 Found 3891 hits with Last Name = 'pan' and Initial = 'p'
Found 3891 hits with Last Name = 'pan' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM97445
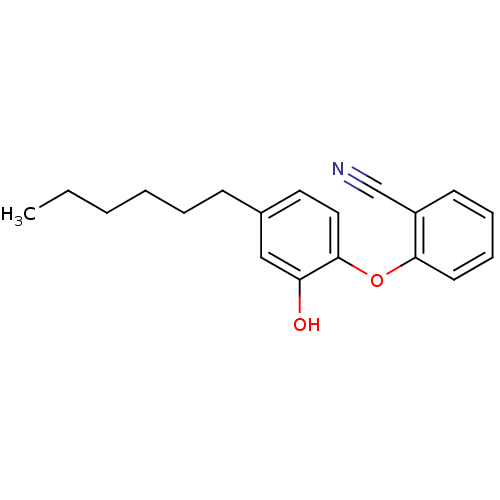
(PT119)Show InChI InChI=1S/C19H21NO2/c1-2-3-4-5-8-15-11-12-19(17(21)13-15)22-18-10-7-6-9-16(18)14-20/h6-7,9-13,21H,2-5,8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM16297
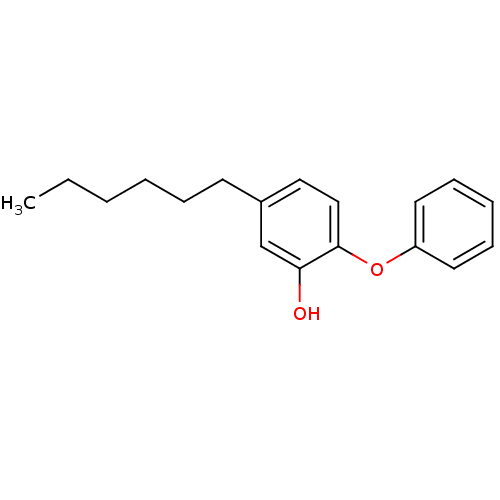
(5-Hexyl-2-phenoxy-phenol | 5-hexyl-2-phenoxylpheno...)Show InChI InChI=1S/C18H22O2/c1-2-3-4-6-9-15-12-13-18(17(19)14-15)20-16-10-7-5-8-11-16/h5,7-8,10-14,19H,2-4,6,9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 622-8 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb14977.x
BindingDB Entry DOI: 10.7270/Q2BR8QP0 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50519598
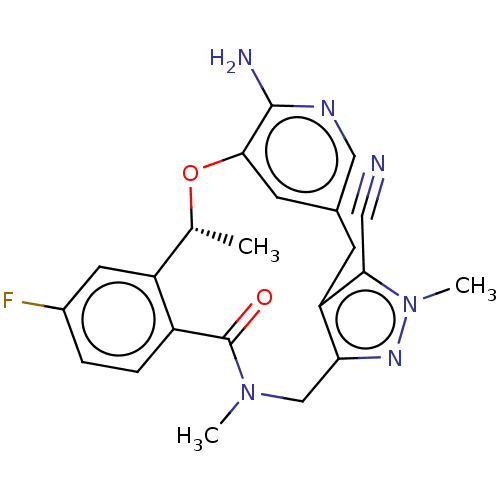
(CHEMBL4436406)Show SMILES C[C@H]1Oc2cc(Cc3c(CN(C)C(=O)c4ccc(F)cc14)nn(C)c3C#N)cnc2N |r| Show InChI InChI=1S/C22H21FN6O2/c1-12-16-8-14(23)4-5-15(16)22(30)28(2)11-18-17(19(9-24)29(3)27-18)6-13-7-20(31-12)21(25)26-10-13/h4-5,7-8,10,12H,6,11H2,1-3H3,(H2,25,26)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) |
J Med Chem 62: 10927-10954 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00446
BindingDB Entry DOI: 10.7270/Q2S185WV |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM16296
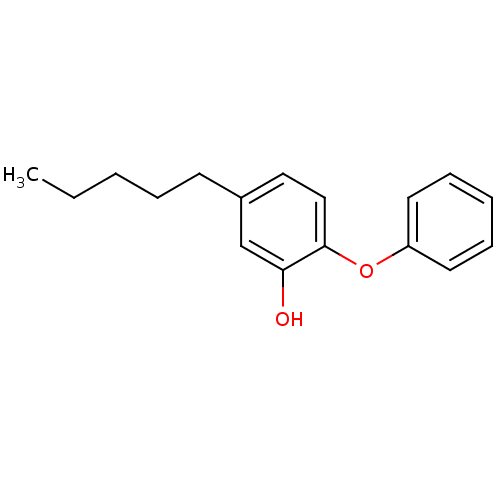
(5-Pentyl-2-phenoxy-phenol | 5-pentyl-2-phenoxylphe...)Show InChI InChI=1S/C17H20O2/c1-2-3-5-8-14-11-12-17(16(18)13-14)19-15-9-6-4-7-10-15/h4,6-7,9-13,18H,2-3,5,8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948
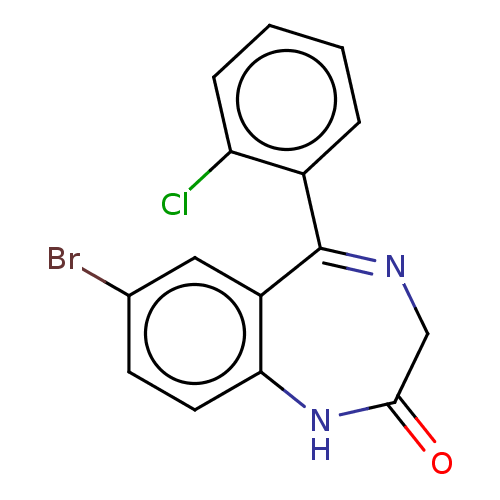
(PHENAZEPAM) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM97444
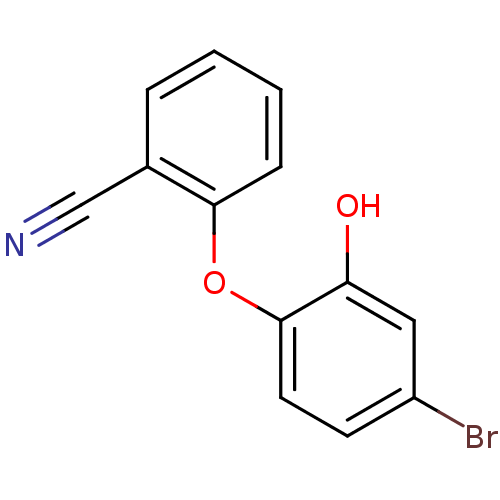
(PT443)Show InChI InChI=1S/C13H8BrNO2/c14-10-5-6-13(11(16)7-10)17-12-4-2-1-3-9(12)8-15/h1-7,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM97443
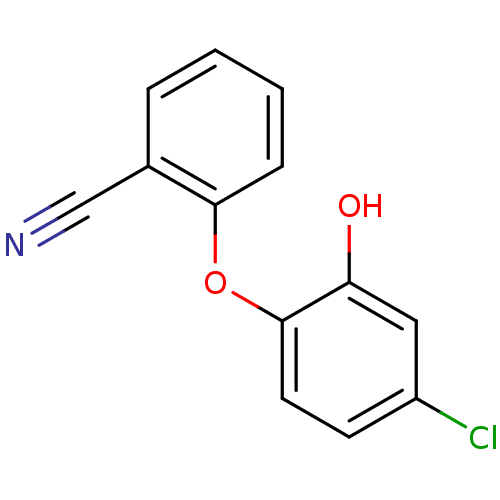
(PT447)Show InChI InChI=1S/C13H8ClNO2/c14-10-5-6-13(11(16)7-10)17-12-4-2-1-3-9(12)8-15/h1-7,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM36540
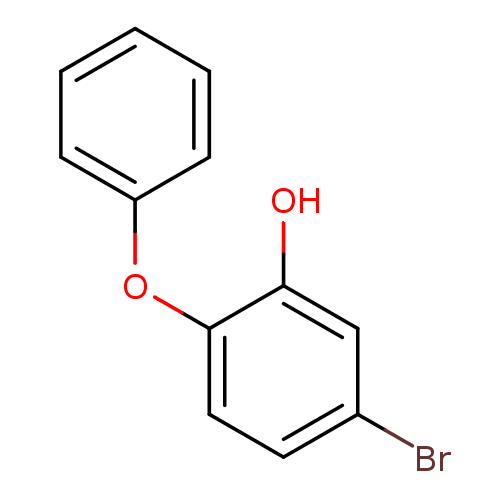
(5-bromo-2-phenoxylphenol | PT103)Show InChI InChI=1S/C12H9BrO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948

(PHENAZEPAM) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50519598

(CHEMBL4436406)Show SMILES C[C@H]1Oc2cc(Cc3c(CN(C)C(=O)c4ccc(F)cc14)nn(C)c3C#N)cnc2N |r| Show InChI InChI=1S/C22H21FN6O2/c1-12-16-8-14(23)4-5-15(16)22(30)28(2)11-18-17(19(9-24)29(3)27-18)6-13-7-20(31-12)21(25)26-10-13/h4-5,7-8,10,12H,6,11H2,1-3H3,(H2,25,26)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ROS1 (unknown origin) |
J Med Chem 62: 10927-10954 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00446
BindingDB Entry DOI: 10.7270/Q2S185WV |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM36543
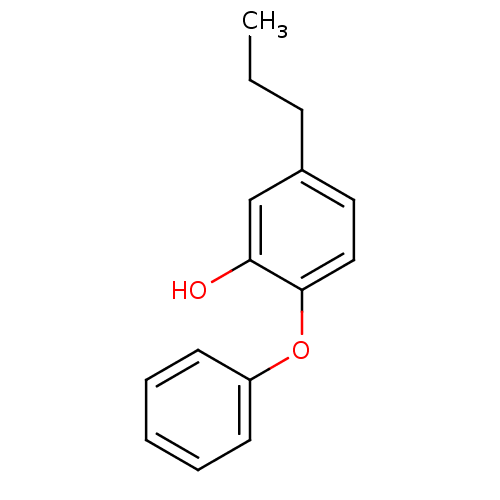
(5-propyl-2-phenoxyphenol | PT02)Show InChI InChI=1S/C15H16O2/c1-2-6-12-9-10-15(14(16)11-12)17-13-7-4-3-5-8-13/h3-5,7-11,16H,2,6H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM16294
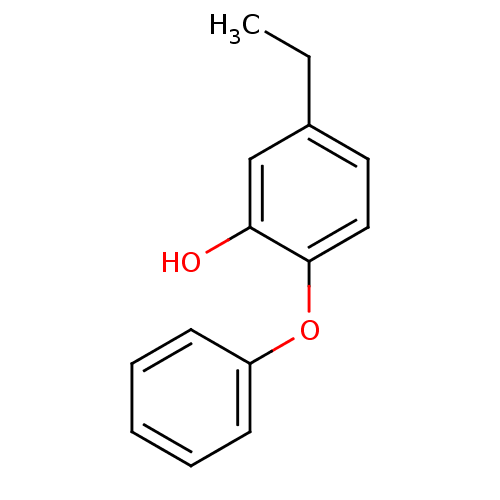
(2PP | 5-Ethyl-2-phenoxy-phenol | 5-butyl-2-phenoxy...)Show InChI InChI=1S/C14H14O2/c1-2-11-8-9-14(13(15)10-11)16-12-6-4-3-5-7-12/h3-10,15H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.0900 | -57.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE RESEARCH FOUNDATION FOR THE STATE UNIVERSITY OF NEW YORK
US Patent
| Assay Description
10 μM saFabI, 15 μM inhibitor, and 500 μM NADPH were preincubated overnight at room temperature followed by a 1:200 dilution into reac... |
US Patent US10071965 (2018)
BindingDB Entry DOI: 10.7270/Q2D79DFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM16294

(2PP | 5-Ethyl-2-phenoxy-phenol | 5-butyl-2-phenoxy...)Show InChI InChI=1S/C14H14O2/c1-2-11-8-9-14(13(15)10-11)16-12-6-4-3-5-7-12/h3-10,15H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of adenosine deaminase |
J Med Chem 24: 1383-5 (1982)
BindingDB Entry DOI: 10.7270/Q22N52T8 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM36539
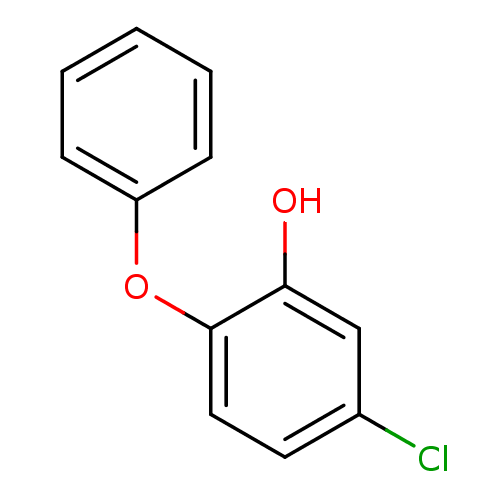
(5-chloro-2-phenoxylphenol | PT52 | US10071965, Com...)Show InChI InChI=1S/C12H9ClO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE RESEARCH FOUNDATION FOR THE STATE UNIVERSITY OF NEW YORK
US Patent
| Assay Description
10 μM saFabI, 15 μM inhibitor, and 500 μM NADPH were preincubated overnight at room temperature followed by a 1:200 dilution into reac... |
US Patent US10071965 (2018)
BindingDB Entry DOI: 10.7270/Q2D79DFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of adenosine deaminase |
J Med Chem 24: 1383-5 (1982)
BindingDB Entry DOI: 10.7270/Q22N52T8 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM97441
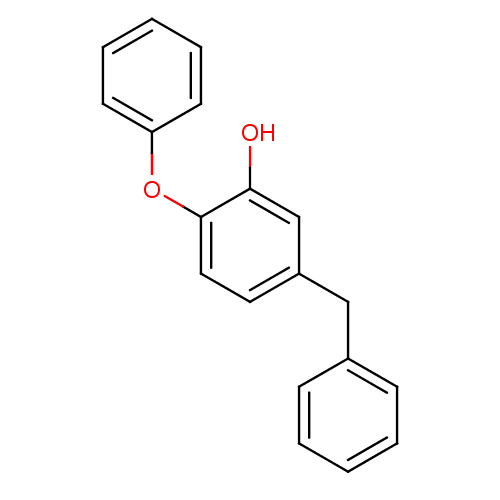
(PT89)Show InChI InChI=1S/C19H16O2/c20-18-14-16(13-15-7-3-1-4-8-15)11-12-19(18)21-17-9-5-2-6-10-17/h1-12,14,20H,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM97440

(PT68)Show InChI InChI=1S/C15H14O2/c1-2-6-12-9-10-15(14(16)11-12)17-13-7-4-3-5-8-13/h2-5,7-11,16H,1,6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM36539

(5-chloro-2-phenoxylphenol | PT52 | US10071965, Com...)Show InChI InChI=1S/C12H9ClO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50373348
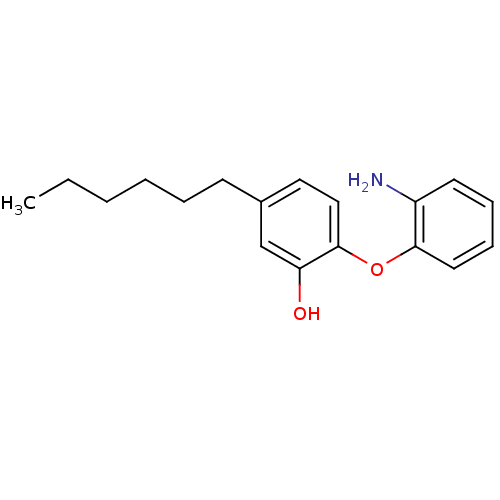
(CHEMBL264434 | PT13)Show InChI InChI=1S/C18H23NO2/c1-2-3-4-5-8-14-11-12-18(16(20)13-14)21-17-10-7-6-9-15(17)19/h6-7,9-13,20H,2-5,8,19H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948

(PHENAZEPAM) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Pro-FMRFamide-related neuropeptide FF
(RAT) | BDBM86140

(CAS_123797 | NPFF | NSC_123797)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-c1ccccc1)-[#6](-[#7])=O Show InChI InChI=1S/C54H76N14O10/c1-32(2)28-41(66-47(72)36(55)29-33-14-6-3-7-15-33)50(75)67-42(31-35-18-10-5-11-19-35)51(76)64-39(23-25-45(57)70)53(78)68-27-13-21-43(68)52(77)63-38(22-24-44(56)69)49(74)62-37(20-12-26-61-54(59)60)48(73)65-40(46(58)71)30-34-16-8-4-9-17-34/h3-11,14-19,32,36-43H,12-13,20-31,55H2,1-2H3,(H2,56,69)(H2,57,70)(H2,58,71)(H,62,74)(H,63,77)(H,64,76)(H,65,73)(H,66,72)(H,67,75)(H4,59,60,61) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Juvantia Pharma, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 825-32 (2003)
Article DOI: 10.1124/jpet.102.047118
BindingDB Entry DOI: 10.7270/Q2WS8RS2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50062051

(1-Dimethylcarbamoyl-6-(4-fluoro-phenyl)-3-[4-(2-me...)Show SMILES COC(=O)c1cc(cc2n(cc(C(=O)c3ccc(Cn4c(C)nc5cnccc45)cc3)c12)C(=O)N(C)C)-c1ccc(F)cc1 Show InChI InChI=1S/C34H28FN5O4/c1-20-37-28-17-36-14-13-29(28)39(20)18-21-5-7-23(8-6-21)32(41)27-19-40(34(43)38(2)3)30-16-24(22-9-11-25(35)12-10-22)15-26(31(27)30)33(42)44-4/h5-17,19H,18H2,1-4H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949
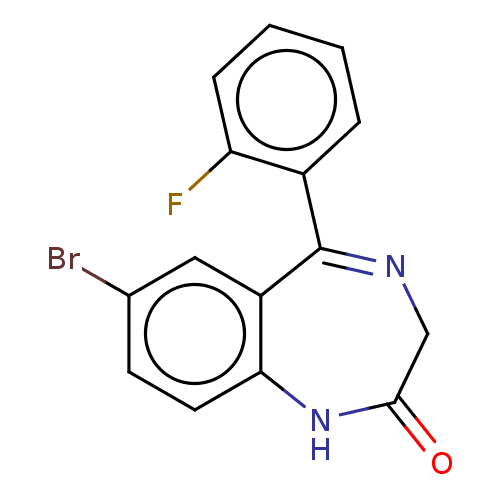
(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50328671
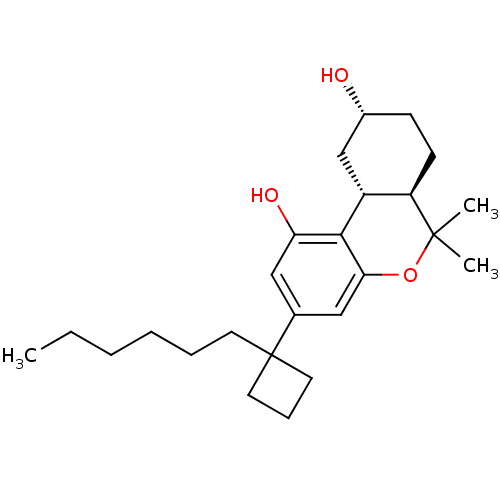
((6aR,9R,10aR)-3-(1-hexylcyclobutyl)-6,6-dimethyl-6...)Show SMILES CCCCCCC1(CCC1)c1cc(O)c2[C@@H]3C[C@H](O)CC[C@H]3C(C)(C)Oc2c1 |r| Show InChI InChI=1S/C25H38O3/c1-4-5-6-7-11-25(12-8-13-25)17-14-21(27)23-19-16-18(26)9-10-20(19)24(2,3)28-22(23)15-17/h14-15,18-20,26-27H,4-13,16H2,1-3H3/t18-,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50062066
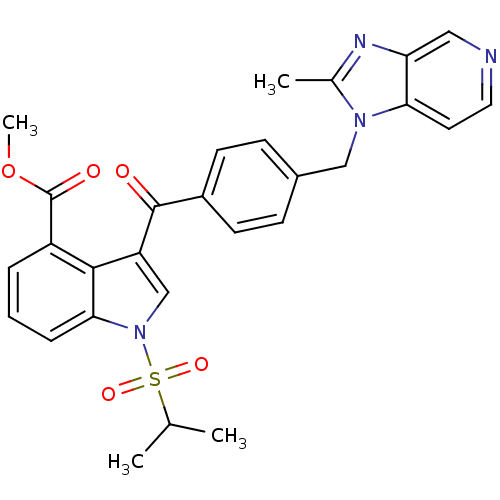
(3-[4-(2-Methyl-imidazo[4,5-c]pyridin-1-ylmethyl)-b...)Show SMILES COC(=O)c1cccc2n(cc(C(=O)c3ccc(Cn4c(C)nc5cnccc45)cc3)c12)S(=O)(=O)C(C)C Show InChI InChI=1S/C28H26N4O5S/c1-17(2)38(35,36)32-16-22(26-21(28(34)37-4)6-5-7-25(26)32)27(33)20-10-8-19(9-11-20)15-31-18(3)30-23-14-29-13-12-24(23)31/h5-14,16-17H,15H2,1-4H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding potency against Platelet activating factor (PAF) receptor using [3H]-C18-PAF as radioligand on rabbit platelet membranes |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM16294

(2PP | 5-Ethyl-2-phenoxy-phenol | 5-butyl-2-phenoxy...)Show InChI InChI=1S/C14H14O2/c1-2-11-8-9-14(13(15)10-11)16-12-6-4-3-5-7-12/h3-10,15H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE RESEARCH FOUNDATION FOR THE STATE UNIVERSITY OF NEW YORK
US Patent
| Assay Description
10 μM saFabI, 15 μM inhibitor, and 500 μM NADPH were preincubated overnight at room temperature followed by a 1:200 dilution into reac... |
US Patent US10071965 (2018)
BindingDB Entry DOI: 10.7270/Q2D79DFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM16294

(2PP | 5-Ethyl-2-phenoxy-phenol | 5-butyl-2-phenoxy...)Show InChI InChI=1S/C14H14O2/c1-2-11-8-9-14(13(15)10-11)16-12-6-4-3-5-7-12/h3-10,15H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE RESEARCH FOUNDATION FOR THE STATE UNIVERSITY OF NEW YORK
US Patent
| Assay Description
10 μM saFabI, 15 μM inhibitor, and 500 μM NADPH were preincubated overnight at room temperature followed by a 1:200 dilution into reac... |
US Patent US10071965 (2018)
BindingDB Entry DOI: 10.7270/Q2D79DFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50056419
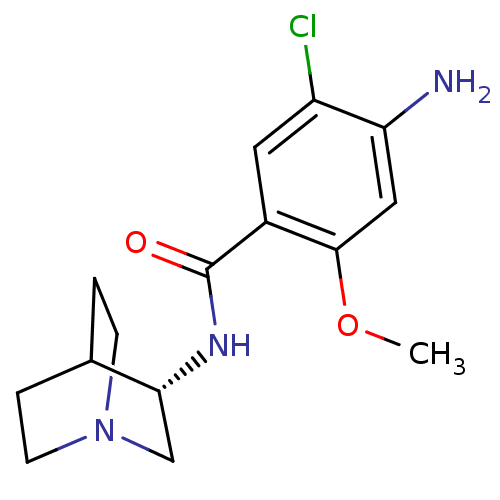
(4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wD:13.13,TLB:12:13:17.16:19.20,(11.11,-14.18,;11.11,-12.63,;9.77,-11.88,;8.43,-12.66,;7.09,-11.89,;5.75,-12.66,;7.09,-10.33,;5.76,-9.57,;8.43,-9.56,;9.75,-10.36,;11.09,-9.59,;11.11,-8.04,;12.44,-10.36,;13.77,-9.59,;14.8,-8.58,;16.63,-8.19,;17.94,-9.45,;16.9,-10.39,;15.57,-9.1,;15.88,-7.77,;16.88,-7.14,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vivo for the antagonistic activity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 2: 1613-1618 (1992)
Article DOI: 10.1016/S0960-894X(00)80441-2
BindingDB Entry DOI: 10.7270/Q2J67HFB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 622-8 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb14977.x
BindingDB Entry DOI: 10.7270/Q2BR8QP0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133408
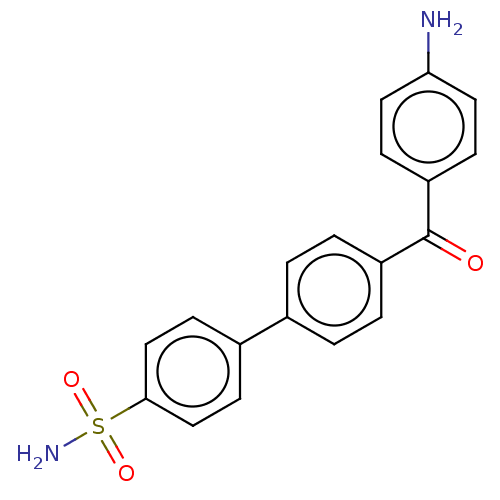
(CHEMBL3632844)Show SMILES Nc1ccc(cc1)C(=O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H16N2O3S/c20-17-9-5-16(6-10-17)19(22)15-3-1-13(2-4-15)14-7-11-18(12-8-14)25(21,23)24/h1-12H,20H2,(H2,21,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 622-8 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb14977.x
BindingDB Entry DOI: 10.7270/Q2BR8QP0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 622-8 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb14977.x
BindingDB Entry DOI: 10.7270/Q2BR8QP0 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133390
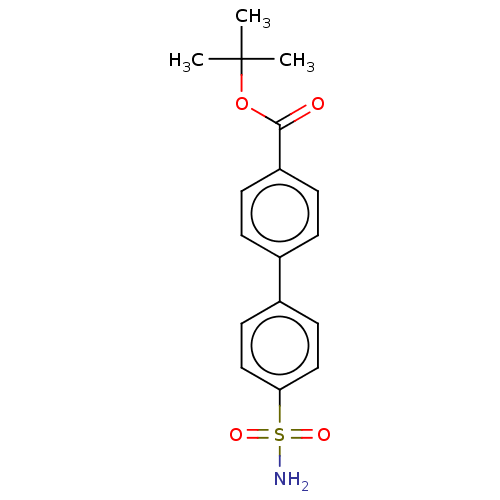
(CHEMBL3632826)Show SMILES CC(C)(C)OC(=O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H19NO4S/c1-17(2,3)22-16(19)14-6-4-12(5-7-14)13-8-10-15(11-9-13)23(18,20)21/h4-11H,1-3H3,(H2,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50286027
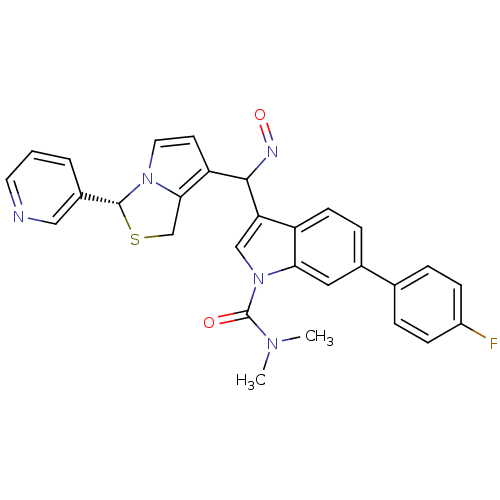
(6-(4-Fluoro-phenyl)-3-[[(Z)-hydroxyimino]-((R)-3-p...)Show SMILES CN(C)C(=O)n1cc(C(N=O)c2ccn3[C@H](SCc23)c2cccnc2)c2ccc(cc12)-c1ccc(F)cc1 Show InChI InChI=1S/C29H24FN5O2S/c1-33(2)29(36)35-16-24(22-10-7-19(14-25(22)35)18-5-8-21(30)9-6-18)27(32-37)23-11-13-34-26(23)17-38-28(34)20-4-3-12-31-15-20/h3-16,27-28H,17H2,1-2H3/t27?,28-/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for platelet activating factor receptor using [3H]-PAF in rabbit platelet membranes |
Bioorg Med Chem Lett 5: 2913-2918 (1995)
Article DOI: 10.1016/0960-894X(95)00511-Q
BindingDB Entry DOI: 10.7270/Q2NC6157 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM36542
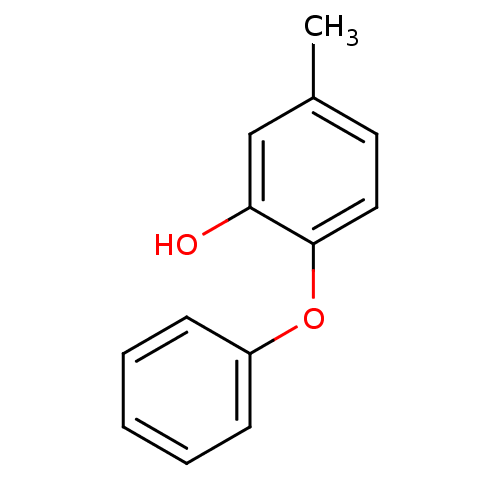
(5-methyl-2-phenoxylphenol | PT53 | US10071965, Com...)Show InChI InChI=1S/C13H12O2/c1-10-7-8-13(12(14)9-10)15-11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stony Brook University
| Assay Description
ThermoFluor experiments were carried out in 96-well plates (Concord) using the CFX96 RealTime PCR Detection system and C1000 Thermal Cycler (BioRad). |
Biochemistry 52: 4217-28 (2013)
Article DOI: 10.1021/bi400413c
BindingDB Entry DOI: 10.7270/Q2610XZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603275
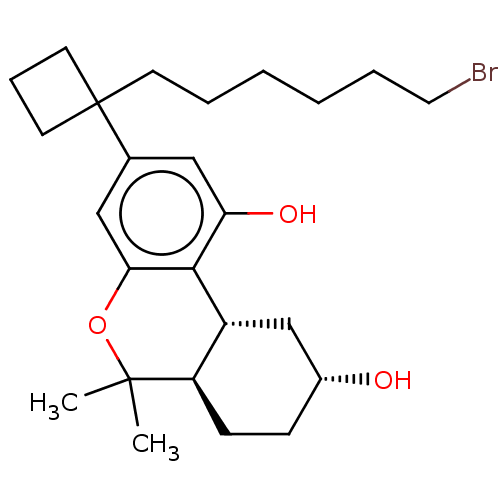
(CHEMBL5187249)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCCBr)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM36542

(5-methyl-2-phenoxylphenol | PT53 | US10071965, Com...)Show InChI InChI=1S/C13H12O2/c1-10-7-8-13(12(14)9-10)15-11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| 0.380 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
THE RESEARCH FOUNDATION FOR THE STATE UNIVERSITY OF NEW YORK
US Patent
| Assay Description
10 μM saFabI, 15 μM inhibitor, and 500 μM NADPH were preincubated overnight at room temperature followed by a 1:200 dilution into reac... |
US Patent US10071965 (2018)
BindingDB Entry DOI: 10.7270/Q2D79DFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 622-8 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb14977.x
BindingDB Entry DOI: 10.7270/Q2BR8QP0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599953

(CHEMBL1451229)Show SMILES Cc1nnc2CN=C(c3ccccc3Cl)c3cc(Br)ccc3-n12 |t:6| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50048485
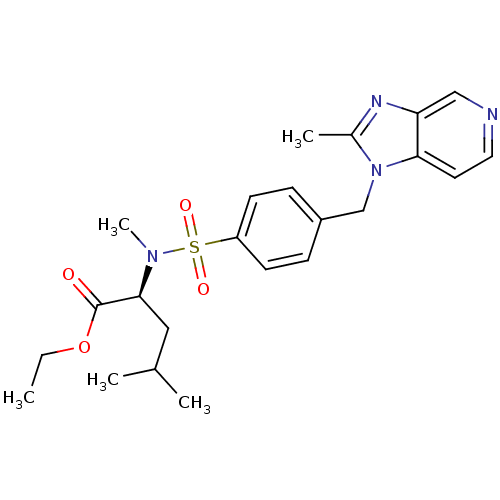
((S)-4-Methyl-2-{methyl-[4-(2-methyl-imidazo[4,5-c]...)Show SMILES CCOC(=O)[C@H](CC(C)C)N(C)S(=O)(=O)c1ccc(Cn2c(C)nc3cnccc23)cc1 Show InChI InChI=1S/C23H30N4O4S/c1-6-31-23(28)22(13-16(2)3)26(5)32(29,30)19-9-7-18(8-10-19)15-27-17(4)25-20-14-24-12-11-21(20)27/h7-12,14,16,22H,6,13,15H2,1-5H3/t22-/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity against PAF receptor in rabbit platelet |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50048485

((S)-4-Methyl-2-{methyl-[4-(2-methyl-imidazo[4,5-c]...)Show SMILES CCOC(=O)[C@H](CC(C)C)N(C)S(=O)(=O)c1ccc(Cn2c(C)nc3cnccc23)cc1 Show InChI InChI=1S/C23H30N4O4S/c1-6-31-23(28)22(13-16(2)3)26(5)32(29,30)19-9-7-18(8-10-19)15-27-17(4)25-20-14-24-12-11-21(20)27/h7-12,14,16,22H,6,13,15H2,1-5H3/t22-/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity against PAF receptor in rabbit platelet |
J Med Chem 41: 74-95 (1998)
Article DOI: 10.1021/jm970389+
BindingDB Entry DOI: 10.7270/Q2MC8Z4D |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133394
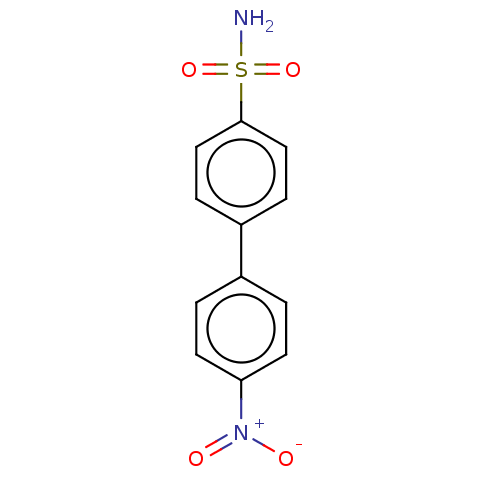
(CHEMBL3632830)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C12H10N2O4S/c13-19(17,18)12-7-3-10(4-8-12)9-1-5-11(6-2-9)14(15)16/h1-8H,(H2,13,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133400
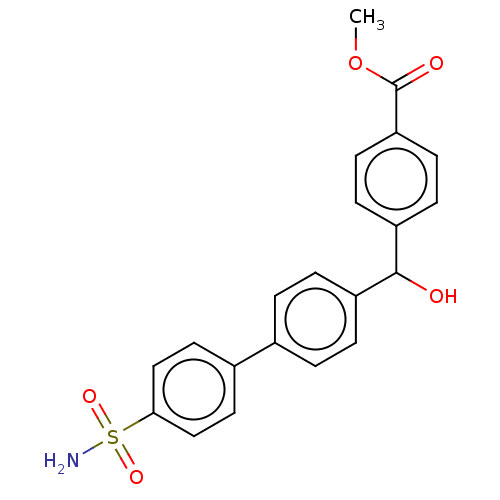
(CHEMBL3632836)Show SMILES COC(=O)c1ccc(cc1)C(O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H19NO5S/c1-27-21(24)18-8-6-17(7-9-18)20(23)16-4-2-14(3-5-16)15-10-12-19(13-11-15)28(22,25)26/h2-13,20,23H,1H3,(H2,22,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50603264
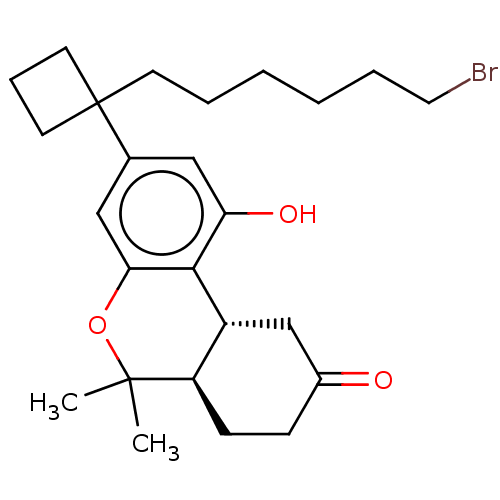
(CHEMBL5206676)Show SMILES [H][C@@]12CC(=O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCCBr)CCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114027
BindingDB Entry DOI: 10.7270/Q2J96BFX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data