

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound was determined against to human cannabinoid receptor 2 in chinese hamster ovary cells | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089369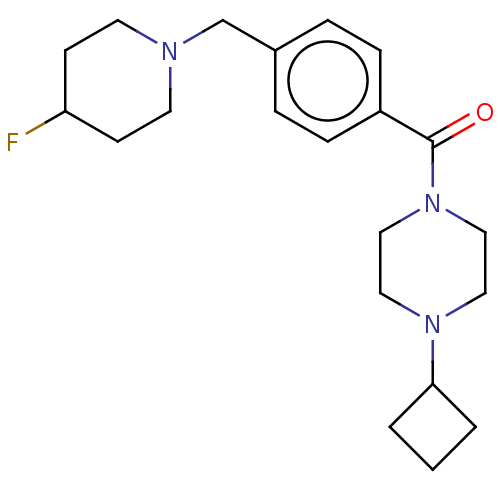 (CHEMBL3577959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089374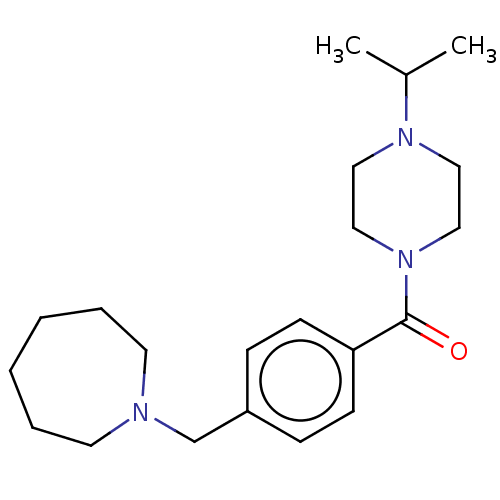 (CHEMBL3577954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50272598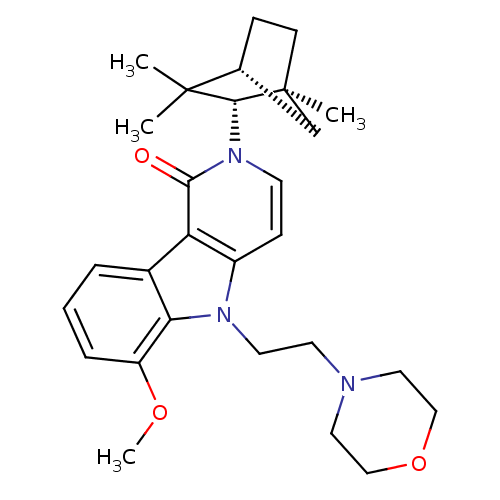 (6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089375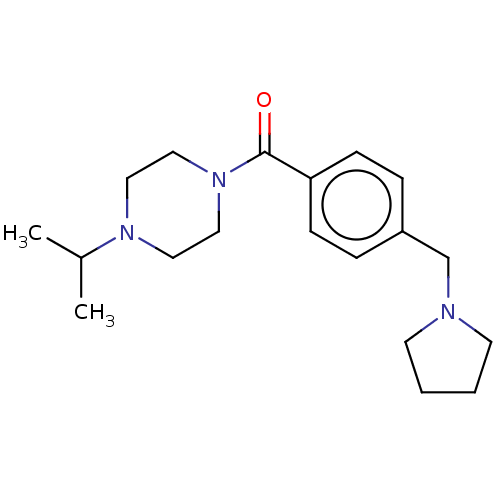 (CHEMBL3577953) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346208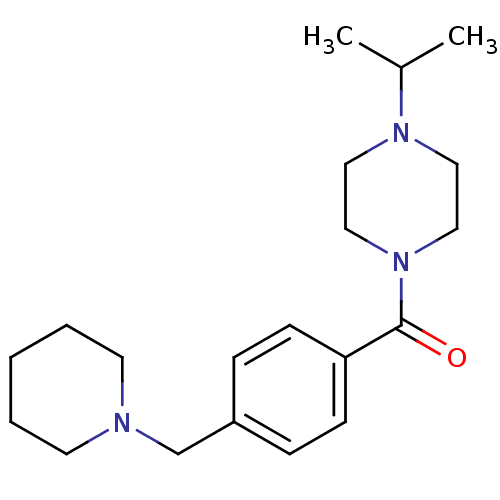 ((1-isopropylpiperidin-4-yl)(4-(piperidin-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089372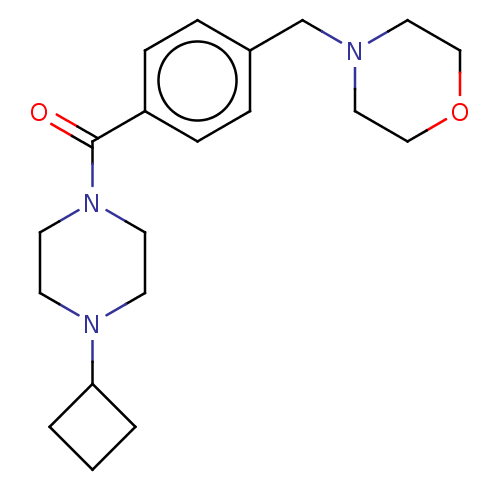 (CHEMBL3577956) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089370 (CHEMBL3577958) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound was determined against to human cannabinoid receptor 1 in chinese hamster ovary cells | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128092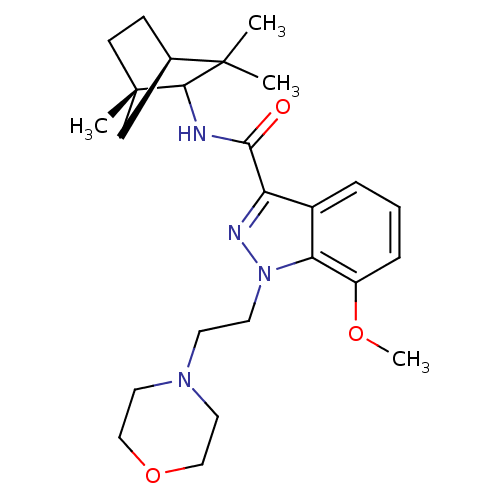 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indazole-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089368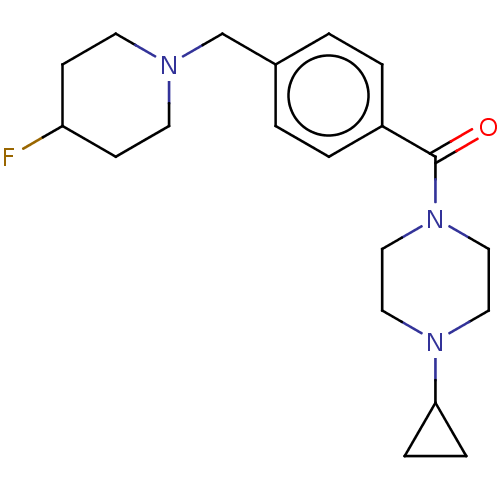 (CHEMBL3577960) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089373 (CHEMBL3577955) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089378 (CHEMBL3577951) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089371 (CHEMBL3577957) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089377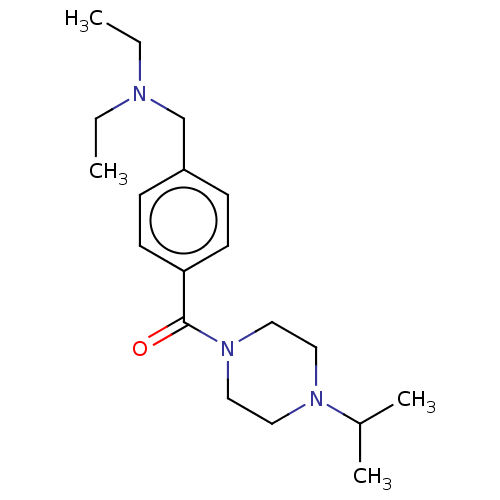 (CHEMBL3577952) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50128095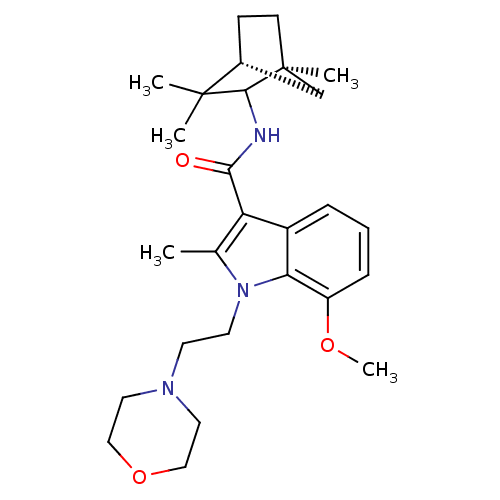 (7-Methoxy-2-methyl-1-(2-morpholin-4-yl-ethyl)-1H-i...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound against human cannabinoid receptor 1 in chinese hamster ovary cells by using radioligand CP-55940 | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128100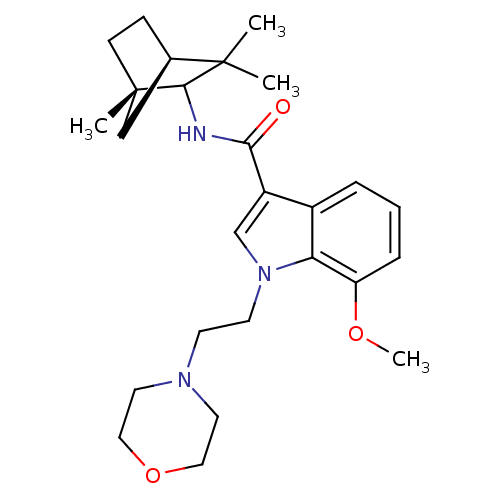 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indole-3-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50272598 (6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound against human cannabinoid receptor 1 in chinese hamster ovary cells by using radioligand CP-55940 | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089383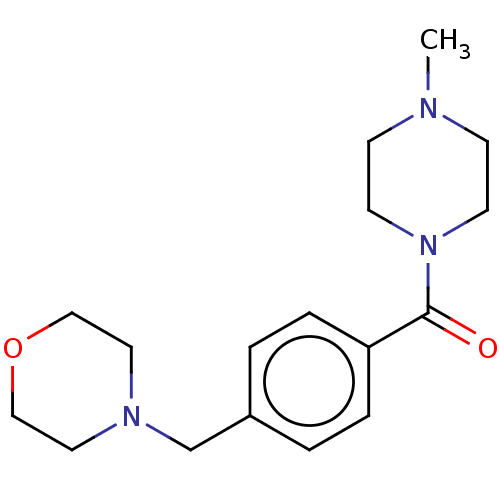 (CHEMBL1707983) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50128092 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indazole-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound against human cannabinoid receptor 1 in chinese hamster ovary cells by using radioligand CP-55940 | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50089384 (CHEMBL3577950) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins | ACS Med Chem Lett 6: 450-4 (2015) Article DOI: 10.1021/ml5005156 BindingDB Entry DOI: 10.7270/Q22F7Q59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128095 (7-Methoxy-2-methyl-1-(2-morpholin-4-yl-ethyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128094 (6-Methoxy-2-(2-methoxy-benzyl)-5-(2-morpholin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128096 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indazole-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128097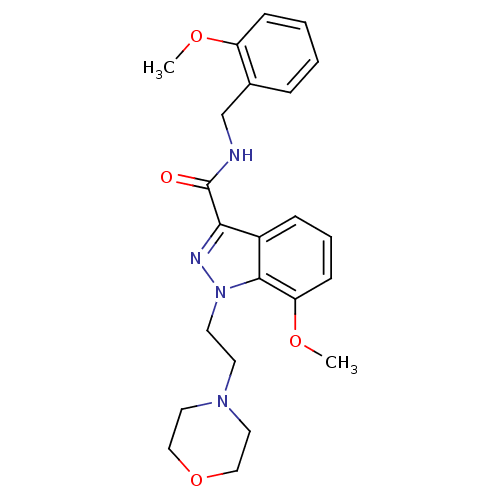 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indazole-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128098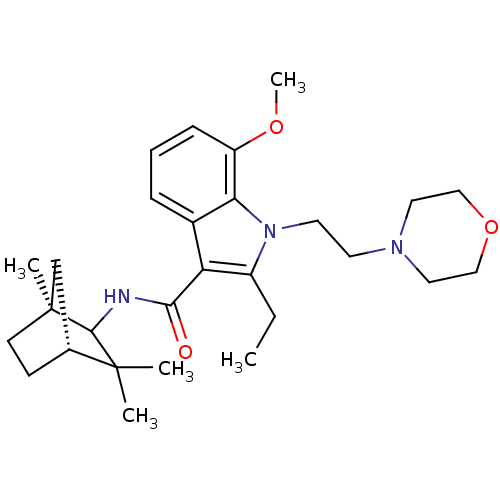 (2-Ethyl-7-methoxy-1-(2-morpholin-4-yl-ethyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50128093 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indazole-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H] | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50128093 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indazole-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound against human cannabinoid receptor 1 in chinese hamster ovary cells by using radioligand CP-55940 | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50128100 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indole-3-c...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound against human cannabinoid receptor 1 in chinese hamster ovary cells by using radioligand CP-55940 | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50128094 (6-Methoxy-2-(2-methoxy-benzyl)-5-(2-morpholin-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound against human cannabinoid receptor 1 in chinese hamster ovary cells by using radioligand CP-55940 | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163251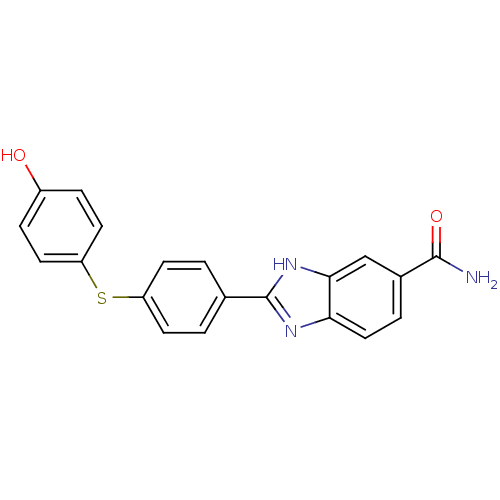 (2-(4-(4-hydroxyphenylthio)phenyl)-1H-benzo[d]imida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163246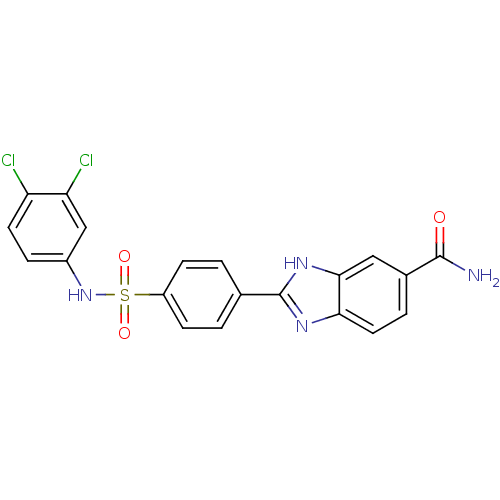 (2-[4-(3,4-Dichloro-phenylsulfamoyl)-phenyl]-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163259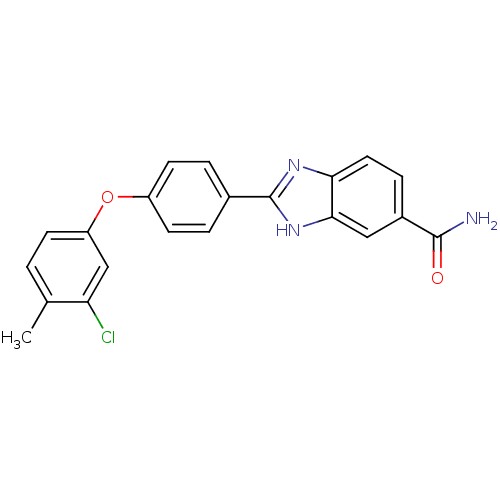 (2-[4-(3-Chloro-4-methyl-phenoxy)-phenyl]-1H-benzoi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163254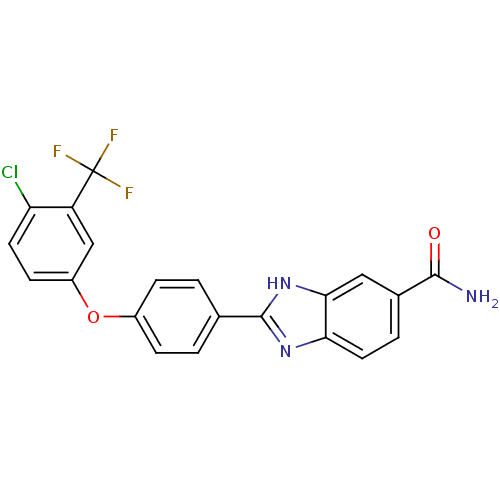 (2-[4-(4-Chloro-3-trifluoromethyl-phenoxy)-phenyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163266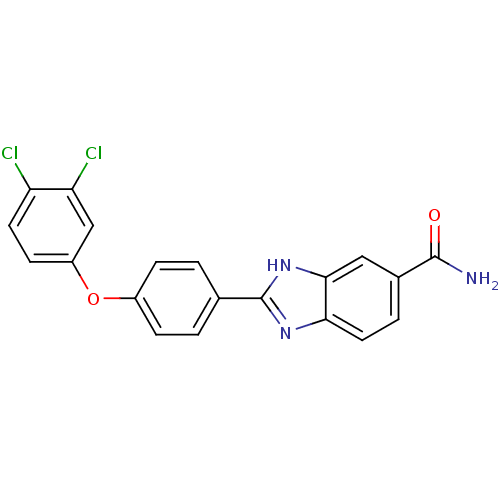 (2-[4-(3,4-Dichloro-phenoxy)-phenyl]-1H-benzoimidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163264 (2-[4-(4-Chloro-benzenesulfonyl)-phenyl]-1H-benzoim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163255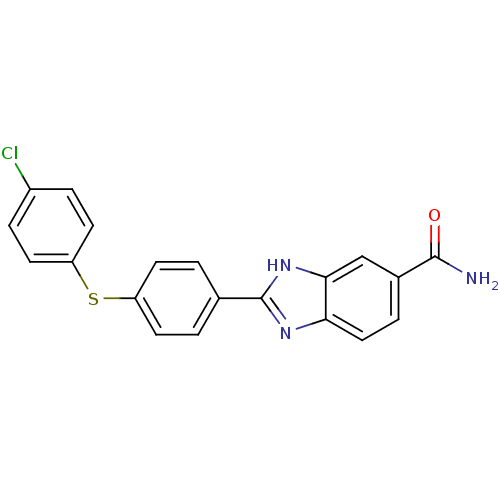 (2-[4-(4-Chloro-phenylsulfanyl)-phenyl]-1H-benzoimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163282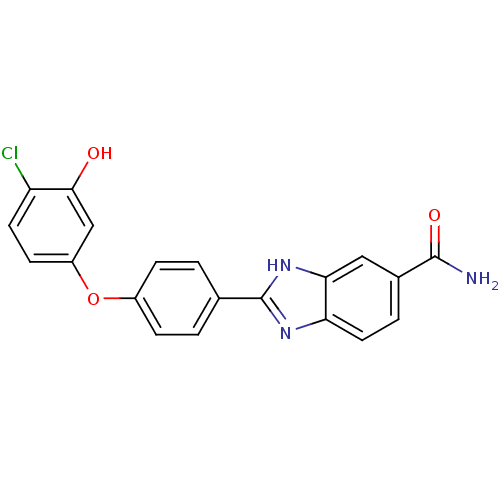 (2-[4-(4-Chloro-3-hydroxy-phenoxy)-phenyl]-1H-benzo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163250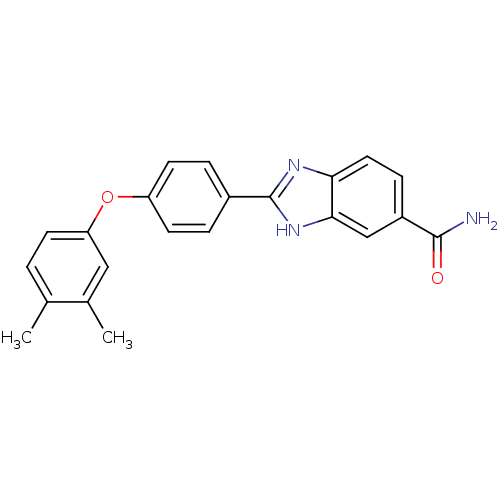 (2-[4-(3,4-Dimethyl-phenoxy)-phenyl]-1H-benzoimidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163278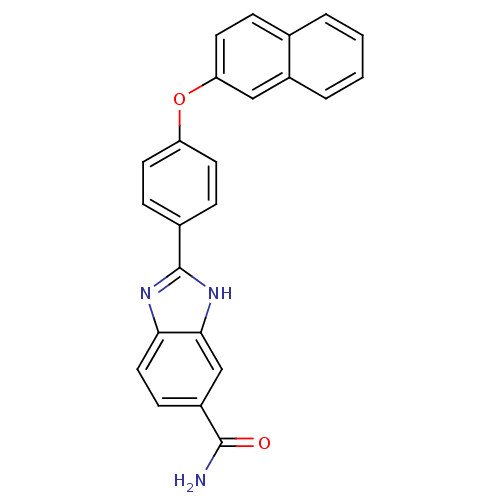 (2-[4-(Naphthalen-2-yloxy)-phenyl]-1H-benzoimidazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163270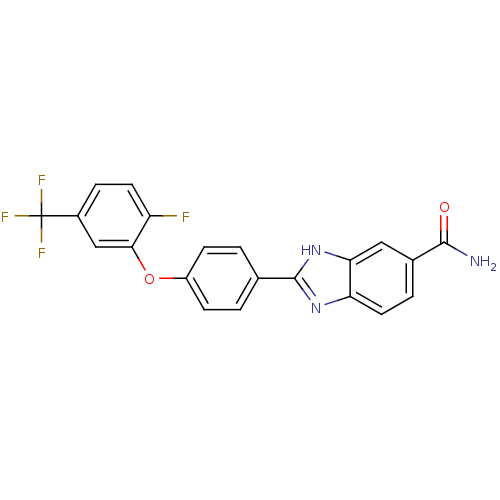 (2-[4-(2-Fluoro-5-trifluoromethyl-phenoxy)-phenyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163269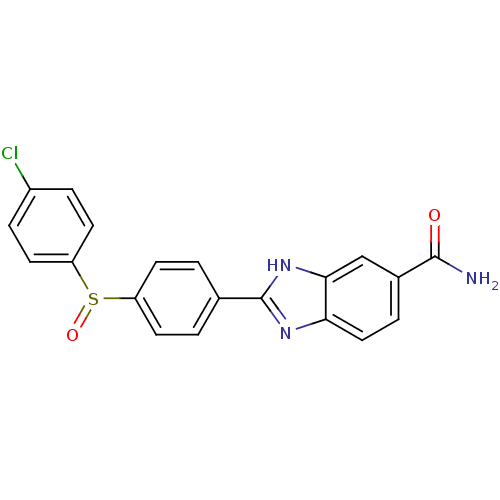 (2-[4-(4-Chloro-benzenesulfinyl)-phenyl]-1H-benzoim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163273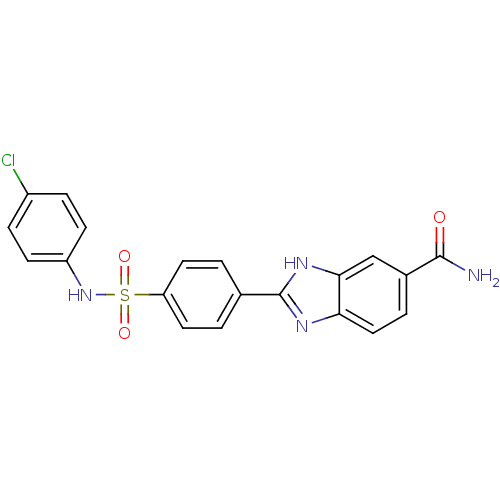 (2-[4-(4-Chloro-phenylsulfamoyl)-phenyl]-1H-benzoim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM34064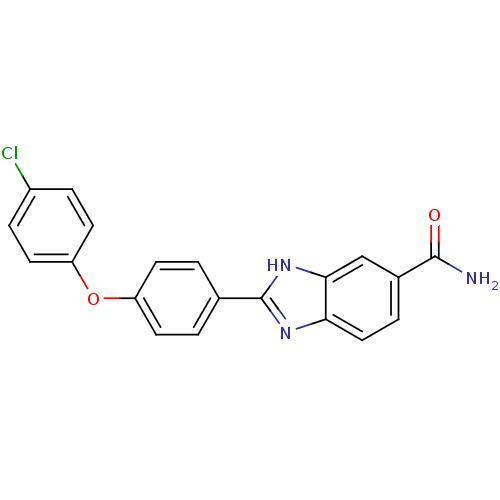 (2-arylbenzimidazole | CHEMBL179583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM34064 (2-arylbenzimidazole | CHEMBL179583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163271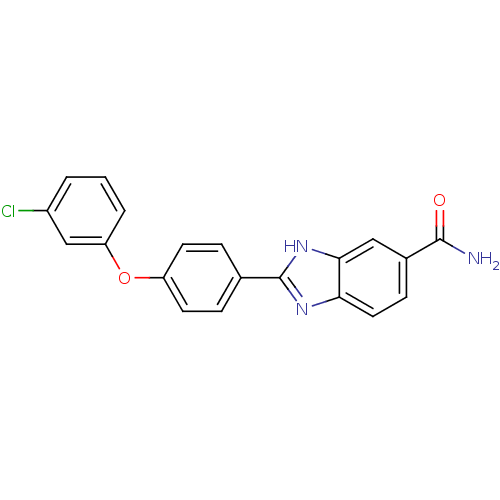 (2-[4-(3-Chloro-phenoxy)-phenyl]-1H-benzoimidazole-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163274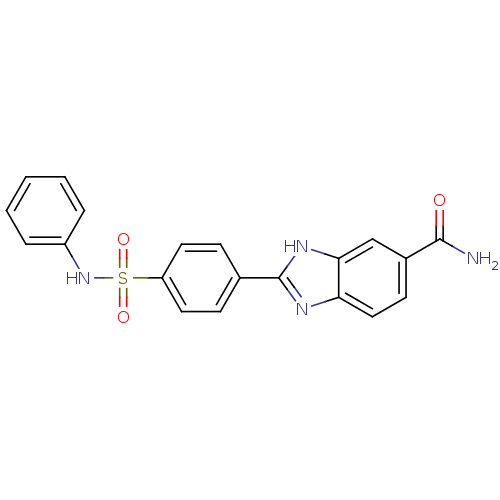 (2-(4-Phenylsulfamoyl-phenyl)-1H-benzoimidazole-5-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163260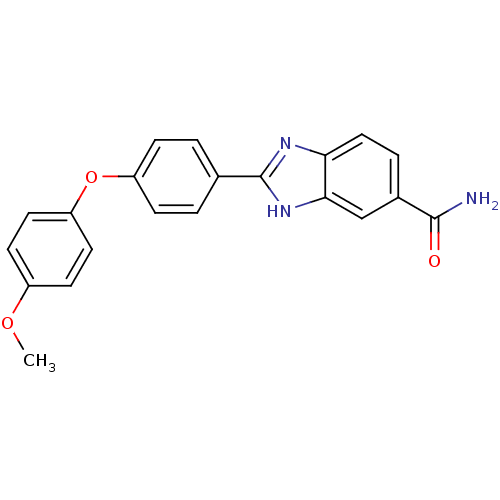 (2-[4-(4-Methoxy-phenoxy)-phenyl]-1H-benzoimidazole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163277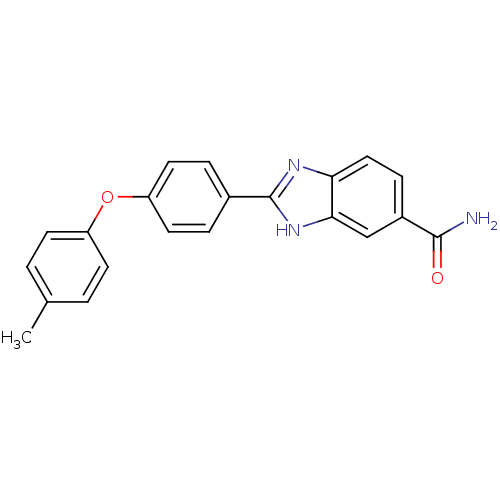 (2-(4-p-Tolyloxy-phenyl)-1H-benzoimidazole-5-carbox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163249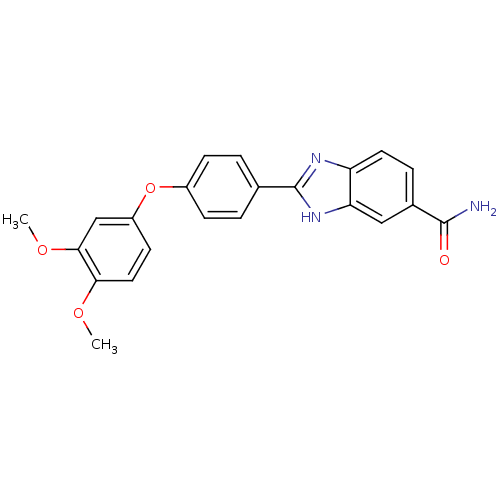 (2-[4-(3,4-Dimethoxy-phenoxy)-phenyl]-1H-benzoimida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |