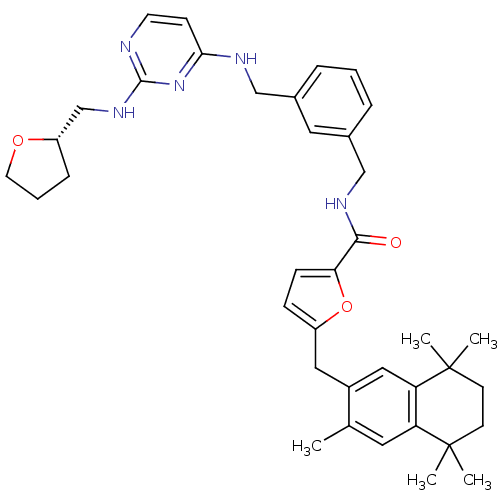
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3ccnc(NC[C@@H]4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-23-27-9-6-8-26(19-27)22-40-34-13-16-39-36(43-34)42-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43)/t30-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380316
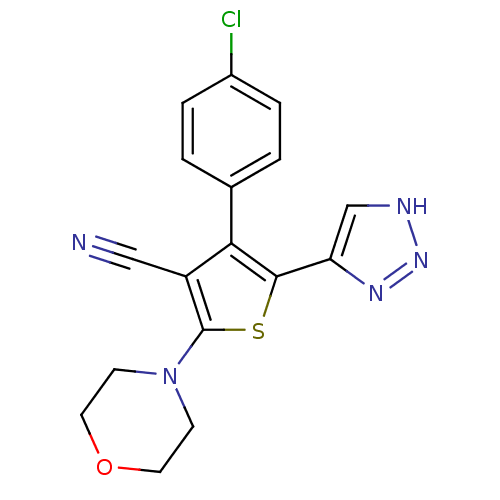
(CHEMBL2017650)Show SMILES Clc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1c[nH]nn1 Show InChI InChI=1S/C17H14ClN5OS/c18-12-3-1-11(2-4-12)15-13(9-19)17(23-5-7-24-8-6-23)25-16(15)14-10-20-22-21-14/h1-4,10H,5-8H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121461
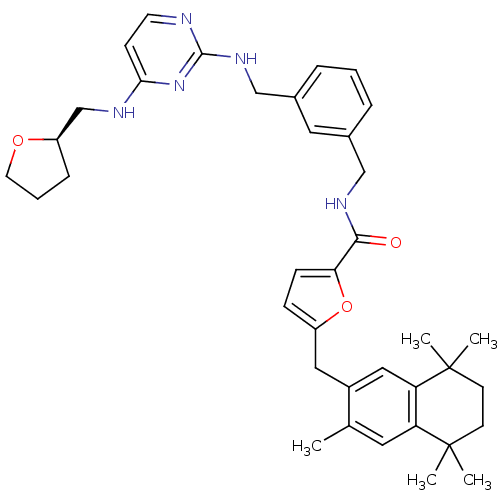
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3nccc(NC[C@H]4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-22-26-8-6-9-27(19-26)23-42-36-39-16-13-34(43-36)40-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43)/t30-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317208
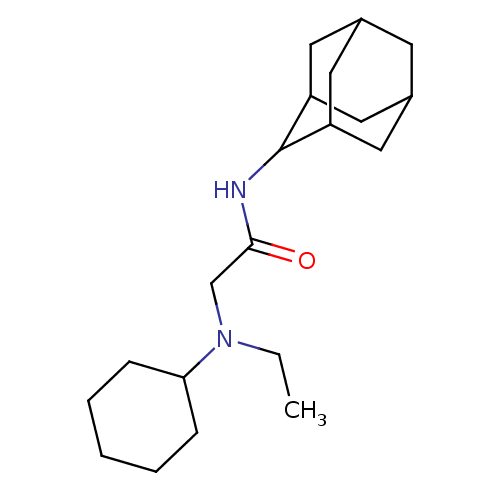
(CHEMBL1088115 | N-(adamantan-2-yl)-2-[cyclohexyl(e...)Show SMILES CCN(CC(=O)NC1C2CC3CC(C2)CC1C3)C1CCCCC1 |TLB:13:12:16:9.8.7,13:8:11.12.14:16,THB:6:7:11.12.14:16,7:8:11:14.15.16,7:15:11:9.13.8,(27.37,5.32,;27.37,3.78,;28.7,3,;28.71,1.46,;30.04,.69,;31.38,1.46,;30.04,-.85,;28.7,-1.62,;28.7,-3.14,;27.29,-3.49,;25.97,-3.01,;24.77,-4.28,;26.26,-3.86,;27.67,-4.42,;26.25,-2.27,;27.3,-1.05,;25.96,-1.52,;30.04,3.78,;31.38,3.01,;32.71,3.78,;32.71,5.31,;31.38,6.08,;30.04,5.31,)| Show InChI InChI=1S/C20H34N2O/c1-2-22(18-6-4-3-5-7-18)13-19(23)21-20-16-9-14-8-15(11-16)12-17(20)10-14/h14-18,20H,2-13H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317216

((2R)-N-(adamantan-2-yl)-1-(cyclohexylmethyl)pyrrol...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(18.88,-25.64,;17.53,-26.39,;17.51,-27.93,;16.16,-28.68,;16.15,-30.21,;14.75,-30.56,;13.41,-30.07,;12.22,-31.35,;13.72,-30.92,;15.13,-31.49,;13.71,-29.34,;14.76,-28.1,;13.4,-28.58,;16.02,-25.88,;14.81,-26.86,;13.57,-25.95,;14.04,-24.49,;15.57,-24.48,;16.47,-23.23,;15.7,-21.89,;16.48,-20.57,;15.71,-19.24,;14.17,-19.23,;13.4,-20.56,;14.17,-21.9,)| Show InChI InChI=1S/C22H36N2O/c25-22(20-7-4-8-24(20)14-15-5-2-1-3-6-15)23-21-18-10-16-9-17(12-18)13-19(21)11-16/h15-21H,1-14H2,(H,23,25)/t16?,17?,18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317218

((2R)-N-(adamantan-2-yl)-1-(3,3,3-trifluoropropyl)p...)Show SMILES FC(F)(F)CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(-5.42,-32.99,;-4.79,-34.4,;-3.25,-34.55,;-4.03,-33.05,;-5.69,-35.65,;-5.05,-37.05,;-5.95,-38.3,;-7.48,-38.31,;-7.95,-39.77,;-6.71,-40.68,;-5.5,-39.7,;-3.99,-40.21,;-2.65,-39.46,;-4.02,-41.75,;-5.36,-42.5,;-5.37,-44.03,;-6.77,-44.38,;-8.11,-43.89,;-9.31,-45.17,;-7.8,-44.74,;-6.39,-45.31,;-7.81,-43.16,;-6.76,-41.92,;-8.12,-42.4,)| Show InChI InChI=1S/C18H27F3N2O/c19-18(20,21)3-5-23-4-1-2-15(23)17(24)22-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,1-10H2,(H,22,24)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317222
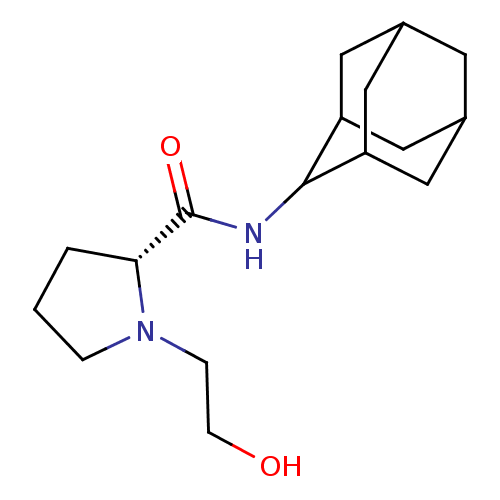
((2R)-N-(adamantan-2-yl)-1-(2-hydroxyethyl)pyrrolid...)Show SMILES OCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(-3.11,5.86,;-3.88,4.52,;-5.43,4.52,;-6.21,3.18,;-7.75,3.17,;-8.21,1.7,;-6.97,.79,;-5.75,1.77,;-4.23,1.26,;-2.88,2.01,;-4.26,-.28,;-5.6,-1.03,;-5.61,-2.57,;-7.02,-2.92,;-8.36,-2.43,;-9.57,-3.71,;-8.06,-3.29,;-6.63,-3.86,;-8.06,-1.69,;-7.01,-.44,;-8.38,-.93,)| Show InChI InChI=1S/C17H28N2O2/c20-5-4-19-3-1-2-15(19)17(21)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16,20H,1-10H2,(H,18,21)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121484
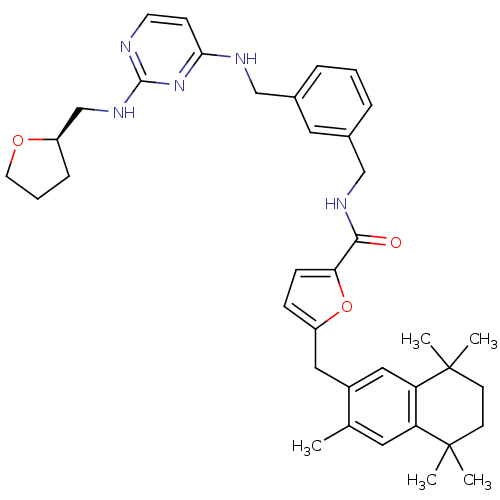
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3ccnc(NC[C@H]4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-23-27-9-6-8-26(19-27)22-40-34-13-16-39-36(43-34)42-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43)/t30-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121484

(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3ccnc(NC[C@H]4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-23-27-9-6-8-26(19-27)22-40-34-13-16-39-36(43-34)42-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43)/t30-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competitive inhibition of GnRH-stimulated extracellular acidification in cells expressing recombinant rat GnRH receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317210

((2R)-N-(adamantan-2-yl)-1-methylpyrrolidine-2-carb...)Show SMILES CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:5.6,TLB:15:14:18:11.10.9,15:10:13.14.16:18,THB:8:9:13.14.16:18,9:10:13:16.17.18,9:17:13:11.15.10,(-4.95,-6.94,;-5.86,-8.19,;-7.4,-8.21,;-7.87,-9.67,;-6.63,-10.58,;-5.41,-9.6,;-3.89,-10.12,;-2.54,-9.36,;-3.91,-11.66,;-5.26,-12.4,;-5.27,-13.94,;-6.68,-14.3,;-8.02,-13.8,;-9.23,-15.09,;-7.71,-14.66,;-6.29,-15.23,;-7.72,-13.07,;-6.67,-11.82,;-8.03,-12.3,)| Show InChI InChI=1S/C16H26N2O/c1-18-4-2-3-14(18)16(19)17-15-12-6-10-5-11(8-12)9-13(15)7-10/h10-15H,2-9H2,1H3,(H,17,19)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121464
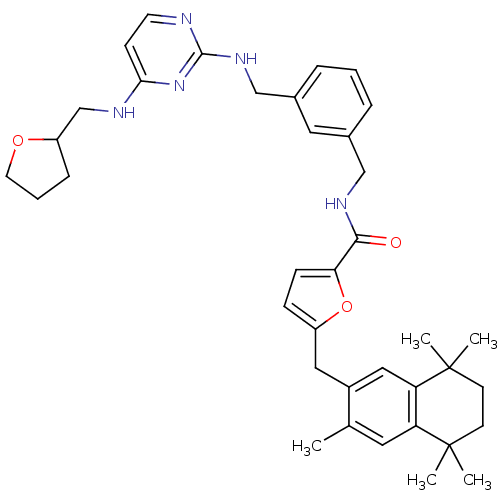
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3nccc(NCC4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-22-26-8-6-9-27(19-26)23-42-36-39-16-13-34(43-36)40-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317219

((2R)-N-(adamantan-2-yl)-1-[(4-chlorophenyl)methyl]...)Show SMILES Clc1ccc(CN2CCC[C@@H]2C(=O)NC2C3CC4CC(C3)CC2C4)cc1 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(2.87,-33.68,;3.64,-35.01,;5.18,-35.02,;5.94,-36.36,;5.16,-37.69,;5.93,-39.03,;5.02,-40.28,;3.49,-40.29,;3.03,-41.75,;4.27,-42.66,;5.48,-41.68,;6.99,-42.19,;8.34,-41.44,;6.96,-43.73,;5.62,-44.48,;5.61,-46.01,;4.21,-46.36,;2.87,-45.87,;1.67,-47.15,;3.18,-46.72,;4.59,-47.29,;3.17,-45.14,;4.22,-43.9,;2.86,-44.38,;3.63,-37.69,;2.86,-36.35,)| Show InChI InChI=1S/C22H29ClN2O/c23-19-5-3-14(4-6-19)13-25-7-1-2-20(25)22(26)24-21-17-9-15-8-16(11-17)12-18(21)10-15/h3-6,15-18,20-21H,1-2,7-13H2,(H,24,26)/t15?,16?,17?,18?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121462
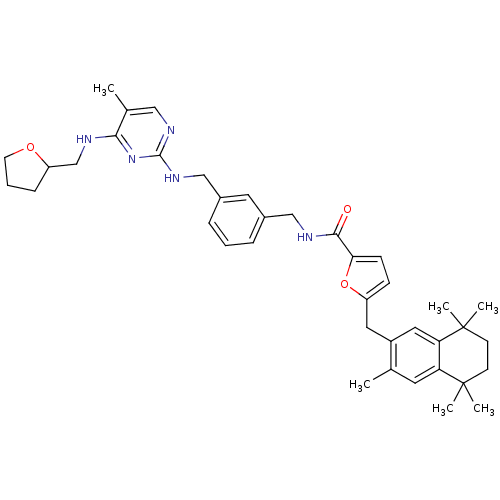
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cnc(NCc2cccc(CNC(=O)c3ccc(Cc4cc5c(cc4C)C(C)(C)CCC5(C)C)o3)c2)nc1NCC1CCCO1 Show InChI InChI=1S/C39H49N5O3/c1-25-17-32-33(39(5,6)15-14-38(32,3)4)20-29(25)19-30-12-13-34(47-30)36(45)41-22-27-9-7-10-28(18-27)23-43-37-42-21-26(2)35(44-37)40-24-31-11-8-16-46-31/h7,9-10,12-13,17-18,20-21,31H,8,11,14-16,19,22-24H2,1-6H3,(H,41,45)(H2,40,42,43,44) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121466
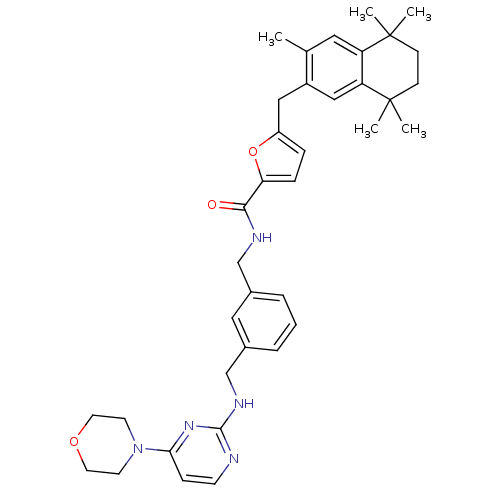
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3nccc(n3)N3CCOCC3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C37H45N5O3/c1-25-19-30-31(37(4,5)13-12-36(30,2)3)22-28(25)21-29-9-10-32(45-29)34(43)39-23-26-7-6-8-27(20-26)24-40-35-38-14-11-33(41-35)42-15-17-44-18-16-42/h6-11,14,19-20,22H,12-13,15-18,21,23-24H2,1-5H3,(H,39,43)(H,38,40,41) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50121472

(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3nccc(NC[C@@H]4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-22-26-8-6-9-27(19-26)23-42-36-39-16-13-34(43-36)40-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121470
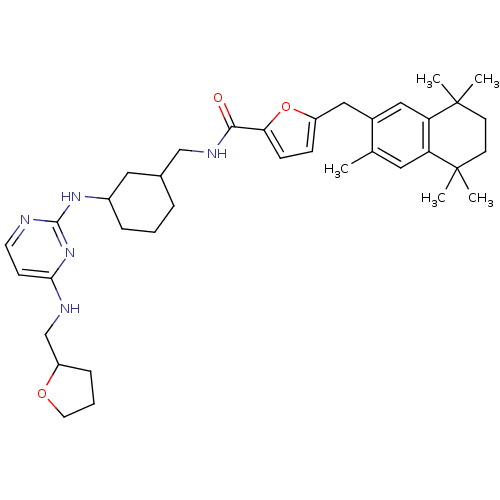
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCC1CCCC(C1)Nc1nccc(NCC3CCCO3)n1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C37H51N5O3/c1-24-18-30-31(37(4,5)15-14-36(30,2)3)21-26(24)20-28-11-12-32(45-28)34(43)40-22-25-8-6-9-27(19-25)41-35-38-16-13-33(42-35)39-23-29-10-7-17-44-29/h11-13,16,18,21,25,27,29H,6-10,14-15,17,19-20,22-23H2,1-5H3,(H,40,43)(H2,38,39,41,42) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50121484

(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3ccnc(NC[C@H]4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-23-27-9-6-8-26(19-27)22-40-34-13-16-39-36(43-34)42-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43)/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317212

((2R)-N-(adamantan-2-yl)-1-(propan-2-yl)pyrrolidine...)Show SMILES CC(C)N1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(12.99,-6.92,;13.62,-8.33,;15.15,-8.49,;12.71,-9.58,;11.18,-9.59,;10.72,-11.05,;11.95,-11.95,;13.17,-10.98,;14.67,-11.49,;16.02,-10.74,;14.65,-13.03,;13.3,-13.78,;13.3,-15.31,;11.9,-15.66,;10.56,-15.17,;9.36,-16.45,;10.87,-16.02,;12.28,-16.59,;10.86,-14.44,;11.9,-13.19,;10.55,-13.68,)| Show InChI InChI=1S/C18H30N2O/c1-11(2)20-5-3-4-16(20)18(21)19-17-14-7-12-6-13(9-14)10-15(17)8-12/h11-17H,3-10H2,1-2H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50121475

(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NCc1cccc(CNc3ccnc(NC[C@@H]4CCCO4)n3)c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C38H47N5O3/c1-25-18-31-32(38(4,5)15-14-37(31,2)3)21-28(25)20-29-11-12-33(46-29)35(44)41-23-27-9-6-8-26(19-27)22-40-34-13-16-39-36(43-34)42-24-30-10-7-17-45-30/h6,8-9,11-13,16,18-19,21,30H,7,10,14-15,17,20,22-24H2,1-5H3,(H,41,44)(H2,39,40,42,43)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50121483
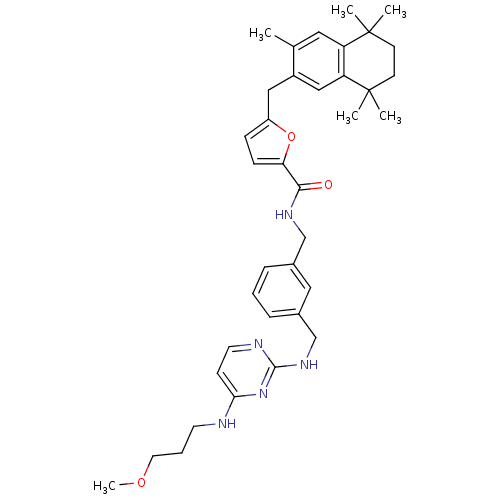
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES COCCCNc1ccnc(NCc2cccc(CNC(=O)c3ccc(Cc4cc5c(cc4C)C(C)(C)CCC5(C)C)o3)c2)n1 Show InChI InChI=1S/C37H47N5O3/c1-25-19-30-31(37(4,5)15-14-36(30,2)3)22-28(25)21-29-11-12-32(45-29)34(43)40-23-26-9-7-10-27(20-26)24-41-35-39-17-13-33(42-35)38-16-8-18-44-6/h7,9-13,17,19-20,22H,8,14-16,18,21,23-24H2,1-6H3,(H,40,43)(H2,38,39,41,42) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards rat gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50121483

(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES COCCCNc1ccnc(NCc2cccc(CNC(=O)c3ccc(Cc4cc5c(cc4C)C(C)(C)CCC5(C)C)o3)c2)n1 Show InChI InChI=1S/C37H47N5O3/c1-25-19-30-31(37(4,5)15-14-36(30,2)3)22-28(25)21-29-11-12-32(45-29)34(43)40-23-26-9-7-10-27(20-26)24-41-35-39-17-13-33(42-35)38-16-8-18-44-6/h7,9-13,17,19-20,22H,8,14-16,18,21,23-24H2,1-6H3,(H,40,43)(H2,38,39,41,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056217
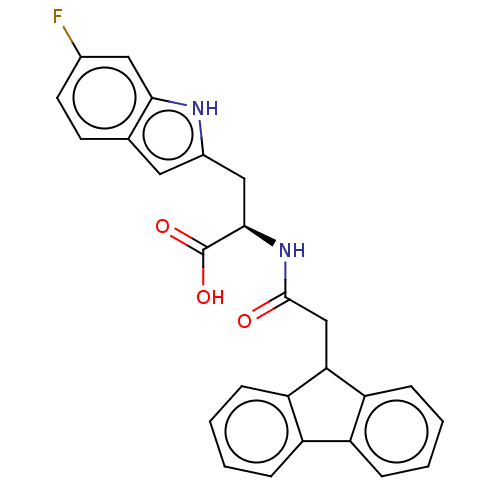
(CHEMBL3353369)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)CC1c2ccccc2-c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50121081
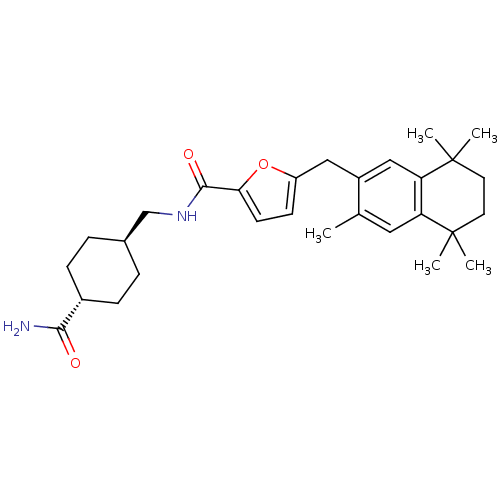
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NC[C@H]1CC[C@@H](CC1)C(N)=O)C(C)(C)CCC2(C)C |wU:20.25,wD:17.18,(9.62,-7.31,;8.27,-6.52,;6.94,-7.29,;5.61,-6.52,;5.61,-4.98,;6.94,-4.21,;8.27,-4.98,;9.62,-4.21,;9.62,-2.67,;10.78,-1.63,;10.16,-.24,;8.62,-.4,;8.29,-1.9,;7.61,.77,;6.1,.47,;8.1,2.22,;7.09,3.38,;5.58,3.08,;4.56,4.25,;3.06,3.96,;2.57,2.49,;3.57,1.33,;5.07,1.63,;1.05,2.19,;.03,3.33,;.56,.72,;4.28,-4.21,;5.61,-3.44,;2.94,-3.44,;2.94,-4.98,;2.94,-6.52,;4.28,-7.29,;5.37,-8.38,;2.78,-7.7,)| Show InChI InChI=1S/C29H40N2O3/c1-18-14-23-24(29(4,5)13-12-28(23,2)3)16-21(18)15-22-10-11-25(34-22)27(33)31-17-19-6-8-20(9-7-19)26(30)32/h10-11,14,16,19-20H,6-9,12-13,15,17H2,1-5H3,(H2,30,32)(H,31,33)/t19-,20- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3467-70 (2002)
BindingDB Entry DOI: 10.7270/Q20K27X7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380312

(CHEMBL2017647)Show InChI InChI=1S/C16H14ClN3O2S/c17-11-3-1-10(2-4-11)13-12(9-18)16(23-14(13)15(19)21)20-5-7-22-8-6-20/h1-4H,5-8H2,(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50121462

(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cnc(NCc2cccc(CNC(=O)c3ccc(Cc4cc5c(cc4C)C(C)(C)CCC5(C)C)o3)c2)nc1NCC1CCCO1 Show InChI InChI=1S/C39H49N5O3/c1-25-17-32-33(39(5,6)15-14-38(32,3)4)20-29(25)19-30-12-13-34(47-30)36(45)41-22-27-9-7-10-28(18-27)23-43-37-42-21-26(2)35(44-37)40-24-31-11-8-16-46-31/h7,9-10,12-13,17-18,20-21,31H,8,11,14-16,19,22-24H2,1-6H3,(H,41,45)(H2,40,42,43,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3635-9 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8DXZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317207

(CHEMBL1088517 | N-cyclohexyl-2-(7,8-dihydro-4H-iso...)Show InChI InChI=1S/C17H27N3O2/c1-2-20(15-6-4-3-5-7-15)17(21)12-19-10-8-14-13-22-18-16(14)9-11-19/h13,15H,2-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50121090
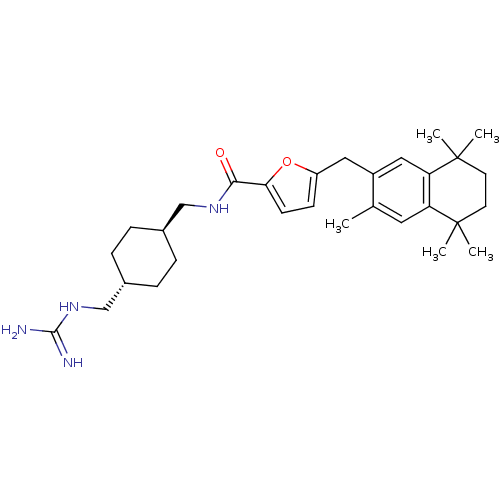
(5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Cc1ccc(o1)C(=O)NC[C@H]1CC[C@H](CNC(N)=N)CC1)C(C)(C)CCC2(C)C |wU:20.22,wD:17.18,(13.73,-14.94,;12.37,-14.16,;11.04,-14.93,;9.71,-14.16,;9.71,-12.62,;11.04,-11.85,;12.37,-12.62,;13.73,-11.85,;13.73,-10.31,;14.89,-9.27,;14.26,-7.89,;12.72,-8.03,;12.4,-9.54,;11.71,-6.87,;10.2,-7.17,;12.21,-5.42,;11.2,-4.25,;9.69,-4.56,;8.68,-3.39,;7.17,-3.67,;6.67,-5.14,;5.16,-5.46,;4.14,-4.3,;4.63,-2.85,;3.6,-1.7,;6.12,-2.54,;7.68,-6.31,;9.18,-6,;8.38,-11.85,;7.05,-11.08,;9.71,-11.08,;7.05,-12.62,;7.05,-14.16,;8.38,-14.93,;9.48,-16.01,;6.89,-15.34,)| Show InChI InChI=1S/C30H44N4O2/c1-19-14-24-25(30(4,5)13-12-29(24,2)3)16-22(19)15-23-10-11-26(36-23)27(35)33-17-20-6-8-21(9-7-20)18-34-28(31)32/h10-11,14,16,20-21H,6-9,12-13,15,17-18H2,1-5H3,(H,33,35)(H4,31,32,34)/t20-,21- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 12: 3467-70 (2002)
BindingDB Entry DOI: 10.7270/Q20K27X7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50380313

(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317224

((2R)-N-(adamantan-2-yl)-1-(oxan-4-ylmethyl)pyrroli...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCOCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(16.19,2.36,;14.84,1.61,;14.82,.07,;13.47,-.68,;13.46,-2.21,;12.06,-2.57,;10.72,-2.07,;9.51,-3.36,;11.02,-2.93,;12.44,-3.5,;11.01,-1.34,;12.06,-.09,;10.7,-.58,;13.33,2.12,;12.11,1.14,;10.87,2.05,;11.33,3.52,;12.87,3.53,;13.64,4.87,;15.19,4.87,;15.96,3.52,;17.5,3.52,;18.28,4.86,;17.51,6.2,;15.96,6.21,)| Show InChI InChI=1S/C21H34N2O2/c24-21(19-2-1-5-23(19)13-14-3-6-25-7-4-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data