 Found 271 hits with Last Name = 'panchal' and Initial = 'ta'
Found 271 hits with Last Name = 'panchal' and Initial = 'ta' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469937
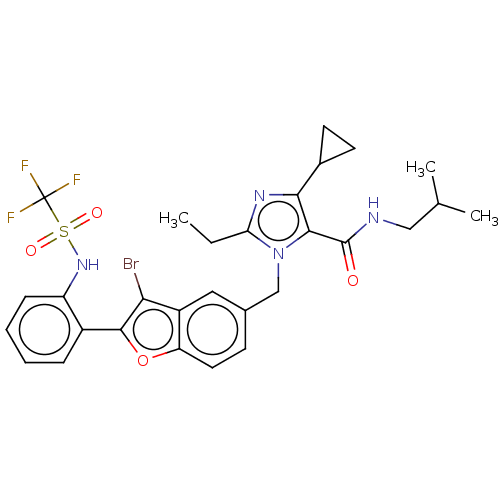
(CHEMBL104493)Show SMILES CCc1nc(C2CC2)c(C(=O)NCC(C)C)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C29H30BrF3N4O4S/c1-4-23-35-25(18-10-11-18)26(28(38)34-14-16(2)3)37(23)15-17-9-12-22-20(13-17)24(30)27(41-22)19-7-5-6-8-21(19)36-42(39,40)29(31,32)33/h5-9,12-13,16,18,36H,4,10-11,14-15H2,1-3H3,(H,34,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444496
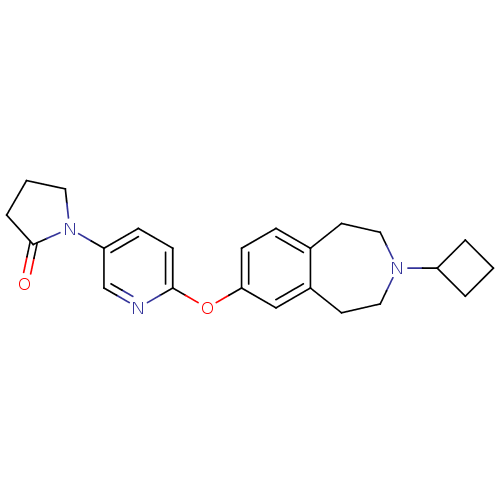
(CHEMBL3092650)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H27N3O2/c27-23-5-2-12-26(23)20-7-9-22(24-16-20)28-21-8-6-17-10-13-25(19-3-1-4-19)14-11-18(17)15-21/h6-9,15-16,19H,1-5,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444491

(CHEMBL3092823)Show InChI InChI=1S/C22H25N3O2/c26-22-10-13-25(22)19-5-7-21(23-15-19)27-20-6-4-16-8-11-24(18-2-1-3-18)12-9-17(16)14-20/h4-7,14-15,18H,1-3,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469896
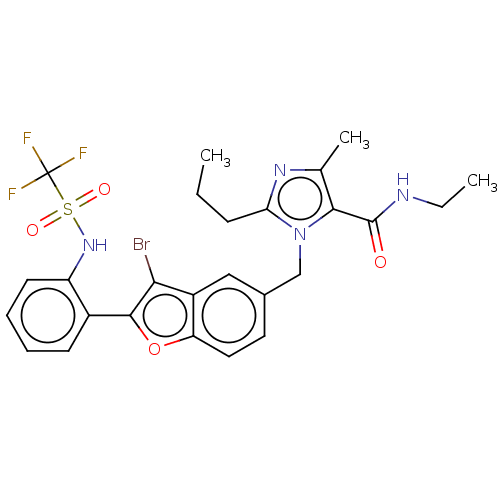
(CHEMBL326910)Show SMILES CCCc1nc(C)c(C(=O)NCC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C26H26BrF3N4O4S/c1-4-8-21-32-15(3)23(25(35)31-5-2)34(21)14-16-11-12-20-18(13-16)22(27)24(38-20)17-9-6-7-10-19(17)33-39(36,37)26(28,29)30/h6-7,9-13,33H,4-5,8,14H2,1-3H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444496

(CHEMBL3092650)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H27N3O2/c27-23-5-2-12-26(23)20-7-9-22(24-16-20)28-21-8-6-17-10-13-25(19-3-1-4-19)14-11-18(17)15-21/h6-9,15-16,19H,1-5,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469939
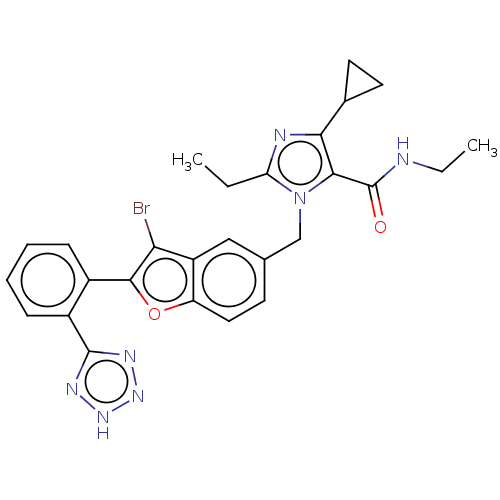
(CHEMBL323125)Show SMILES CCNC(=O)c1c(nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1-c1nn[nH]n1)C1CC1 Show InChI InChI=1S/C27H26BrN7O2/c1-3-21-30-23(16-10-11-16)24(27(36)29-4-2)35(21)14-15-9-12-20-19(13-15)22(28)25(37-20)17-7-5-6-8-18(17)26-31-33-34-32-26/h5-9,12-13,16H,3-4,10-11,14H2,1-2H3,(H,29,36)(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469890

(CHEMBL63072)Show SMILES CCc1nc(C2CC2)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C26H24BrF3N4O4S/c1-3-20-32-22(15-9-10-15)23(25(35)31-2)34(20)13-14-8-11-19-17(12-14)21(27)24(38-19)16-6-4-5-7-18(16)33-39(36,37)26(28,29)30/h4-8,11-12,15,33H,3,9-10,13H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444500
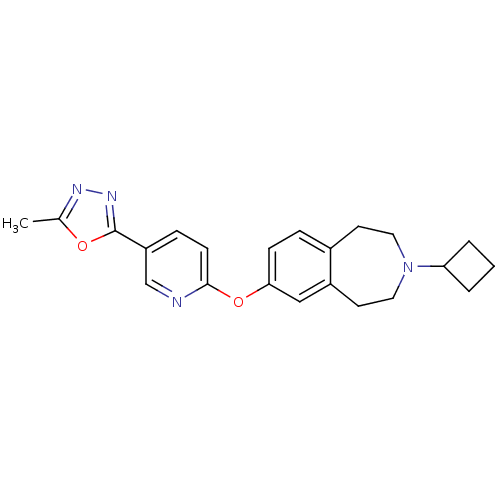
(CHEMBL3092834)Show SMILES Cc1nnc(o1)-c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C22H24N4O2/c1-15-24-25-22(27-15)18-6-8-21(23-14-18)28-20-7-5-16-9-11-26(19-3-2-4-19)12-10-17(16)13-20/h5-8,13-14,19H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469901

(GR-138950 | SAPRISARTAN)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H22BrF3N4O4S/c1-2-19-31-21(14-8-9-14)22(24(30)34)33(19)12-13-7-10-18-16(11-13)20(26)23(37-18)15-5-3-4-6-17(15)32-38(35,36)25(27,28)29/h3-7,10-11,14,32H,2,8-9,12H2,1H3,(H2,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469888
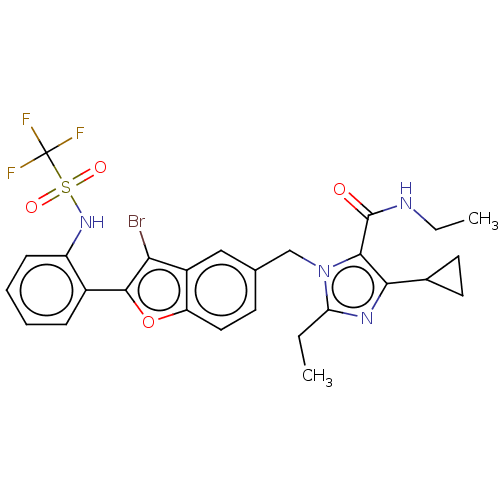
(CHEMBL104667)Show SMILES CCNC(=O)c1c(nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F)C1CC1 Show InChI InChI=1S/C27H26BrF3N4O4S/c1-3-21-33-23(16-10-11-16)24(26(36)32-4-2)35(21)14-15-9-12-20-18(13-15)22(28)25(39-20)17-7-5-6-8-19(17)34-40(37,38)27(29,30)31/h5-9,12-13,16,34H,3-4,10-11,14H2,1-2H3,(H,32,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444507
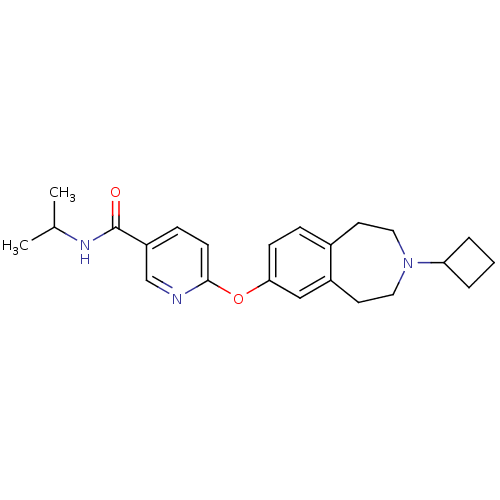
(CHEMBL3092826)Show SMILES CC(C)NC(=O)c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H29N3O2/c1-16(2)25-23(27)19-7-9-22(24-15-19)28-21-8-6-17-10-12-26(20-4-3-5-20)13-11-18(17)14-21/h6-9,14-16,20H,3-5,10-13H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444509
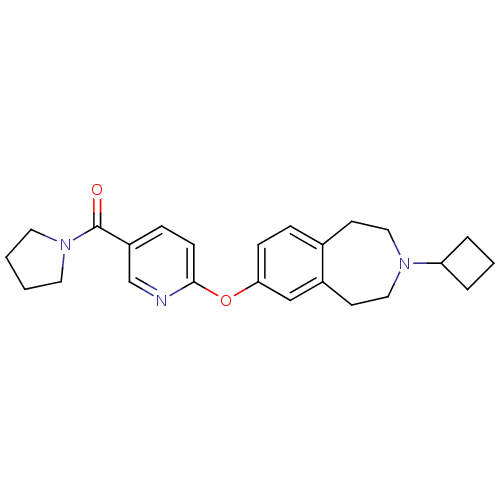
(CHEMBL3092840)Show SMILES O=C(N1CCCC1)c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C24H29N3O2/c28-24(27-12-1-2-13-27)20-7-9-23(25-17-20)29-22-8-6-18-10-14-26(21-4-3-5-21)15-11-19(18)16-22/h6-9,16-17,21H,1-5,10-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444505
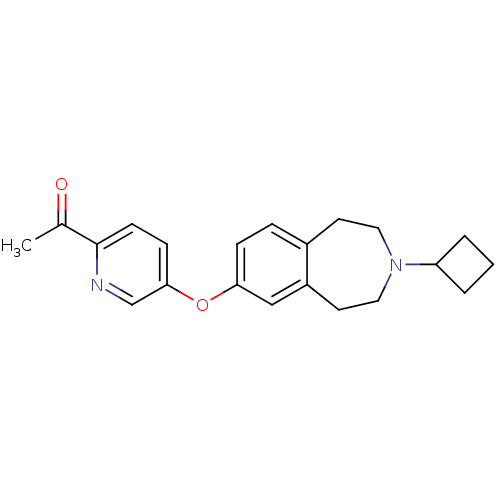
(CHEMBL3092828)Show InChI InChI=1S/C21H24N2O2/c1-15(24)21-8-7-20(14-22-21)25-19-6-5-16-9-11-23(18-3-2-4-18)12-10-17(16)13-19/h5-8,13-14,18H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50247054
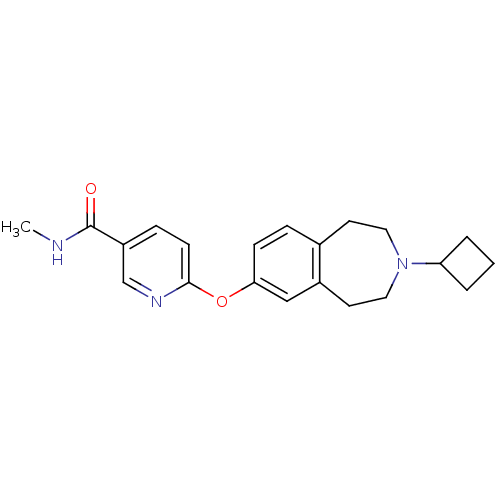
(6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C21H25N3O2/c1-22-21(25)17-6-8-20(23-14-17)26-19-7-5-15-9-11-24(18-3-2-4-18)12-10-16(15)13-19/h5-8,13-14,18H,2-4,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444489
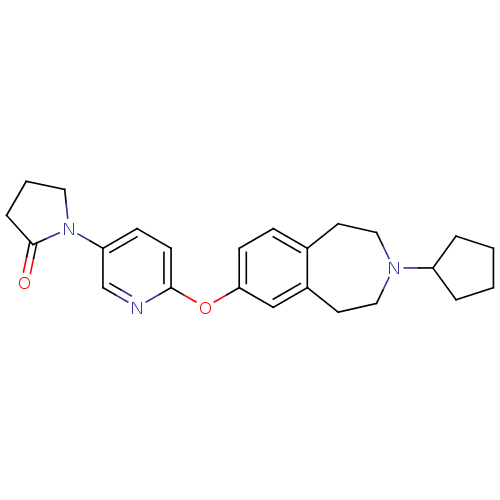
(CHEMBL3092825)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCCC2)nc1 Show InChI InChI=1S/C24H29N3O2/c28-24-6-3-13-27(24)21-8-10-23(25-17-21)29-22-9-7-18-11-14-26(15-12-19(18)16-22)20-4-1-2-5-20/h7-10,16-17,20H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444495
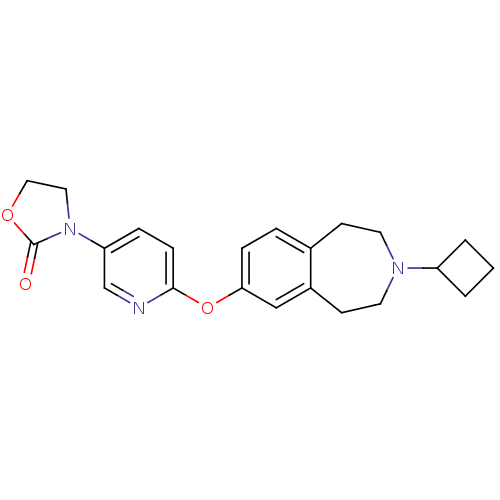
(CHEMBL3092651)Show SMILES O=C1OCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C22H25N3O3/c26-22-25(12-13-27-22)19-5-7-21(23-15-19)28-20-6-4-16-8-10-24(18-2-1-3-18)11-9-17(16)14-20/h4-7,14-15,18H,1-3,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469886

(CHEMBL107006)Show SMILES CCc1nc(CC)n(Cc2ccc3oc(c(Br)c3c2)-c2ccccc2NS(=O)(=O)C(F)(F)F)c1C(N)=O Show InChI InChI=1S/C24H22BrF3N4O4S/c1-3-16-21(23(29)33)32(19(4-2)30-16)12-13-9-10-18-15(11-13)20(25)22(36-18)14-7-5-6-8-17(14)31-37(34,35)24(26,27)28/h5-11,31H,3-4,12H2,1-2H3,(H2,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469892

(CHEMBL107081)Show SMILES CCCc1nc(C)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C24H22BrF3N4O4S/c1-3-6-19-30-13(2)21(23(29)33)32(19)12-14-9-10-18-16(11-14)20(25)22(36-18)15-7-4-5-8-17(15)31-37(34,35)24(26,27)28/h4-5,7-11,31H,3,6,12H2,1-2H3,(H2,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50346209

(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444494
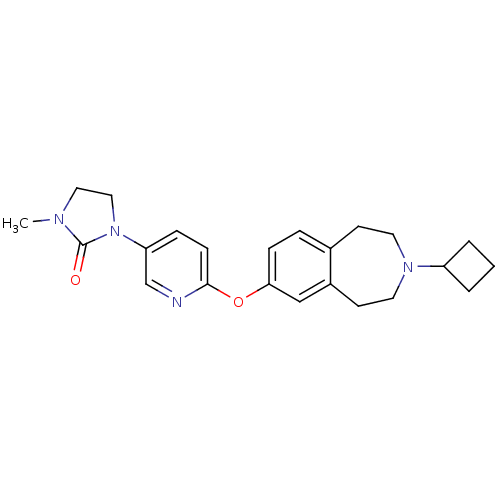
(CHEMBL3092820)Show SMILES CN1CCN(C1=O)c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H28N4O2/c1-25-13-14-27(23(25)28)20-6-8-22(24-16-20)29-21-7-5-17-9-11-26(19-3-2-4-19)12-10-18(17)15-21/h5-8,15-16,19H,2-4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469940
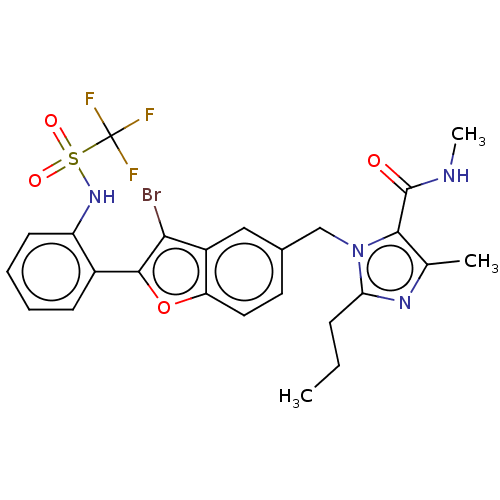
(CHEMBL105887)Show SMILES CCCc1nc(C)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H24BrF3N4O4S/c1-4-7-20-31-14(2)22(24(34)30-3)33(20)13-15-10-11-19-17(12-15)21(26)23(37-19)16-8-5-6-9-18(16)32-38(35,36)25(27,28)29/h5-6,8-12,32H,4,7,13H2,1-3H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469897

(CHEMBL104281)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(c(c2c1)C(F)(F)F)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C26H22F6N4O4S/c1-2-19-34-21(14-8-9-14)22(24(33)37)36(19)12-13-7-10-18-16(11-13)20(25(27,28)29)23(40-18)15-5-3-4-6-17(15)35-41(38,39)26(30,31)32/h3-7,10-11,14,35H,2,8-9,12H2,1H3,(H2,33,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444506
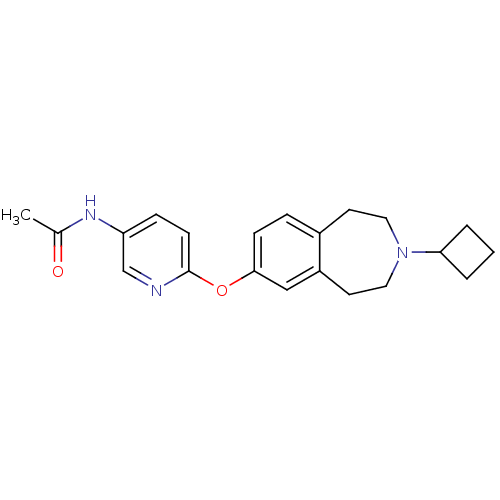
(CHEMBL3092827)Show InChI InChI=1S/C21H25N3O2/c1-15(25)23-18-6-8-21(22-14-18)26-20-7-5-16-9-11-24(19-3-2-4-19)12-10-17(16)13-20/h5-8,13-14,19H,2-4,9-12H2,1H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469893

(CHEMBL104418)Show SMILES CCCc1nc(Cl)c(C(=O)NCC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H23BrClF3N4O4S/c1-3-7-19-32-23(27)21(24(35)31-4-2)34(19)13-14-10-11-18-16(12-14)20(26)22(38-18)15-8-5-6-9-17(15)33-39(36,37)25(28,29)30/h5-6,8-12,33H,3-4,7,13H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50346209

(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469934

(CHEMBL324196)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(c(Cl)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H22ClF3N4O4S/c1-2-19-31-21(14-8-9-14)22(24(30)34)33(19)12-13-7-10-18-16(11-13)20(26)23(37-18)15-5-3-4-6-17(15)32-38(35,36)25(27,28)29/h3-7,10-11,14,32H,2,8-9,12H2,1H3,(H2,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469935
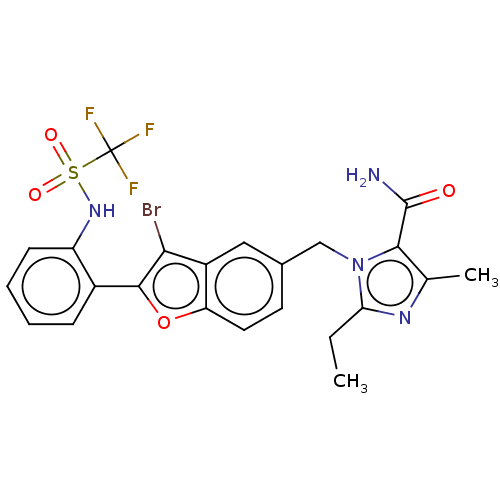
(CHEMBL62909)Show SMILES CCc1nc(C)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C23H20BrF3N4O4S/c1-3-18-29-12(2)20(22(28)32)31(18)11-13-8-9-17-15(10-13)19(24)21(35-17)14-6-4-5-7-16(14)30-36(33,34)23(25,26)27/h4-10,30H,3,11H2,1-2H3,(H2,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469889

(CHEMBL62553)Show SMILES CCNC(=O)c1c(C)nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H24BrF3N4O4S/c1-4-20-31-14(3)22(24(34)30-5-2)33(20)13-15-10-11-19-17(12-15)21(26)23(37-19)16-8-6-7-9-18(16)32-38(35,36)25(27,28)29/h6-12,32H,4-5,13H2,1-3H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469938
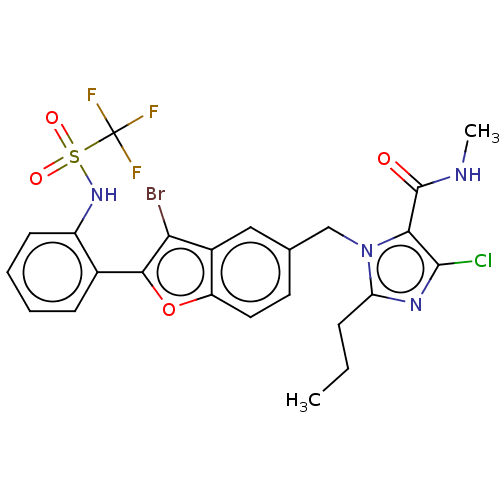
(CHEMBL322989)Show SMILES CCCc1nc(Cl)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C24H21BrClF3N4O4S/c1-3-6-18-31-22(26)20(23(34)30-2)33(18)12-13-9-10-17-15(11-13)19(25)21(37-17)14-7-4-5-8-16(14)32-38(35,36)24(27,28)29/h4-5,7-11,32H,3,6,12H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469887

(CHEMBL107043)Show SMILES CCCCc1nc(Cl)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H23BrClF3N4O4S/c1-3-4-9-19-32-23(27)21(24(35)31-2)34(19)13-14-10-11-18-16(12-14)20(26)22(38-18)15-7-5-6-8-17(15)33-39(36,37)25(28,29)30/h5-8,10-12,33H,3-4,9,13H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444493

(CHEMBL3092821)Show SMILES O[C@H]1CN(C(=O)C1)c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 |r| Show InChI InChI=1S/C23H27N3O3/c27-20-13-23(28)26(15-20)19-5-7-22(24-14-19)29-21-6-4-16-8-10-25(18-2-1-3-18)11-9-17(16)12-21/h4-7,12,14,18,20,27H,1-3,8-11,13,15H2/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469899

(CHEMBL107967)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(c(CC)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C27H27F3N4O4S/c1-3-17-19-13-15(14-34-22(4-2)32-23(16-10-11-16)24(34)26(31)35)9-12-21(19)38-25(17)18-7-5-6-8-20(18)33-39(36,37)27(28,29)30/h5-9,12-13,16,33H,3-4,10-11,14H2,1-2H3,(H2,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469900

(CHEMBL104252)Show SMILES CCNC(=O)c1c(Cl)nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C24H21BrClF3N4O4S/c1-3-18-31-22(26)20(23(34)30-4-2)33(18)12-13-9-10-17-15(11-13)19(25)21(37-17)14-7-5-6-8-16(14)32-38(35,36)24(27,28)29/h5-11,32H,3-4,12H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444510

(CHEMBL3092839)Show InChI InChI=1S/C20H23N3O2/c21-20(24)16-5-7-19(22-13-16)25-18-6-4-14-8-10-23(17-2-1-3-17)11-9-15(14)12-18/h4-7,12-13,17H,1-3,8-11H2,(H2,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469891

(CHEMBL322516)Show SMILES CCCc1nc(Cl)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C23H19BrClF3N4O4S/c1-2-5-17-30-21(25)19(22(29)33)32(17)11-12-8-9-16-14(10-12)18(24)20(36-16)13-6-3-4-7-15(13)31-37(34,35)23(26,27)28/h3-4,6-10,31H,2,5,11H2,1H3,(H2,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444492
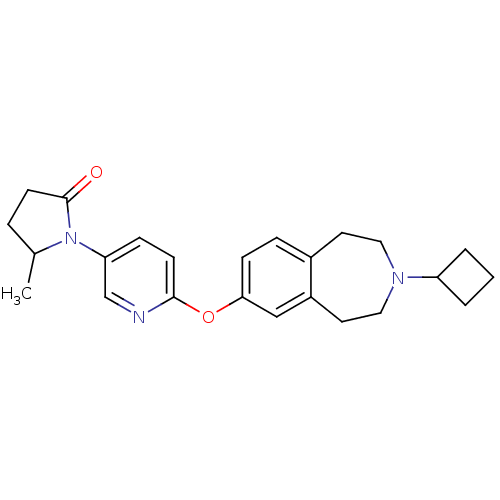
(CHEMBL3092822)Show SMILES CC1CCC(=O)N1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C24H29N3O2/c1-17-5-10-24(28)27(17)21-7-9-23(25-16-21)29-22-8-6-18-11-13-26(20-3-2-4-20)14-12-19(18)15-22/h6-9,15-17,20H,2-5,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444499

(CHEMBL3092835)Show SMILES Cc1noc(C)c1-c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C24H27N3O2/c1-16-24(17(2)29-26-16)20-7-9-23(25-15-20)28-22-8-6-18-10-12-27(21-4-3-5-21)13-11-19(18)14-22/h6-9,14-15,21H,3-5,10-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444497
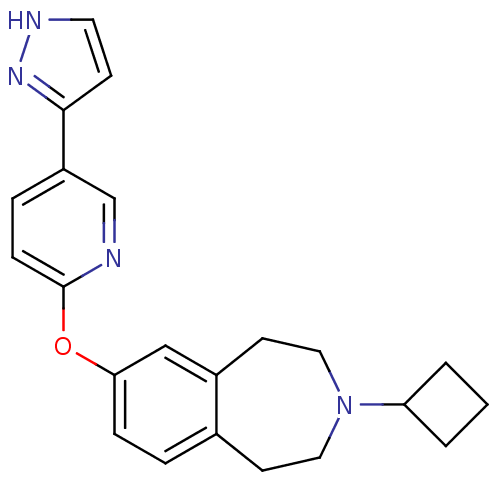
(CHEMBL3092649)Show SMILES C1CC(C1)N1CCc2ccc(Oc3ccc(cn3)-c3cc[nH]n3)cc2CC1 Show InChI InChI=1S/C22H24N4O/c1-2-19(3-1)26-12-9-16-4-6-20(14-17(16)10-13-26)27-22-7-5-18(15-23-22)21-8-11-24-25-21/h4-8,11,14-15,19H,1-3,9-10,12-13H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444490
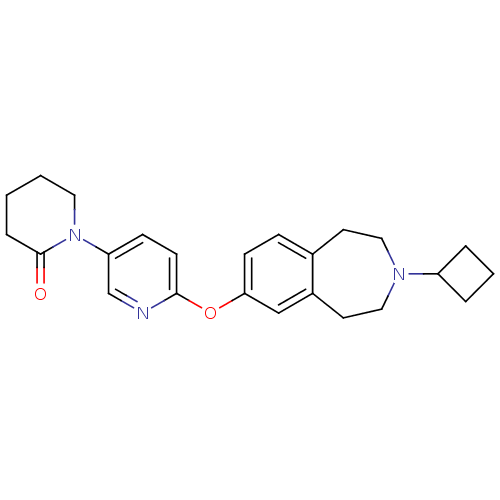
(CHEMBL3092824)Show SMILES O=C1CCCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C24H29N3O2/c28-24-6-1-2-13-27(24)21-8-10-23(25-17-21)29-22-9-7-18-11-14-26(20-4-3-5-20)15-12-19(18)16-22/h7-10,16-17,20H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469894
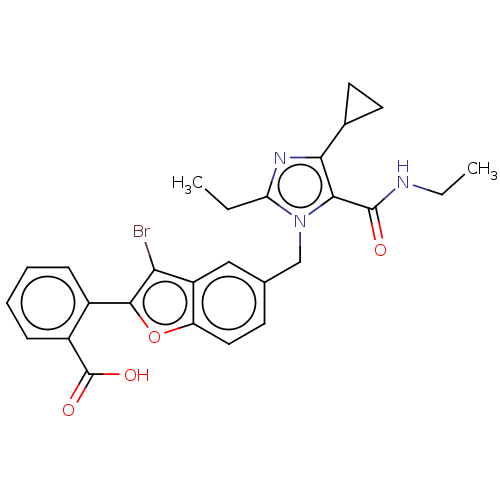
(CHEMBL323315)Show SMILES CCNC(=O)c1c(nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1C(O)=O)C1CC1 Show InChI InChI=1S/C27H26BrN3O4/c1-3-21-30-23(16-10-11-16)24(26(32)29-4-2)31(21)14-15-9-12-20-19(13-15)22(28)25(35-20)17-7-5-6-8-18(17)27(33)34/h5-9,12-13,16H,3-4,10-11,14H2,1-2H3,(H,29,32)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469884

(CHEMBL293447)Show SMILES CCc1nc(C)c(C(=O)N(C)C)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H24BrF3N4O4S/c1-5-20-30-14(2)22(24(34)32(3)4)33(20)13-15-10-11-19-17(12-15)21(26)23(37-19)16-8-6-7-9-18(16)31-38(35,36)25(27,28)29/h6-12,31H,5,13H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469933
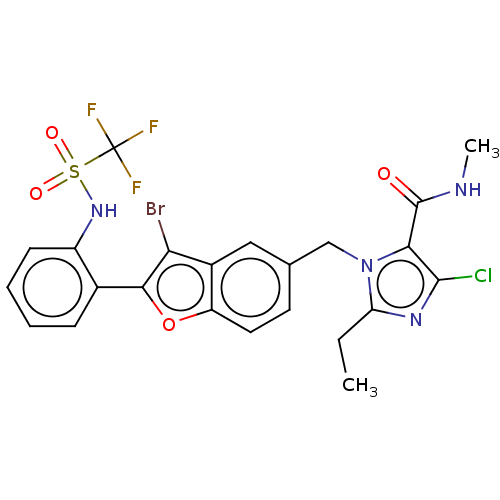
(CHEMBL323378)Show SMILES CCc1nc(Cl)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C23H19BrClF3N4O4S/c1-3-17-30-21(25)19(22(33)29-2)32(17)11-12-8-9-16-14(10-12)18(24)20(36-16)13-6-4-5-7-15(13)31-37(34,35)23(26,27)28/h4-10,31H,3,11H2,1-2H3,(H,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50247054

(6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C21H25N3O2/c1-22-21(25)17-6-8-20(23-14-17)26-19-7-5-15-9-11-24(18-3-2-4-18)12-10-16(15)13-19/h5-8,13-14,18H,2-4,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444498
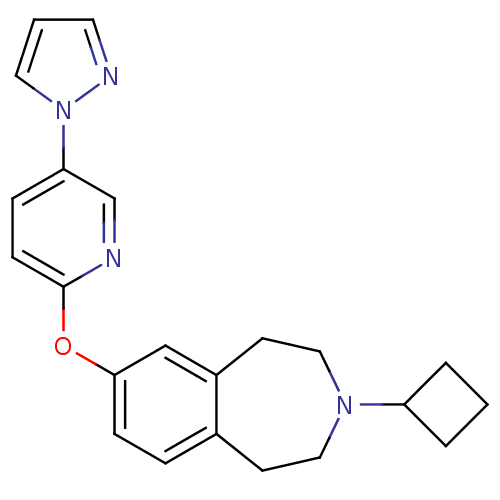
(CHEMBL3092648)Show SMILES C1CC(C1)N1CCc2ccc(Oc3ccc(cn3)-n3cccn3)cc2CC1 Show InChI InChI=1S/C22H24N4O/c1-3-19(4-1)25-13-9-17-5-7-21(15-18(17)10-14-25)27-22-8-6-20(16-23-22)26-12-2-11-24-26/h2,5-8,11-12,15-16,19H,1,3-4,9-10,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469941
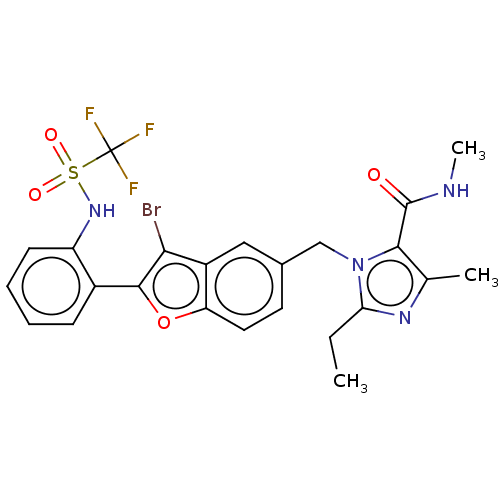
(CHEMBL292040)Show SMILES CCc1nc(C)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C24H22BrF3N4O4S/c1-4-19-30-13(2)21(23(33)29-3)32(19)12-14-9-10-18-16(11-14)20(25)22(36-18)15-7-5-6-8-17(15)31-37(34,35)24(26,27)28/h5-11,31H,4,12H2,1-3H3,(H,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469895
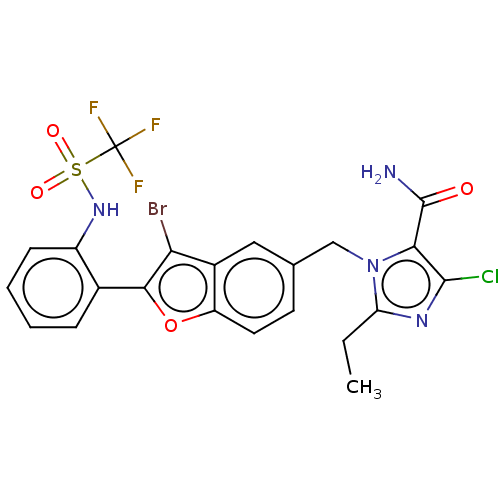
(CHEMBL104626)Show SMILES CCc1nc(Cl)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C22H17BrClF3N4O4S/c1-2-16-29-20(24)18(21(28)32)31(16)10-11-7-8-15-13(9-11)17(23)19(35-15)12-5-3-4-6-14(12)30-36(33,34)22(25,26)27/h3-9,30H,2,10H2,1H3,(H2,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444502
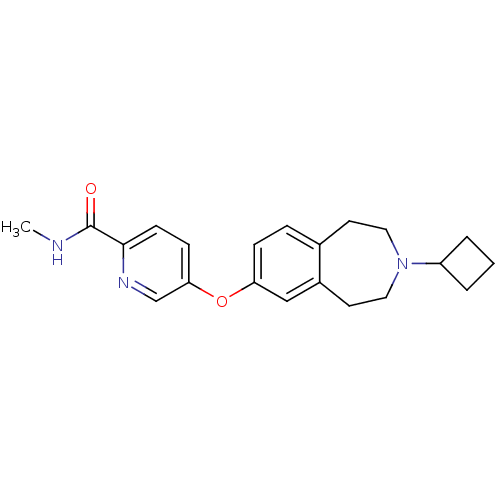
(CHEMBL3092831)Show InChI InChI=1S/C21H25N3O2/c1-22-21(25)20-8-7-19(14-23-20)26-18-6-5-15-9-11-24(17-3-2-4-17)12-10-16(15)13-18/h5-8,13-14,17H,2-4,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444501

(CHEMBL3092832)Show InChI InChI=1S/C22H27N3O2/c1-2-23-22(26)21-9-8-20(15-24-21)27-19-7-6-16-10-12-25(18-4-3-5-18)13-11-17(16)14-19/h6-9,14-15,18H,2-5,10-13H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444511

(CHEMBL3092838)Show InChI InChI=1S/C21H23N3O/c1-15-18(14-22)6-8-21(23-15)25-20-7-5-16-9-11-24(19-3-2-4-19)12-10-17(16)13-20/h5-8,13,19H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469936
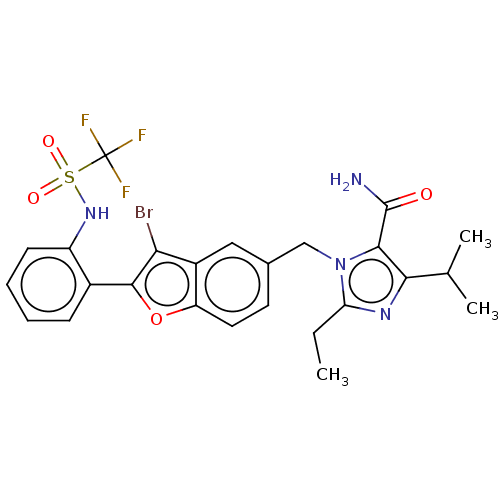
(CHEMBL108115)Show SMILES CCc1nc(C(C)C)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H24BrF3N4O4S/c1-4-19-31-21(13(2)3)22(24(30)34)33(19)12-14-9-10-18-16(11-14)20(26)23(37-18)15-7-5-6-8-17(15)32-38(35,36)25(27,28)29/h5-11,13,32H,4,12H2,1-3H3,(H2,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data