

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052440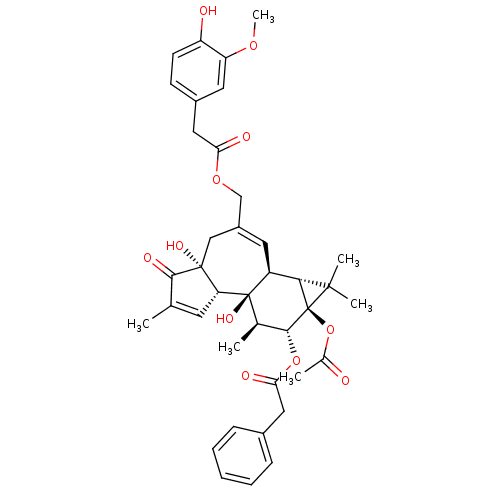 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory constant for RTX binding to rat spinal cord | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory constant for RTX binding to porcine spinal cord | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory constant for RTX binding to human spinal cord | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22473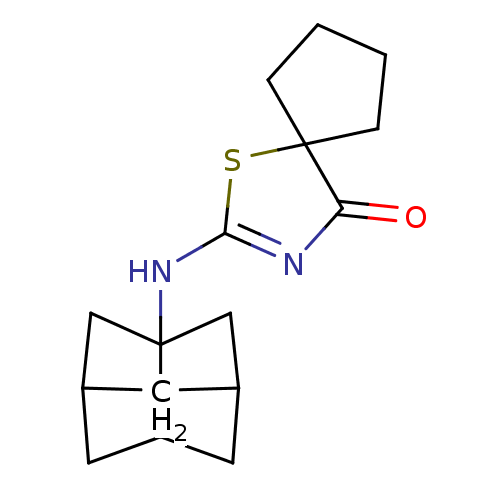 (2-(hexahydro-2,5-methanopentalen-3a(1H)-ylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22467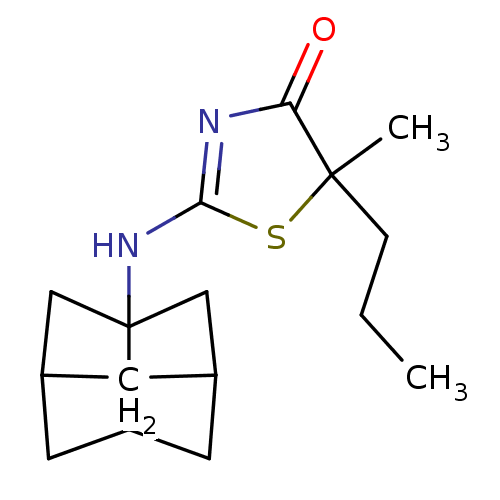 (2-Amino-1,3-thiazol-4(5H)-one, 6h | 5-methyl-5-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22466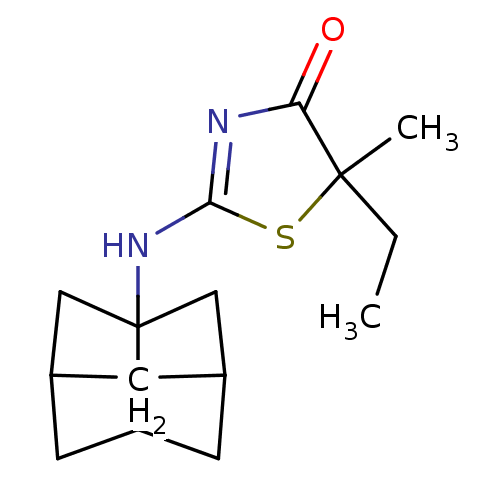 (2-Amino-1,3-thiazol-4(5H)-one, 5h | 5-ethyl-5-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22472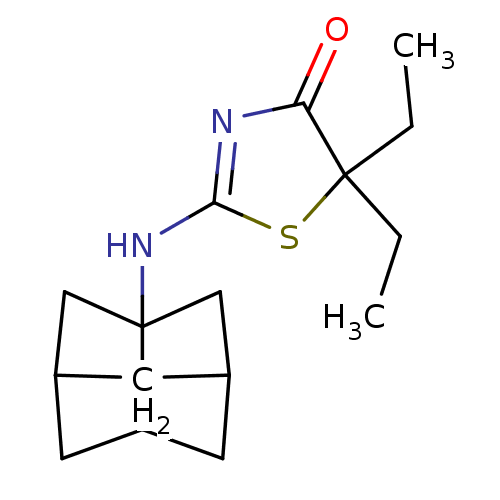 (2-Amino-1,3-thiazol-4(5H)-one, 7b | 5,5-diethyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22471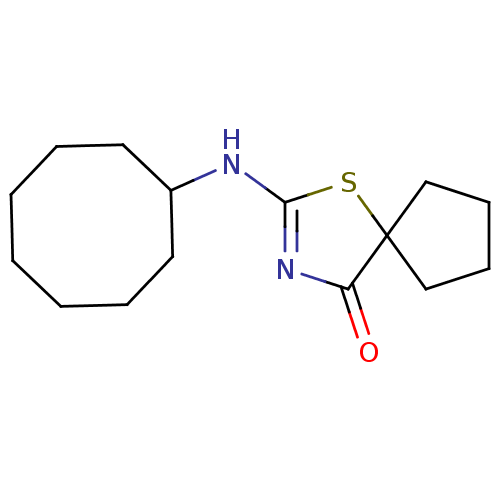 (2-(cyclooctylamino)-1-thia-3-azaspiro[4.4]non-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22474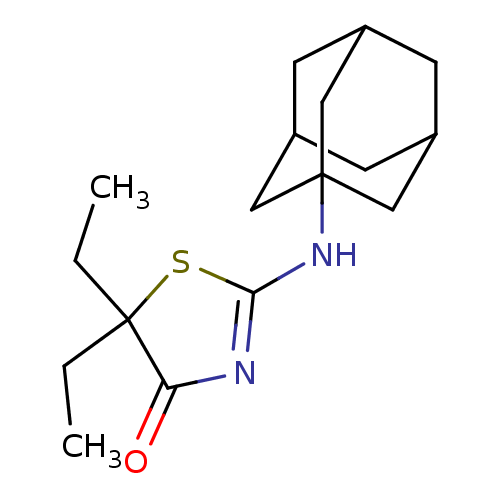 (2-(adamantan-1-ylamino)-5,5-diethyl-4,5-dihydro-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22469 (2-(adamantan-1-ylamino)-5-methyl-5-propyl-4,5-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22468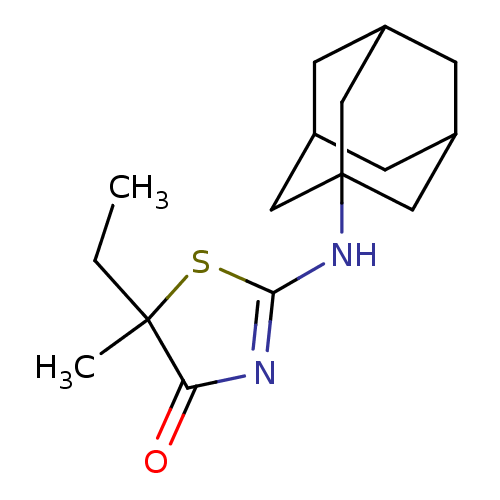 (2-(adamantan-1-ylamino)-5-ethyl-5-methyl-4,5-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22463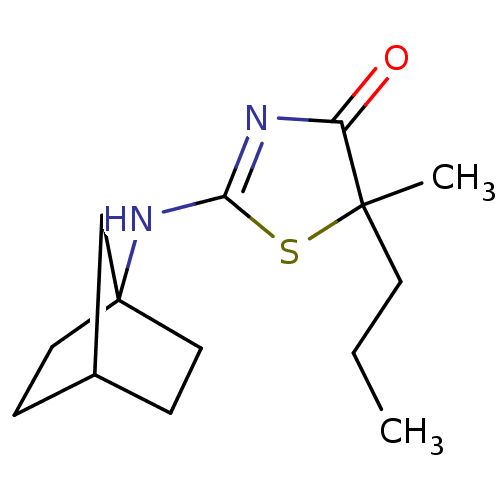 (2-Amino-1,3-thiazol-4(5H)-one, 6f | 2-{bicyclo[2.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22470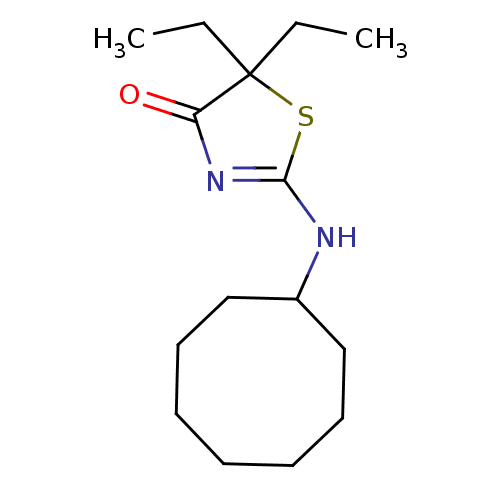 (2-(cyclooctylamino)-5,5-diethyl-4,5-dihydro-1,3-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22475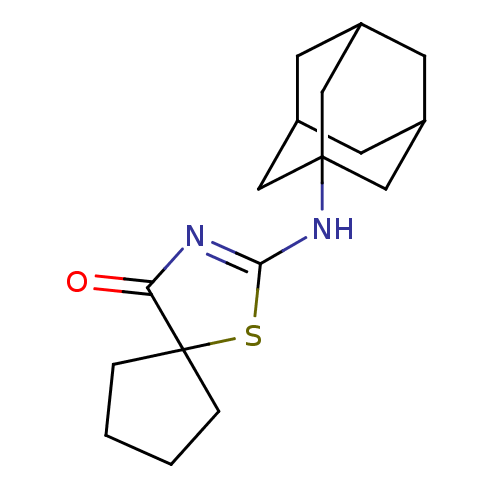 (2-(adamantan-1-ylamino)-1-thia-3-azaspiro[4.4]non-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22459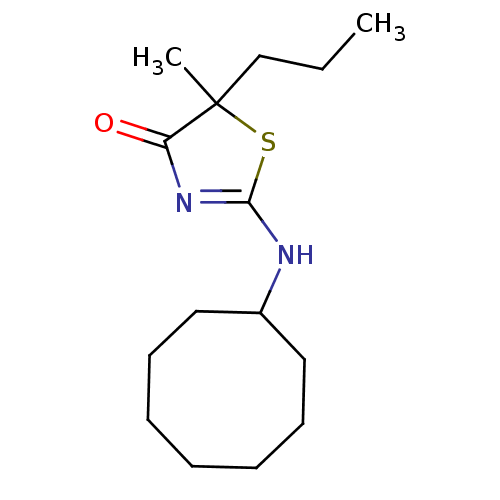 (2-(cyclooctylamino)-5-methyl-5-propyl-4,5-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 28 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22457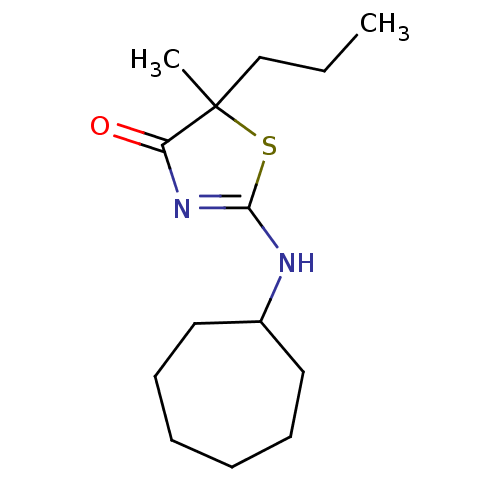 (2-(cycloheptylamino)-5-methyl-5-propyl-4,5-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22455 (2-Amino-1,3-thiazol-4(5H)-one, 6b | 2-[(cyclohexyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22461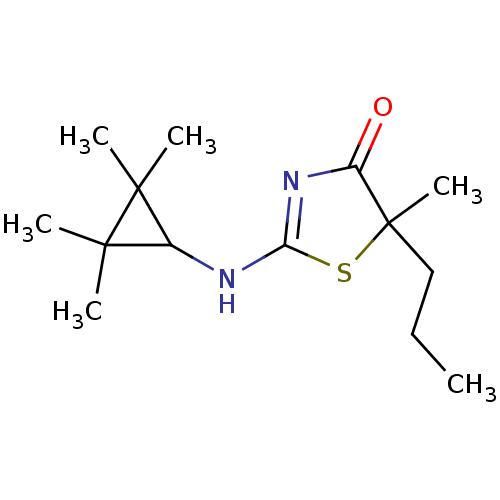 (2-Amino-1,3-thiazol-4(5H)-one, 6e | 5-methyl-5-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22458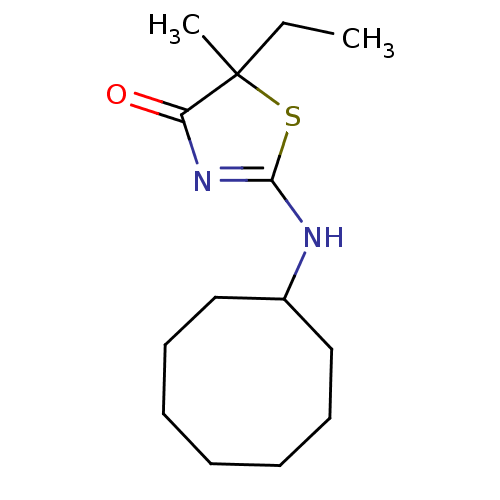 (2-(cyclooctylamino)-5-ethyl-5-methyl-4,5-dihydro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22453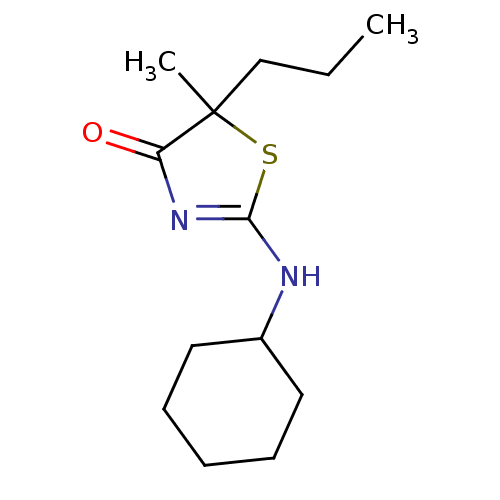 (2-(cyclohexylamino)-5-methyl-5-propyl-4,5-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22460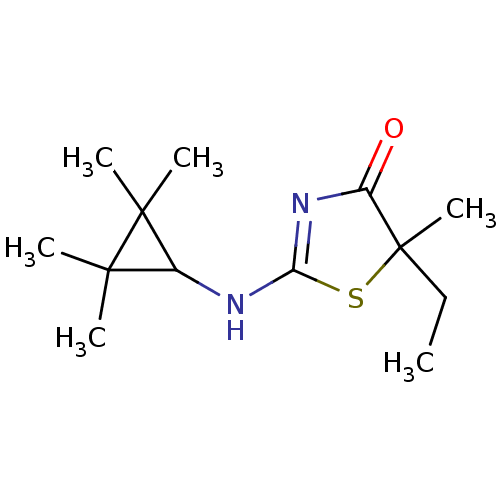 (2-Amino-1,3-thiazol-4(5H)-one, 5e | 5-ethyl-5-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22454 (2-Amino-1,3-thiazol-4(5H)-one, 5b | 2-[(cyclohexyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 64 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22462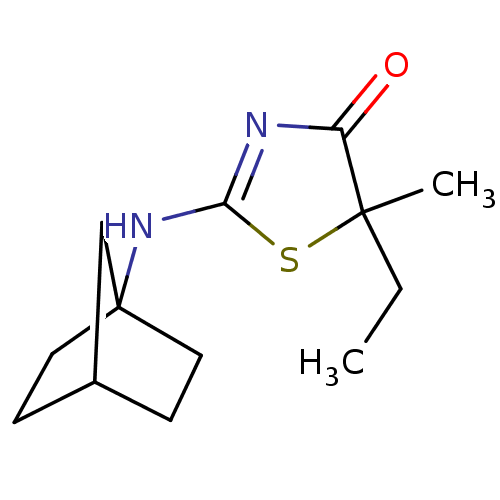 (2-Amino-1,3-thiazol-4(5H)-one, 5f | 2-{bicyclo[2.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 73 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22456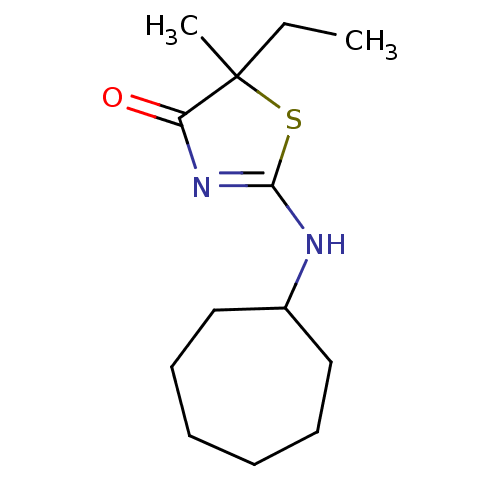 (2-(cycloheptylamino)-5-ethyl-5-methyl-4,5-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 82 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM22473 (2-(hexahydro-2,5-methanopentalen-3a(1H)-ylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description Enzyme assays were performed using purified recombinant enzymes, incubating with test compound and [3H]cortisone/NADPH. After incubation, the amount ... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22451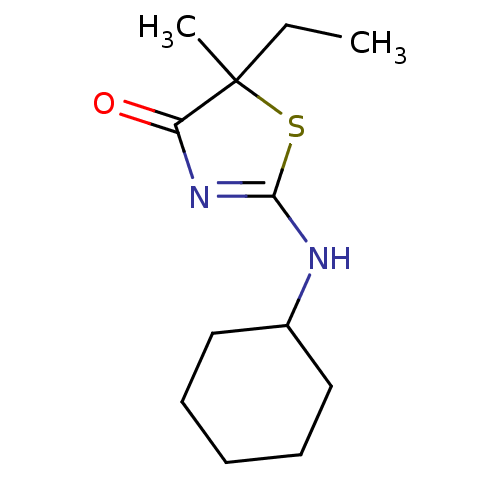 (2-(cyclohexylamino)-5-ethyl-5-methyl-4,5-dihydro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 126 | -39.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22465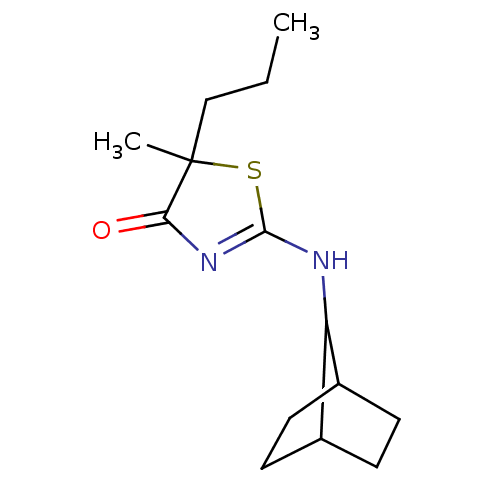 (2-Amino-1,3-thiazol-4(5H)-one, 6g | 2-{bicyclo[2.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 149 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM22473 (2-(hexahydro-2,5-methanopentalen-3a(1H)-ylamino)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 180 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description Enzyme assays were performed using purified recombinant enzymes, incubating with test compound and [3H]cortisone/NADPH. After incubation, the amount ... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052439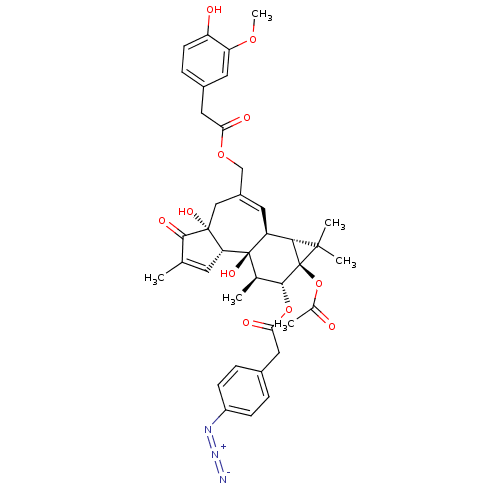 ((4-Azido-phenyl)-acetic acid (1aR,1bS,4aR,7aS,7bS,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052437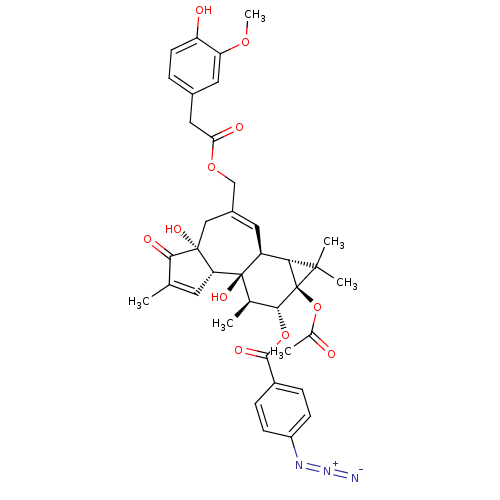 (4-Azido-benzoic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052443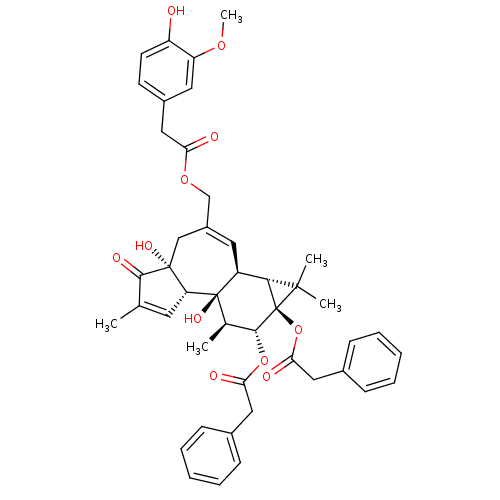 (CHEMBL320485 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM22464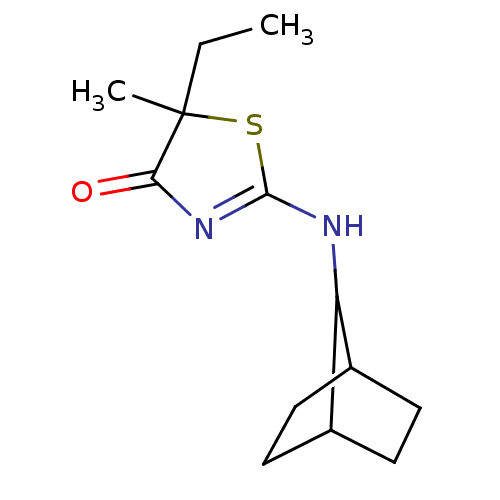 (2-Amino-1,3-thiazol-4(5H)-one, 5g | 2-{bicyclo[2.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 372 | -36.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Biovitrum AB | Assay Description The 11beta-HSD1 enzyme assay was carried out in the replica plates of the compounds in reaction buffer containing substrate mixture [3H]-cortisone/NA... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052445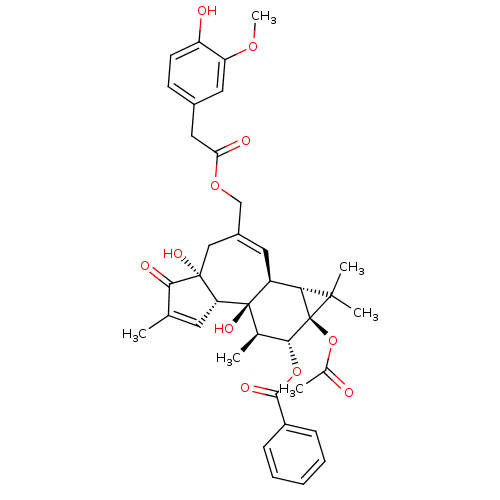 (Benzoic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-9a-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052441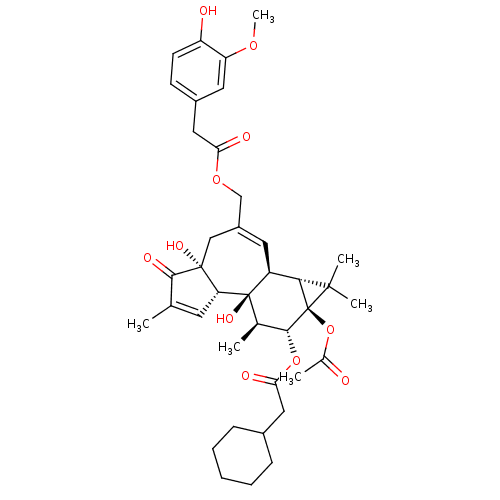 (CHEMBL317025 | Cyclohexyl-acetic acid (1aR,1bS,4aR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX binding from Vanilloid receptor of rat dorsal Root Ganglion (DRG) membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20284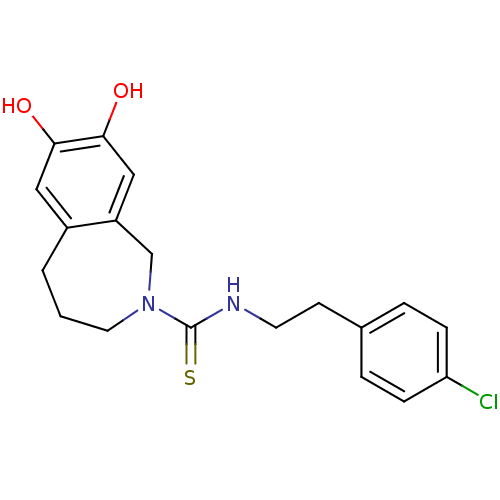 (CHEMBL391997 | CPZ | Capsazepine | N-[2-(4-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052447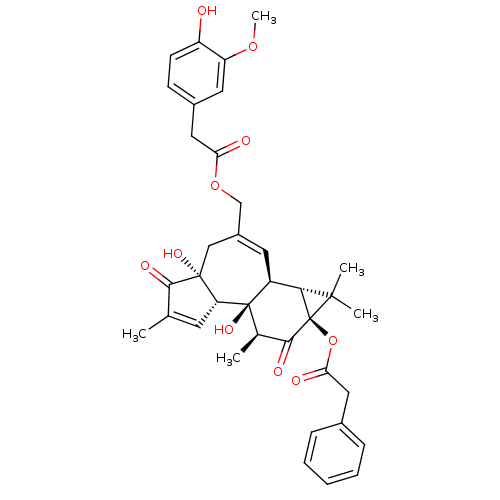 (CHEMBL322621 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052446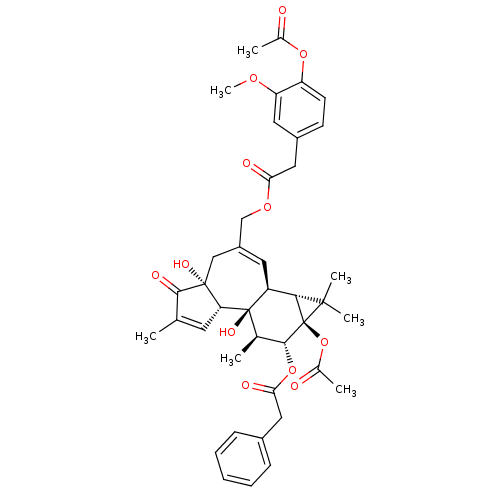 (CHEMBL319644 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20461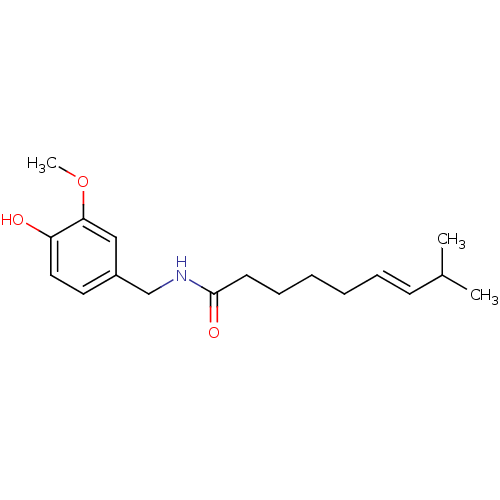 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of [3H]- RTX binding to vanilloid receptor of rat urinary bladder | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of [3H]- RTX binding to Vanilloid receptors from pig spinal cord | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052448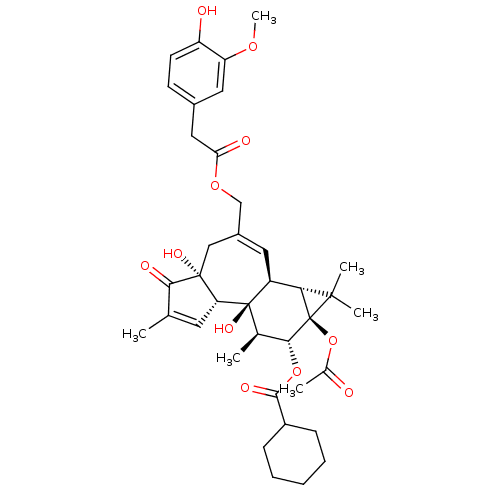 (CHEMBL107248 | Cyclohexanecarboxylic acid (1aR,1bS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of [3H]- RTX binding to Vanilloid receptors from human spinal cord | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052444 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (1aR,1bS,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052438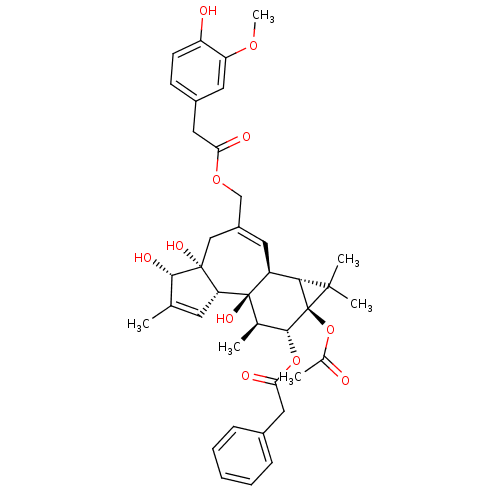 (CHEMBL320175 | Phenyl-acetic acid (1aR,1bS,4aR,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acylamino-acid-releasing enzyme (Sus scrofa) | BDBM50382729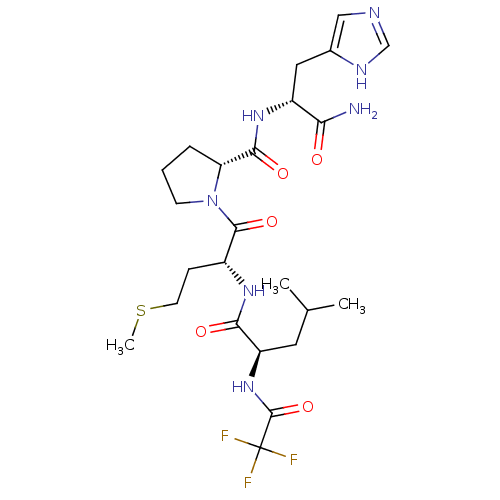 (CHEMBL2023560) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini Curated by ChEMBL | Assay Description Inhibition of pig APEH using acetyl-Ala-pNA as substrate incubated for 2 mins prior to substrate addition by spectrophotometry | J Med Chem 55: 2102-11 (2012) Article DOI: 10.1021/jm2013375 BindingDB Entry DOI: 10.7270/Q2BZ672F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM22473 (2-(hexahydro-2,5-methanopentalen-3a(1H)-ylamino)-1...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB | Assay Description The assay was performed at room temperature in a 96-well microtiter plate containing substrate mixture [3H]cortisol/NAD+, inhibitors, and 11beta-HSD2... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM22472 (2-Amino-1,3-thiazol-4(5H)-one, 7b | 5,5-diethyl-2-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB | Assay Description The assay was performed at room temperature in a 96-well microtiter plate containing substrate mixture [3H]cortisol/NAD+, inhibitors, and 11beta-HSD2... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM22471 (2-(cyclooctylamino)-1-thia-3-azaspiro[4.4]non-2-en...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB | Assay Description The assay was performed at room temperature in a 96-well microtiter plate containing substrate mixture [3H]cortisol/NAD+, inhibitors, and 11beta-HSD2... | J Med Chem 51: 2933-43 (2008) Article DOI: 10.1021/jm701551j BindingDB Entry DOI: 10.7270/Q2HM56Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |