

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50186527 ((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3859-63 (2006) Article DOI: 10.1016/j.bmcl.2006.04.031 BindingDB Entry DOI: 10.7270/Q2QV3M44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221547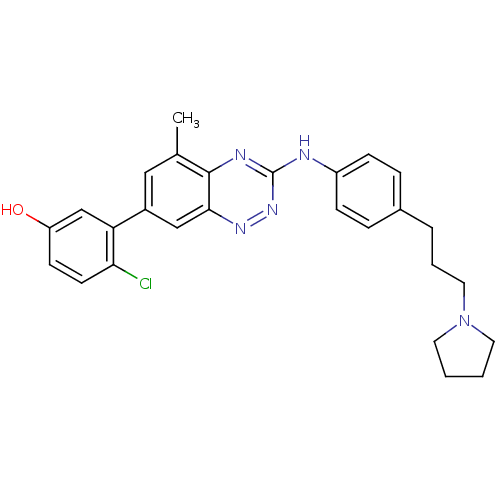 (4-chloro-3-(5-methyl-3-(4-(3-(pyrrolidin-1-yl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl | Bioorg Med Chem Lett 17: 5812-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.043 BindingDB Entry DOI: 10.7270/Q2M32VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408198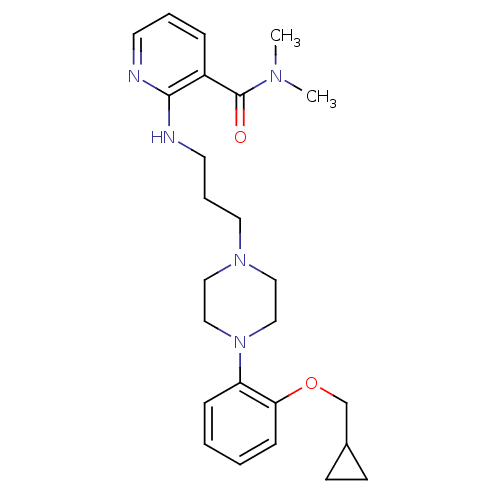 (CHEMBL91278) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126304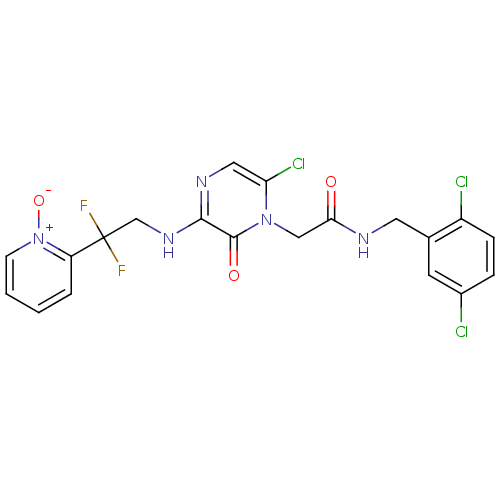 (2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | Bioorg Med Chem Lett 13: 1353-7 (2003) BindingDB Entry DOI: 10.7270/Q2833RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50186522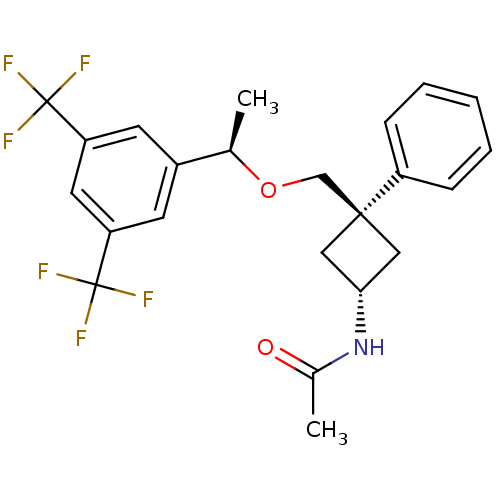 (CHEMBL379072 | trans-N-{3-[(R)-1-(3,5-bis-trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3859-63 (2006) Article DOI: 10.1016/j.bmcl.2006.04.031 BindingDB Entry DOI: 10.7270/Q2QV3M44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50060964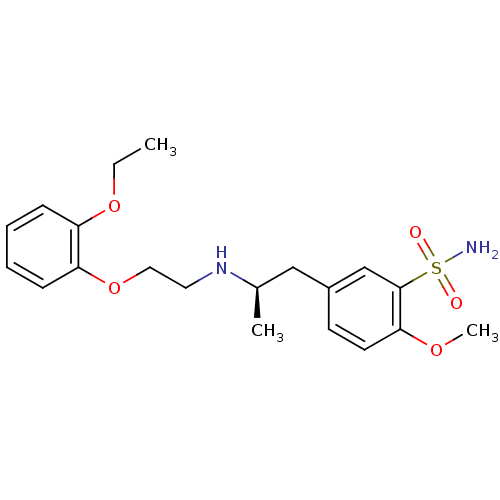 ((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408201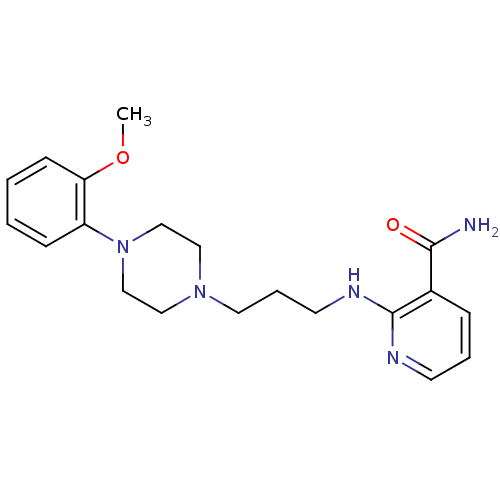 (CHEMBL88512) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328970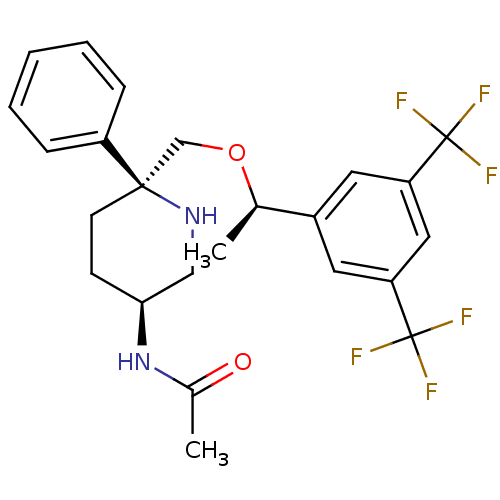 (CHEMBL1270066 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126303 (2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | Bioorg Med Chem Lett 13: 1353-7 (2003) BindingDB Entry DOI: 10.7270/Q2833RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126309 (CHEMBL29744 | N-(3-Bromo-benzyl)-2-{6-chloro-3-[2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | Bioorg Med Chem Lett 13: 1353-7 (2003) BindingDB Entry DOI: 10.7270/Q2833RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328981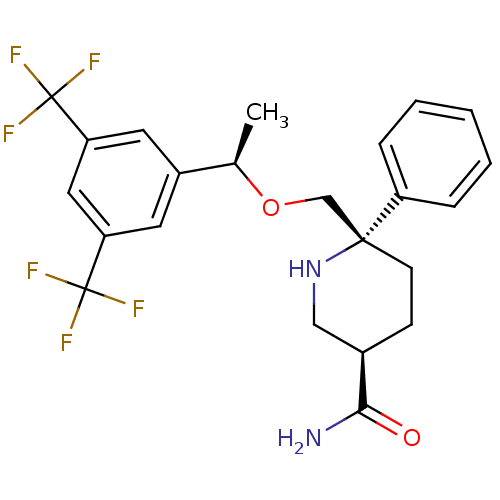 ((3R,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243427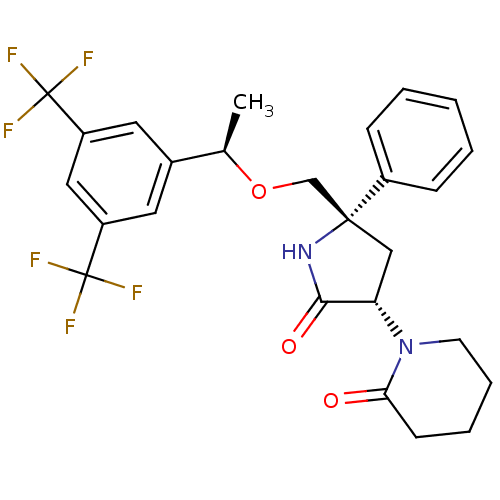 (1-((3S,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243425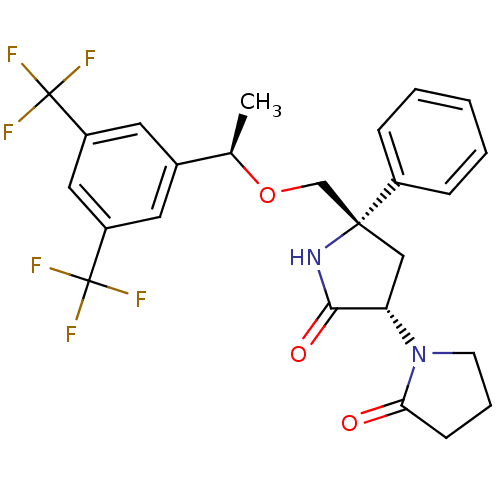 (1-((3S,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243428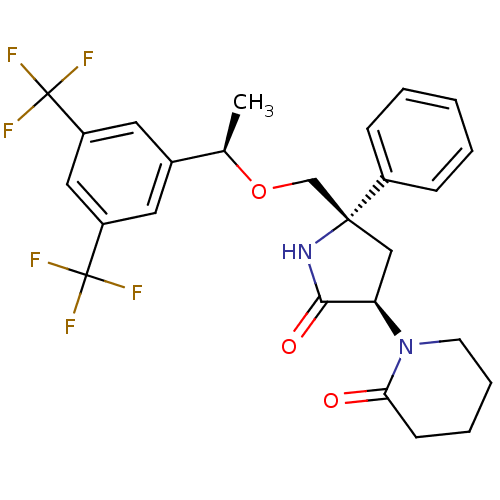 (1-((3R,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116745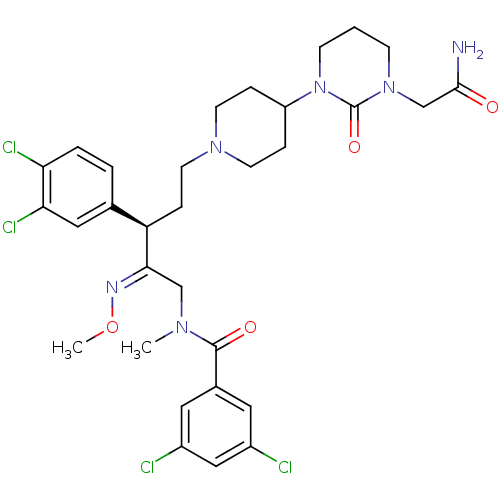 (CHEMBL78284 | N-{(R)-5-[4-(3-Carbamoylmethyl-2-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against recombinant human tachykinin receptor 2 in CHO cells using [3H]-NKA as radioligand | Bioorg Med Chem Lett 12: 2355-8 (2002) BindingDB Entry DOI: 10.7270/Q26T0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27210 ((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27208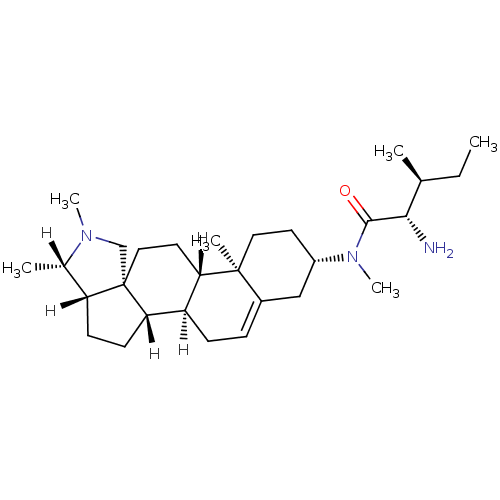 ((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328980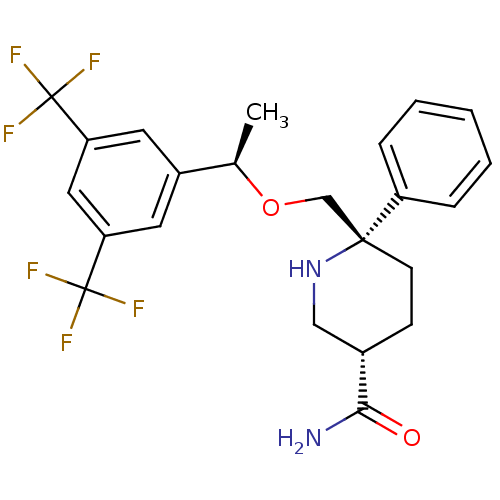 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50393252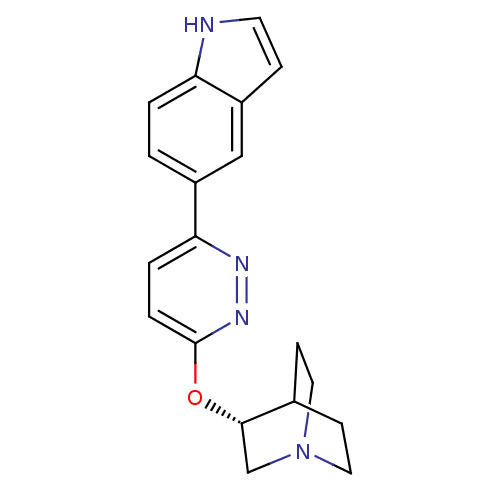 (CHEMBL2151570) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mahidol University Curated by ChEMBL | Assay Description Displacement of [3H]A-585539 from alpha7 nAChR in human cerebral cortex membranes after 75 mins by scintillation counting | ACS Med Chem Lett 7: 890-895 (2016) Article DOI: 10.1021/acsmedchemlett.6b00146 BindingDB Entry DOI: 10.7270/Q2ZW1QDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328973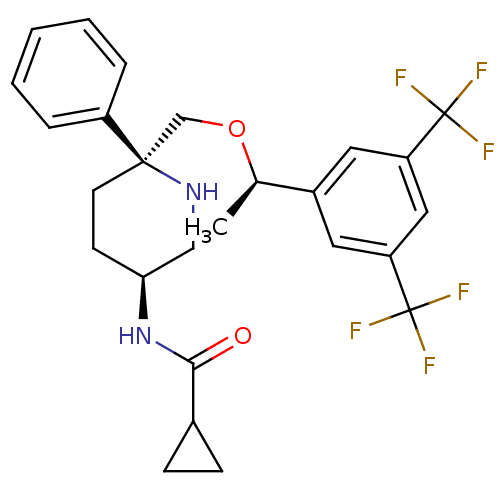 (CHEMBL1270175 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50252829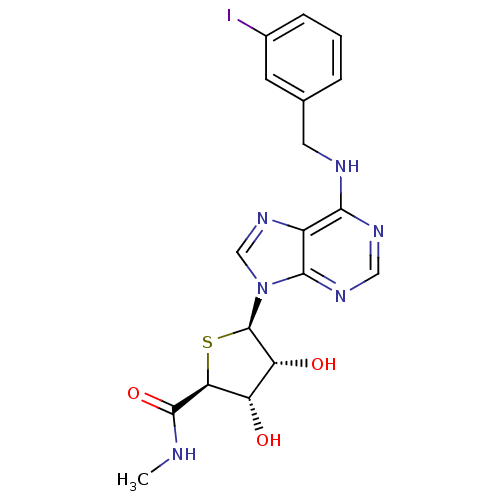 ((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-9H-purin-9-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328982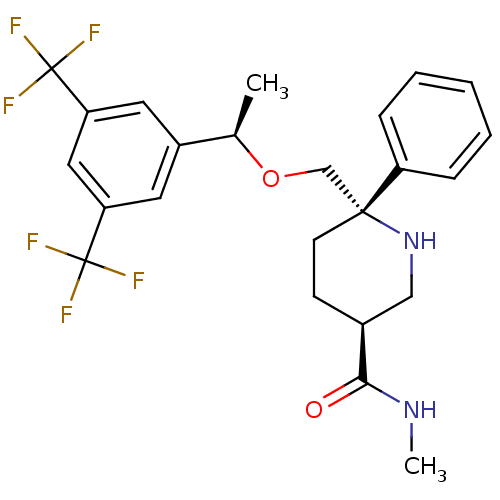 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328974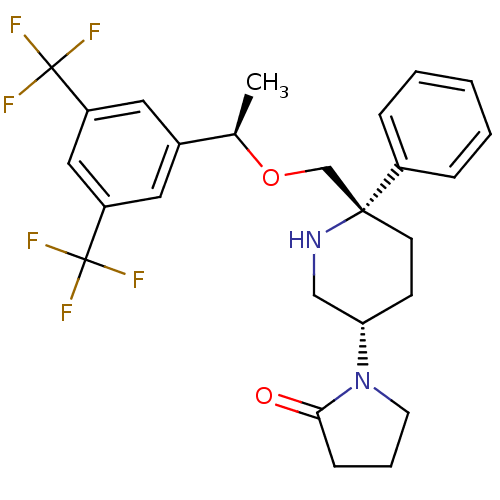 (1-((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126300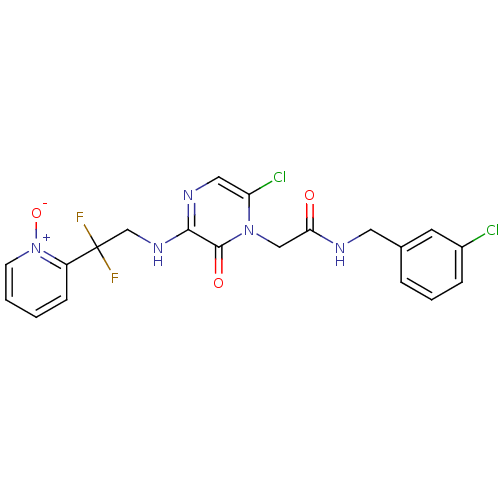 (CHEMBL25845 | N-(3-Chloro-benzyl)-2-{6-chloro-3-[2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | Bioorg Med Chem Lett 13: 1353-7 (2003) BindingDB Entry DOI: 10.7270/Q2833RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328979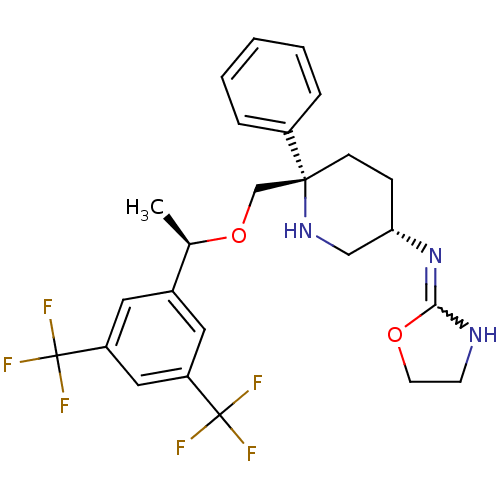 (CHEMBL1270465 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328972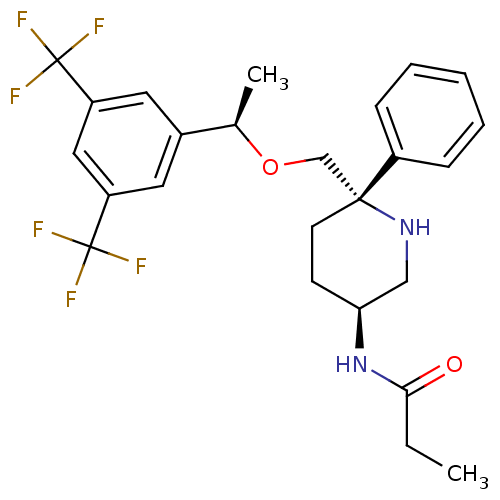 (CHEMBL1270174 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50116745 (CHEMBL78284 | N-{(R)-5-[4-(3-Carbamoylmethyl-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against recombinant human tachykinin receptor 1 in CHO cells using [3H]-Sar SP as radioligand | Bioorg Med Chem Lett 12: 2355-8 (2002) BindingDB Entry DOI: 10.7270/Q26T0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50116722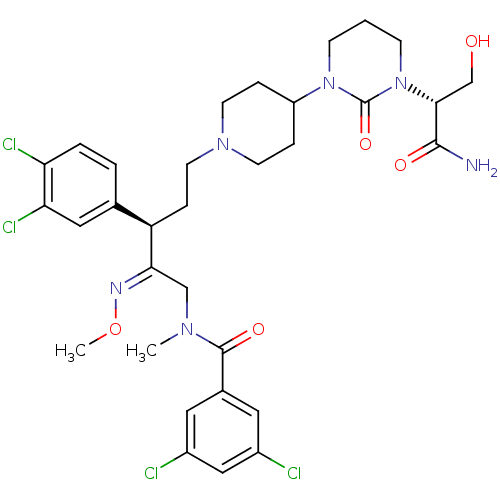 (CHEMBL263243 | N-{(R)-5-{4-[3-((R)-1-Carbamoyl-2-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against recombinant human tachykinin receptor 3 in CHO cells using [125I]-[MePhe]-NKB as radioligand | Bioorg Med Chem Lett 12: 2355-8 (2002) BindingDB Entry DOI: 10.7270/Q26T0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50116717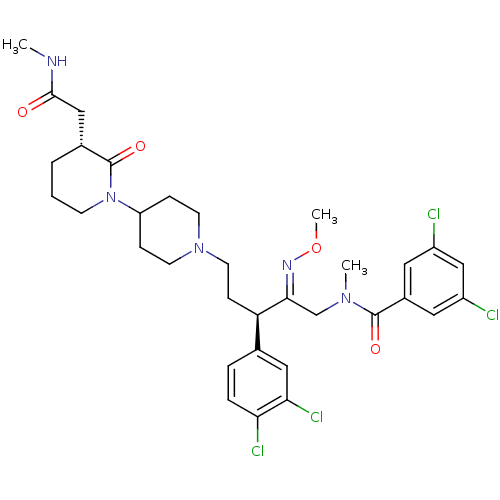 (3,5-Dichloro-N-[(R)-3-(3,4-dichloro-phenyl)-2-[(Z)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against recombinant human tachykinin receptor 3 in CHO cells using [125I]-[MePhe]-NKB as radioligand | Bioorg Med Chem Lett 12: 2355-8 (2002) BindingDB Entry DOI: 10.7270/Q26T0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50116736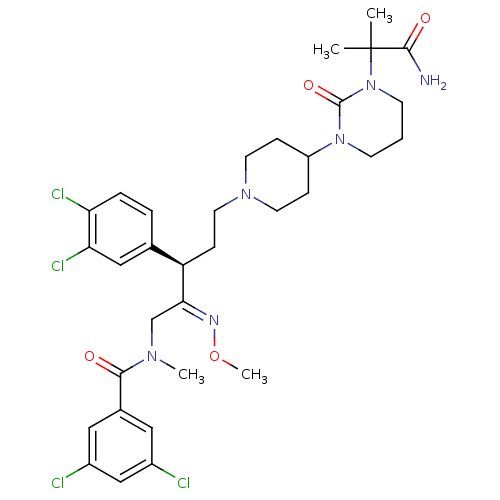 (CHEMBL74956 | N-{(R)-5-{4-[3-(1-Carbamoyl-1-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against recombinant human tachykinin receptor 3 in CHO cells using [125I]-[MePhe]-NKB as radioligand | Bioorg Med Chem Lett 12: 2355-8 (2002) BindingDB Entry DOI: 10.7270/Q26T0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328988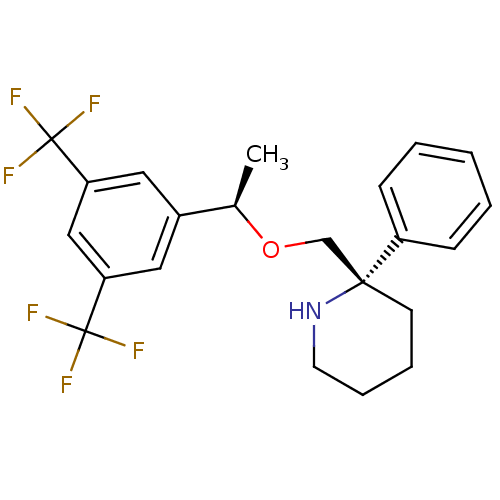 ((S)-2-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243376 (CHEMBL452532 | N-((3R,5S)-5-(((R)-1-(3,5-bis(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243375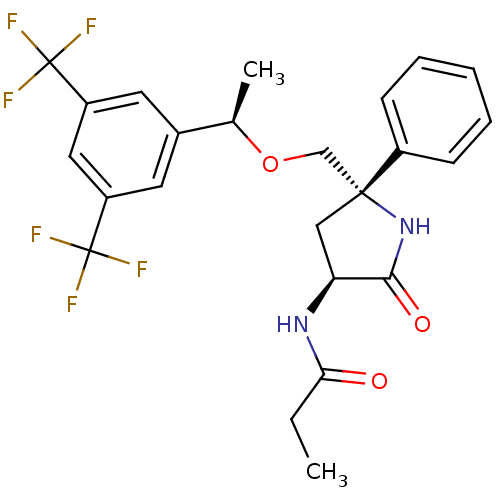 (CHEMBL451765 | N-((3S,5S)-5-(((R)-1-(3,5-bis(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50243337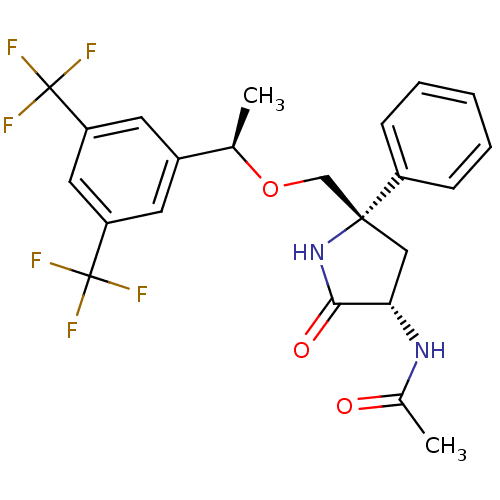 (CHEMBL452270 | N-((3S,5S)-5-(((R)-1-(3,5-bis(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 4168-71 (2008) Article DOI: 10.1016/j.bmcl.2008.05.082 BindingDB Entry DOI: 10.7270/Q2D79B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50186525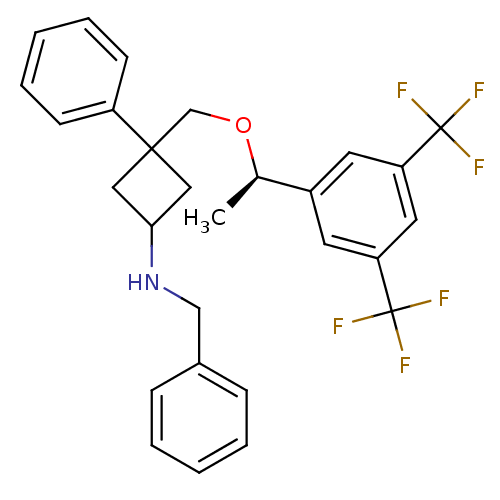 ((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3859-63 (2006) Article DOI: 10.1016/j.bmcl.2006.04.031 BindingDB Entry DOI: 10.7270/Q2QV3M44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50186528 (CHEMBL212112 | cis-1-{3-[(R)-1-(3,5-bis-trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3859-63 (2006) Article DOI: 10.1016/j.bmcl.2006.04.031 BindingDB Entry DOI: 10.7270/Q2QV3M44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221559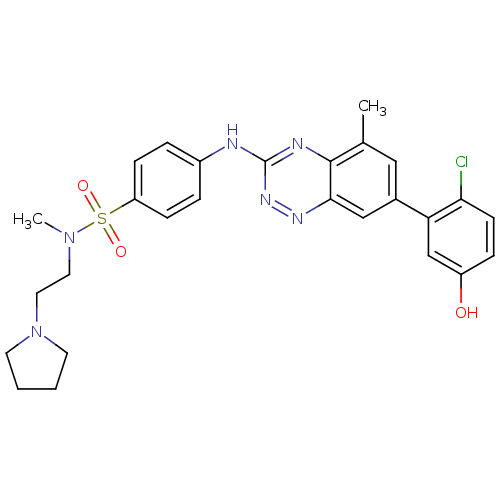 (4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl | Bioorg Med Chem Lett 17: 5812-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.043 BindingDB Entry DOI: 10.7270/Q2M32VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328978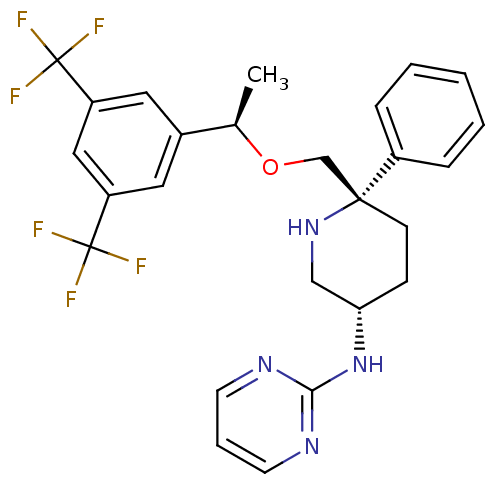 (CHEMBL1270464 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from rat adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328986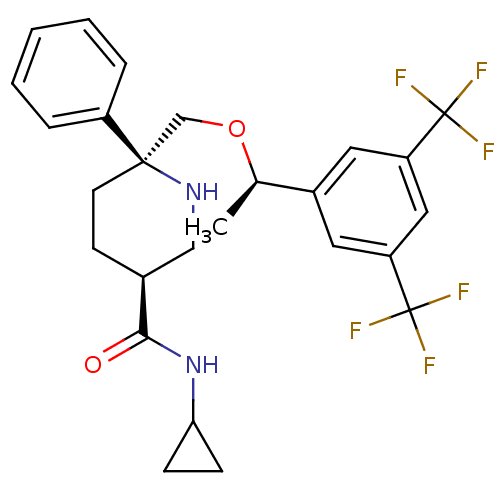 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328971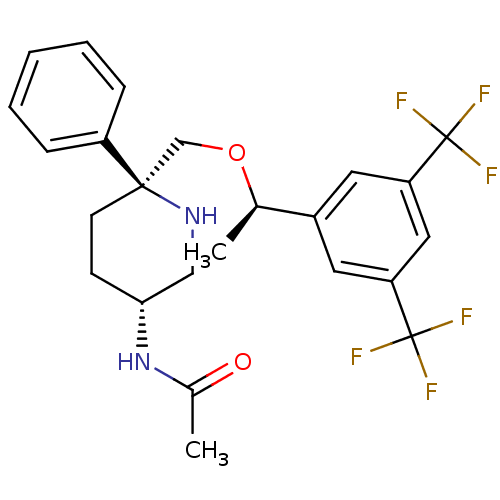 (CHEMBL1270067 | N-((3R,6S)-6-(((R)-1-(3,5-bis(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50328984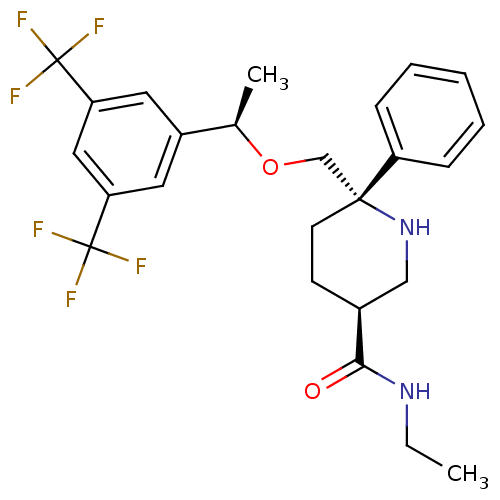 ((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6313-5 (2010) Article DOI: 10.1016/j.bmcl.2010.08.059 BindingDB Entry DOI: 10.7270/Q23X86VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27209 ((2R,3R)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197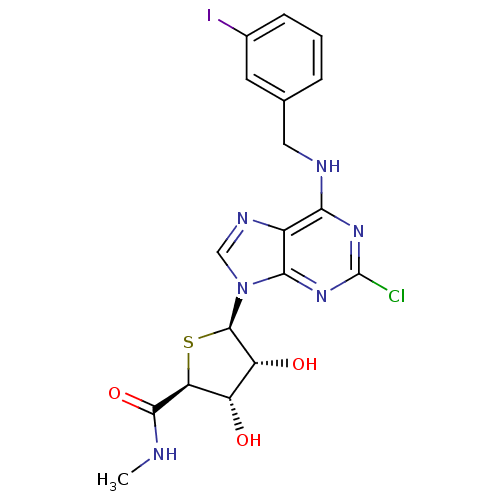 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]ABMECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 1612-6 (2008) Article DOI: 10.1016/j.bmcl.2008.01.070 BindingDB Entry DOI: 10.7270/Q2RN38QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem 17: 3733-8 (2009) Article DOI: 10.1016/j.bmc.2009.03.034 BindingDB Entry DOI: 10.7270/Q2V69JMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408246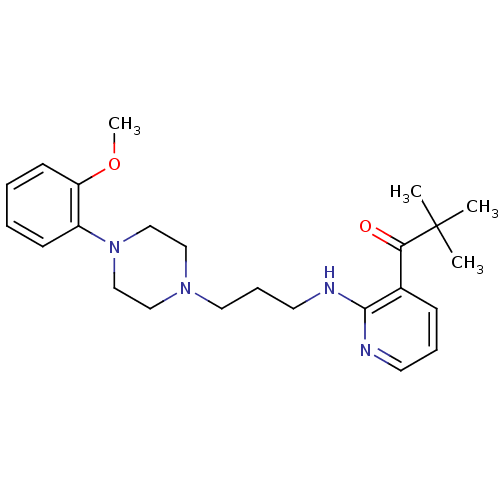 (CHEMBL92261) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408248 (CHEMBL330060) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221565 (4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl | Bioorg Med Chem Lett 17: 5812-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.043 BindingDB Entry DOI: 10.7270/Q2M32VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9198 total ) | Next | Last >> |