 Found 148 hits with Last Name = 'pandya' and Initial = 'v'
Found 148 hits with Last Name = 'pandya' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM302860
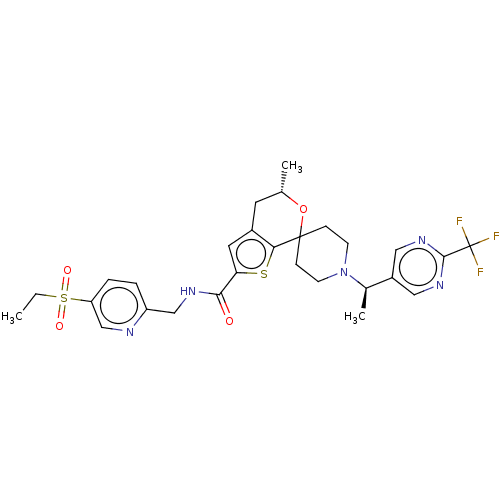
((2S)-1-(3-Thienyl)propan-2-ol | US9598431, 3)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3C[C@H](C)OC4(CCN(CC4)[C@H](C)c4cnc(nc4)C(F)(F)F)c3s2)nc1 |r| Show InChI InChI=1S/C28H32F3N5O4S2/c1-4-42(38,39)22-6-5-21(32-16-22)15-33-25(37)23-12-19-11-17(2)40-27(24(19)41-23)7-9-36(10-8-27)18(3)20-13-34-26(35-14-20)28(29,30)31/h5-6,12-14,16-18H,4,7-11,15H2,1-3H3,(H,33,37)/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM351740
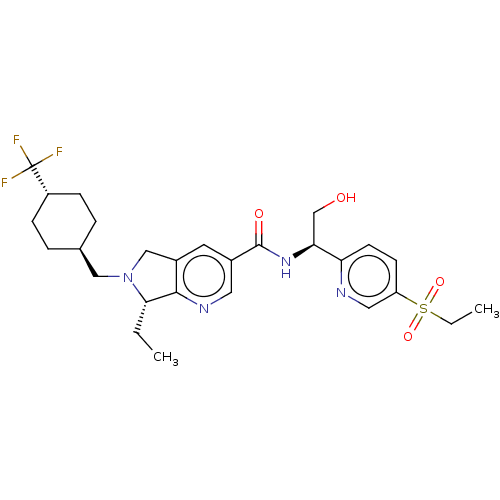
(US9796710, Compound 33)Show SMILES CC[C@@H]1N(C[C@H]2CC[C@@H](CC2)C(F)(F)F)Cc2cc(cnc12)C(=O)N[C@@H](CO)c1ccc(cn1)S(=O)(=O)CC |r,wU:8.11,25.27,wD:5.4,2.1,(-5.82,-4.43,;-4.33,-4.03,;-3.93,-2.54,;-4.84,-1.3,;-6.38,-1.3,;-7.15,.04,;-6.38,1.37,;-7.15,2.7,;-8.69,2.7,;-9.46,1.37,;-8.69,.04,;-9.46,4.04,;-8.69,5.37,;-11,4.04,;-10.23,5.37,;-3.93,-.05,;-2.47,-.53,;-1.13,.24,;.2,-.53,;.2,-2.07,;-1.13,-2.84,;-2.47,-2.07,;1.53,.24,;1.53,1.78,;2.87,-.53,;4.2,.24,;4.2,1.78,;5.53,2.55,;5.53,-.53,;6.87,.24,;8.2,-.53,;8.27,-2.15,;6.87,-2.84,;5.53,-2.07,;9.59,-2.93,;8.8,-4.26,;10.38,-1.61,;10.91,-3.72,;12.26,-2.97,)| Show InChI InChI=1S/C27H35F3N4O4S/c1-3-24-25-19(15-34(24)14-17-5-7-20(8-6-17)27(28,29)30)11-18(12-32-25)26(36)33-23(16-35)22-10-9-21(13-31-22)39(37,38)4-2/h9-13,17,20,23-24,35H,3-8,14-16H2,1-2H3,(H,33,36)/t17-,20-,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385806
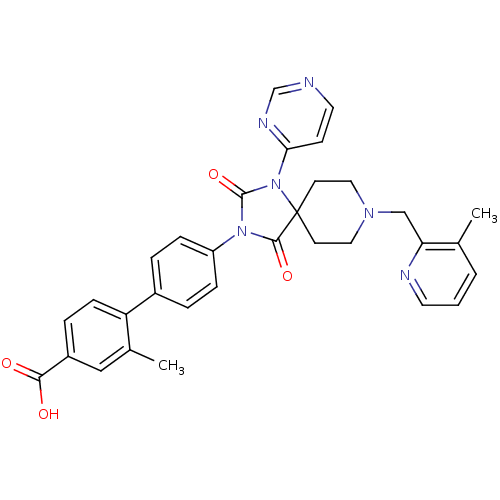
(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD1 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385806

(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316499

(CHEMBL4167582)Show SMILES OC(=O)CCNC(=O)c1cc(O)c(cn1)C(=O)NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H21N3O5/c27-19-13-18(23(31)24-12-11-20(28)29)25-14-17(19)22(30)26-21(15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,13-14,21H,11-12H2,(H,24,31)(H,25,27)(H,26,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316852

(CHEMBL4171446)Show SMILES Oc1nc(ncc1NC(=O)Cc1ccc(cc1)-c1ccccc1)-n1cccn1 Show InChI InChI=1S/C21H17N5O2/c27-19(13-15-7-9-17(10-8-15)16-5-2-1-3-6-16)24-18-14-22-21(25-20(18)28)26-12-4-11-23-26/h1-12,14H,13H2,(H,24,27)(H,22,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316816
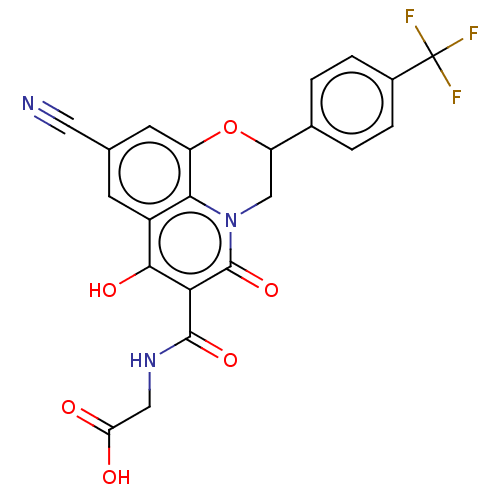
(CHEMBL4161223)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3OC(Cn(c23)c1=O)c1ccc(cc1)C(F)(F)F)C#N Show InChI InChI=1S/C22H14F3N3O6/c23-22(24,25)12-3-1-11(2-4-12)15-9-28-18-13(5-10(7-26)6-14(18)34-15)19(31)17(21(28)33)20(32)27-8-16(29)30/h1-6,15,31H,8-9H2,(H,27,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316816

(CHEMBL4161223)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3OC(Cn(c23)c1=O)c1ccc(cc1)C(F)(F)F)C#N Show InChI InChI=1S/C22H14F3N3O6/c23-22(24,25)12-3-1-11(2-4-12)15-9-28-18-13(5-10(7-26)6-14(18)34-15)19(31)17(21(28)33)20(32)27-8-16(29)30/h1-6,15,31H,8-9H2,(H,27,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN3
(Homo sapiens (Human)) | BDBM50317545
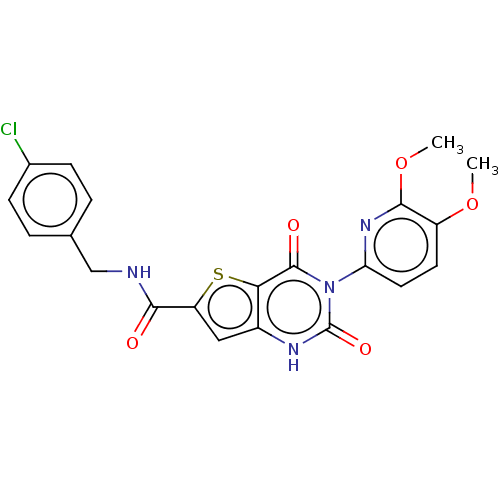
(CHEMBL4173371)Show SMILES COc1ccc(nc1OC)-n1c(=O)[nH]c2cc(sc2c1=O)C(=O)NCc1ccc(Cl)cc1 Show InChI InChI=1S/C21H17ClN4O5S/c1-30-14-7-8-16(25-19(14)31-2)26-20(28)17-13(24-21(26)29)9-15(32-17)18(27)23-10-11-3-5-12(22)6-4-11/h3-9H,10H2,1-2H3,(H,23,27)(H,24,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD3 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN3
(Homo sapiens (Human)) | BDBM50446903
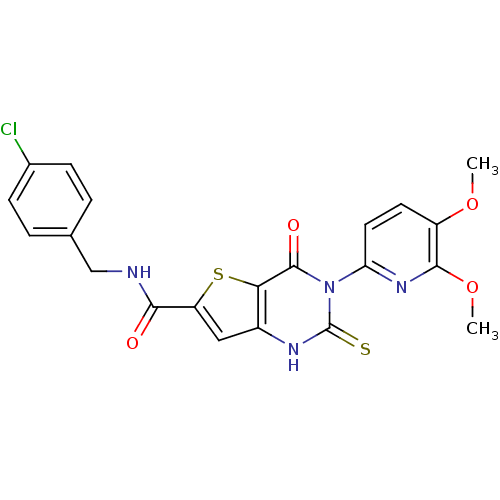
(CHEMBL3115293)Show SMILES COc1ccc(nc1OC)-n1c(=S)[nH]c2cc(sc2c1=O)C(=O)NCc1ccc(Cl)cc1 Show InChI InChI=1S/C21H17ClN4O4S2/c1-29-14-7-8-16(25-19(14)30-2)26-20(28)17-13(24-21(26)31)9-15(32-17)18(27)23-10-11-3-5-12(22)6-4-11/h3-9H,10H2,1-2H3,(H,23,27)(H,24,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD3 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50321025

(CHEMBL4170335)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3NC(Cn(c23)c1=O)c1ccc(cc1)C(F)(F)F)C#N Show InChI InChI=1S/C22H15F3N4O5/c23-22(24,25)12-3-1-11(2-4-12)15-9-29-18-13(5-10(7-26)6-14(18)28-15)19(32)17(21(29)34)20(33)27-8-16(30)31/h1-6,15,28,32H,8-9H2,(H,27,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50594741
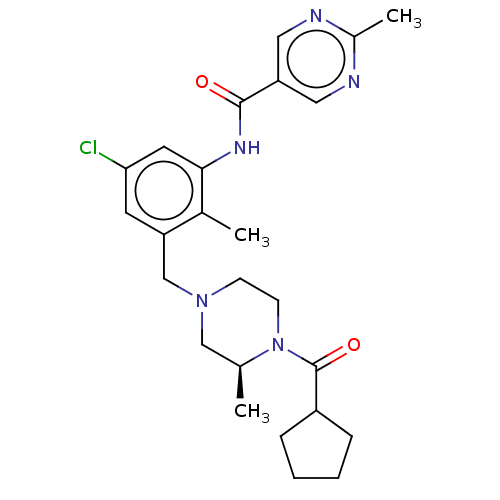
(CHEMBL5205088)Show SMILES C[C@H]1CN(Cc2cc(Cl)cc(NC(=O)c3cnc(C)nc3)c2C)CCN1C(=O)C1CCCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316844

(CHEMBL4162417)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3SC(Cn(c23)c1=O)c1ccc(cc1)C(F)(F)F)C#N Show InChI InChI=1S/C22H14F3N3O5S/c23-22(24,25)12-3-1-11(2-4-12)15-9-28-18-13(5-10(7-26)6-14(18)34-15)19(31)17(21(28)33)20(32)27-8-16(29)30/h1-6,15,31H,8-9H2,(H,27,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
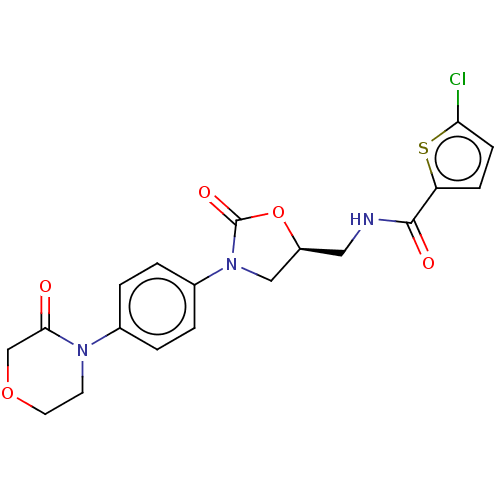
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl hydroxylase EGLN3
(Homo sapiens (Human)) | BDBM50385806

(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD3 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50317465
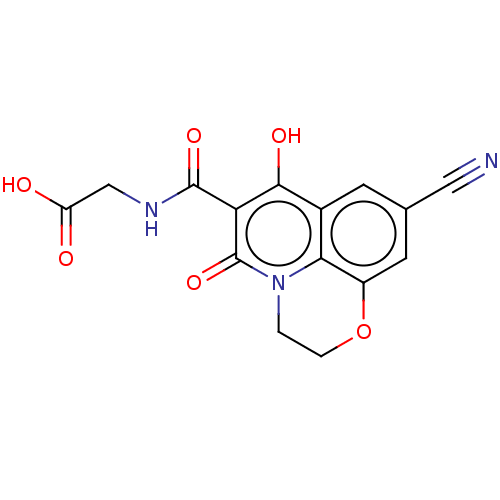
(CHEMBL4165865)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3OCCn(c23)c1=O)C#N Show InChI InChI=1S/C15H11N3O6/c16-5-7-3-8-12-9(4-7)24-2-1-18(12)15(23)11(13(8)21)14(22)17-6-10(19)20/h3-4,21H,1-2,6H2,(H,17,22)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | CHEMBL4099342

| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50318459

(CHEMBL4159227)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3CC(Cn(c23)c1=O)c1ccc(cc1)C(F)(F)F)C#N Show InChI InChI=1S/C23H16F3N3O5/c24-23(25,26)15-3-1-12(2-4-15)14-7-13-5-11(8-27)6-16-19(13)29(10-14)22(34)18(20(16)32)21(33)28-9-17(30)31/h1-6,14,32H,7,9-10H2,(H,28,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | CHEMBL5276516
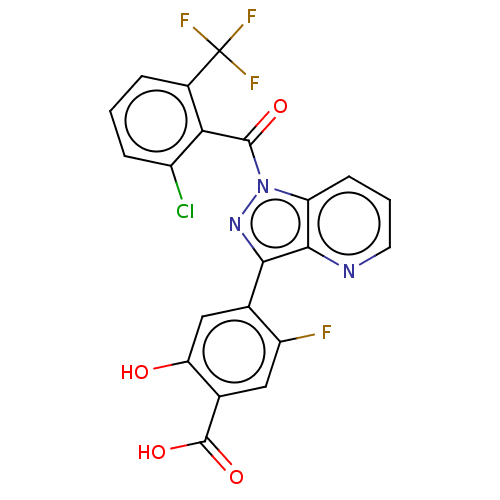
| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50509565
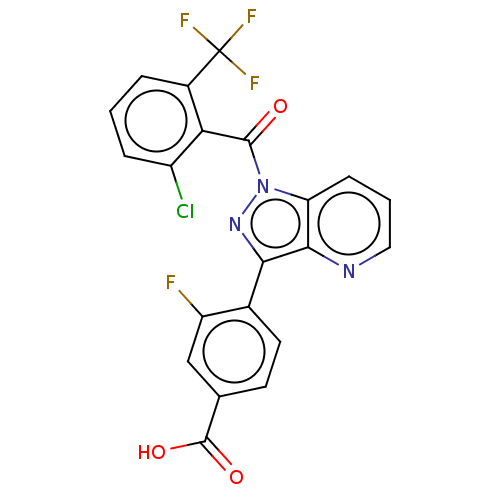
(CHEMBL3558130)Show SMILES OC(=O)c1ccc(-c2nn(C(=O)c3c(Cl)cccc3C(F)(F)F)c3cccnc23)c(F)c1 Show InChI InChI=1S/C21H10ClF4N3O3/c22-13-4-1-3-12(21(24,25)26)16(13)19(30)29-15-5-2-8-27-18(15)17(28-29)11-7-6-10(20(31)32)9-14(11)23/h1-9H,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | CHEMBL5270756

| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | CHEMBL5277587
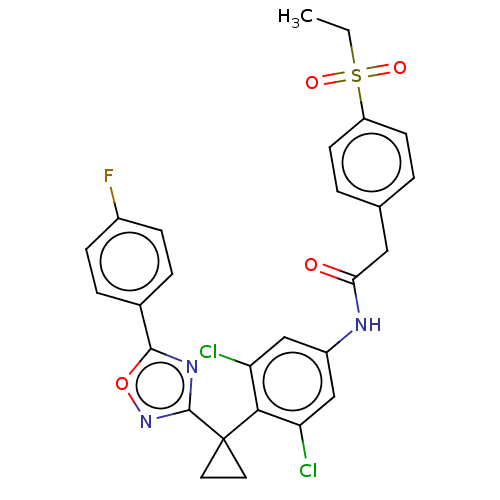
| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392595
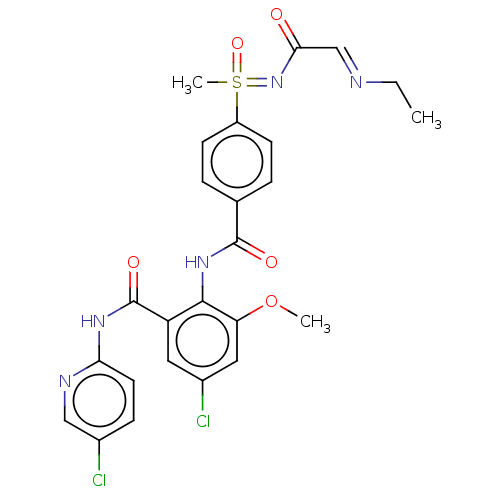
(CHEMBL2153392)Show SMILES CC\N=C\C(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1c(OC)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H25Cl2N5O5S/c1-4-28-14-22(33)32-38(3,36)18-8-5-15(6-9-18)24(34)31-23-19(11-17(27)12-20(23)37-2)25(35)30-21-10-7-16(26)13-29-21/h5-14,38H,4H2,1-3H3,(H,31,34)(H,29,30,35)(H,32,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | CHEMBL5272739

| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392593
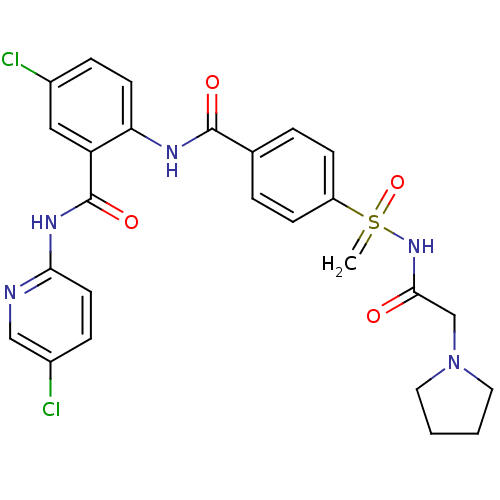
(CHEMBL2153383)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)S(=C)(=O)NC(=O)CN2CCCC2)nc1 Show InChI InChI=1S/C26H25Cl2N5O4S/c1-38(37,32-24(34)16-33-12-2-3-13-33)20-8-4-17(5-9-20)25(35)30-22-10-6-18(27)14-21(22)26(36)31-23-11-7-19(28)15-29-23/h4-11,14-15H,1-3,12-13,16H2,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392596
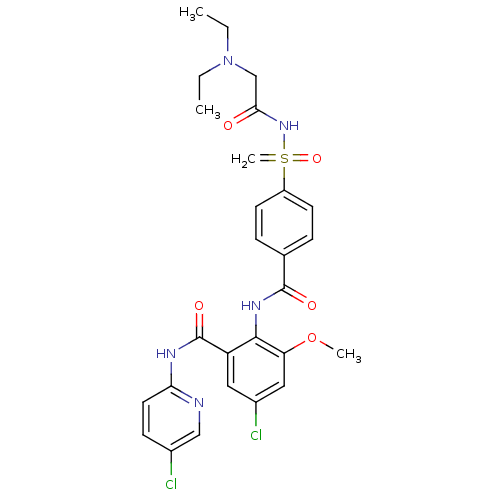
(CHEMBL2153393)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1c(OC)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C27H29Cl2N5O5S/c1-5-34(6-2)16-24(35)33-40(4,38)20-10-7-17(8-11-20)26(36)32-25-21(13-19(29)14-22(25)39-3)27(37)31-23-12-9-18(28)15-30-23/h7-15H,4-6,16H2,1-3H3,(H,32,36)(H,30,31,37)(H,33,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50317547

(CHEMBL4163812)Show SMILES OC(=O)CNC(=O)c1c(O)n(C2CCCCC2)c(=O)n(C2CCCCC2)c1=O Show InChI InChI=1S/C19H27N3O6/c23-14(24)11-20-16(25)15-17(26)21(12-7-3-1-4-8-12)19(28)22(18(15)27)13-9-5-2-6-10-13/h12-13,26H,1-11H2,(H,20,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of PHD1 (unknown origin) |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM412666

((S)-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-7-is...)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cnc3[C@H](C(C)C)N(C[C@H]4CC[C@@H](CC4)C(F)(F)F)Cc3c2)nc1 |r,wU:26.29,wD:17.17,23.22,(9.73,-1.18,;8.39,-1.95,;7.06,-1.18,;6.29,-2.52,;7.83,.15,;5.72,-.41,;5.68,1.15,;4.34,1.93,;3.01,1.15,;1.68,1.92,;.34,1.15,;-.99,1.92,;-.99,3.46,;-2.33,1.15,;-2.33,-.39,;-3.66,-1.16,;-4.99,-.39,;-6.46,-.86,;-6.86,-2.35,;-5.77,-3.44,;-8.34,-2.75,;-7.36,.38,;-8.9,.38,;-9.67,1.72,;-8.9,3.05,;-9.67,4.39,;-11.21,4.39,;-11.98,3.05,;-11.21,1.72,;-11.98,5.72,;-13.52,5.72,;-11.21,7.05,;-10.44,5.72,;-6.46,1.63,;-4.99,1.15,;-3.66,1.92,;3.01,-.39,;4.34,-1.16,)| Show InChI InChI=1S/C27H35F3N4O3S/c1-4-38(36,37)23-10-9-22(31-14-23)13-33-26(35)19-11-20-16-34(25(17(2)3)24(20)32-12-19)15-18-5-7-21(8-6-18)27(28,29)30/h9-12,14,17-18,21,25H,4-8,13,15-16H2,1-3H3,(H,33,35)/t18-,21-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| PDB
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50509558
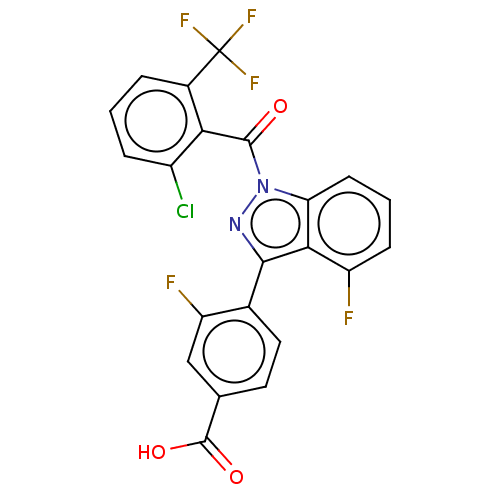
(CHEMBL4449356)Show SMILES OC(=O)c1ccc(-c2nn(C(=O)c3c(Cl)cccc3C(F)(F)F)c3cccc(F)c23)c(F)c1 Show InChI InChI=1S/C22H10ClF5N2O3/c23-13-4-1-3-12(22(26,27)28)17(13)20(31)30-16-6-2-5-14(24)18(16)19(29-30)11-8-7-10(21(32)33)9-15(11)25/h1-9H,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392590
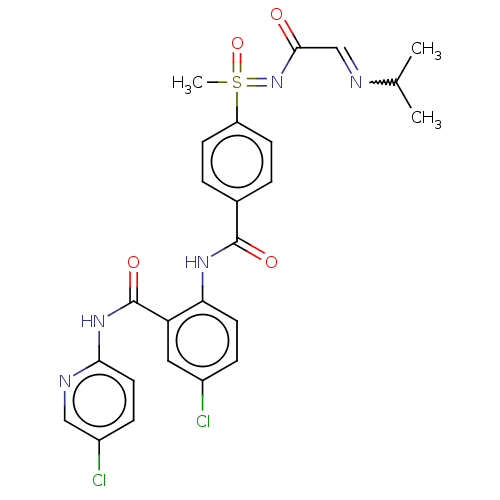
(CHEMBL2153378)Show SMILES CC(C)N=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:4.4| Show InChI InChI=1S/C25H25Cl2N5O4S/c1-15(2)28-14-23(33)32-37(3,36)19-8-4-16(5-9-19)24(34)30-21-10-6-17(26)12-20(21)25(35)31-22-11-7-18(27)13-29-22/h4-15,37H,1-3H3,(H,30,34)(H,29,31,35)(H,32,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM350280
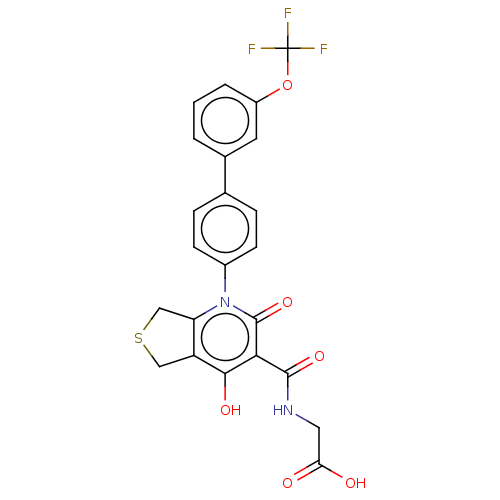
(2-(4-Hydroxy-2-oxo-1-(3'- (trifluoromethoxy)-[1,1'...)Show SMILES OC(=O)CNC(=O)c1c(O)c2CSCc2n(-c2ccc(cc2)-c2cccc(OC(F)(F)F)c2)c1=O Show InChI InChI=1S/C23H17F3N2O6S/c24-23(25,26)34-15-3-1-2-13(8-15)12-4-6-14(7-5-12)28-17-11-35-10-16(17)20(31)19(22(28)33)21(32)27-9-18(29)30/h1-8,31H,9-11H2,(H,27,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | CHEMBL5270326
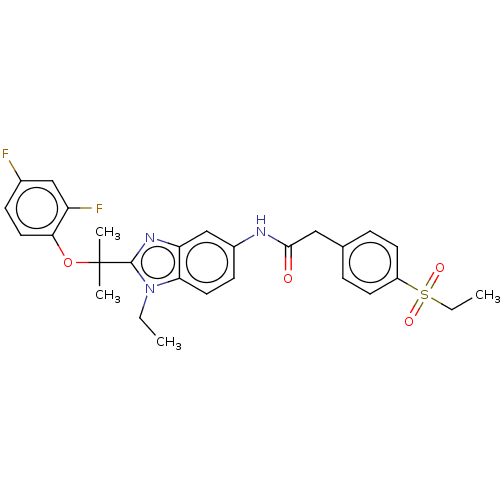
| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM189897
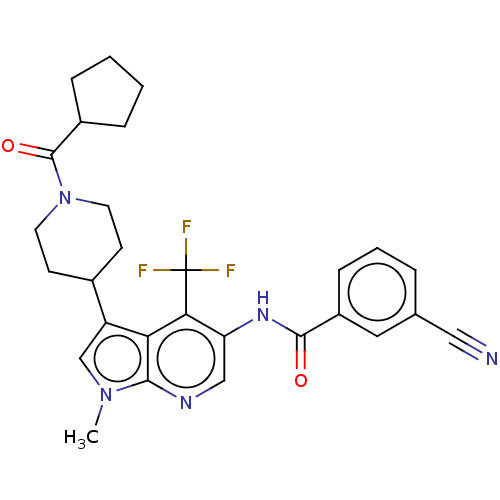
(US10227346, Example 23 | US10426135, Example 23 | ...)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2c(c(NC(=O)c3cccc(c3)C#N)cnc12)C(F)(F)F Show InChI InChI=1S/C28H28F3N5O2/c1-35-16-21(18-9-11-36(12-10-18)27(38)19-6-2-3-7-19)23-24(28(29,30)31)22(15-33-25(23)35)34-26(37)20-8-4-5-17(13-20)14-32/h4-5,8,13,15-16,18-19H,2-3,6-7,9-12H2,1H3,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | CHEMBL5280104
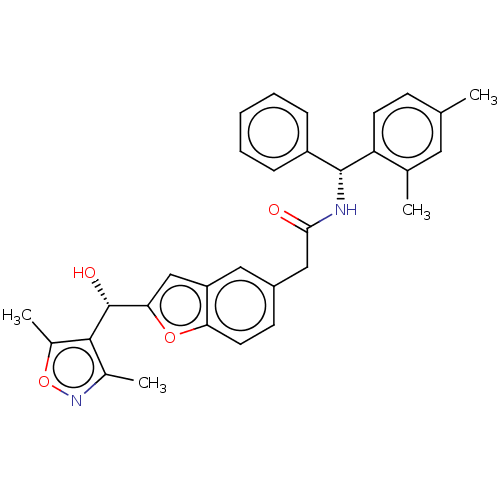
| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
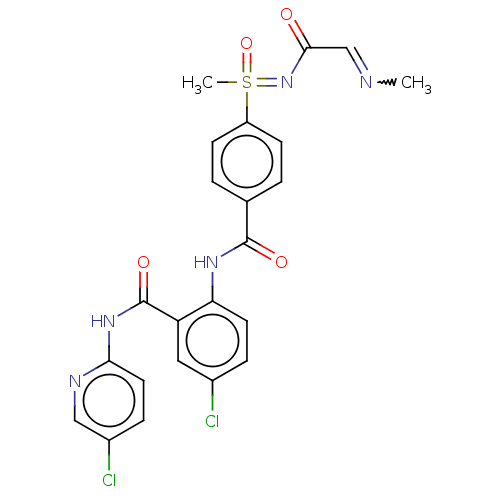
(Homo sapiens (Human)) | BDBM50392588
(CHEMBL2153376)Show SMILES CN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:1.0| Show InChI InChI=1S/C23H21Cl2N5O4S/c1-26-13-21(31)30-35(2,34)17-7-3-14(4-8-17)22(32)28-19-9-5-15(24)11-18(19)23(33)29-20-10-6-16(25)12-27-20/h3-13,35H,1-2H3,(H,28,32)(H,27,29,33)(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50318681

(CHEMBL4162309)Show SMILES OC(=O)CNC(=O)c1c(O)c2COCCc2n(Cc2nc3ccccc3s2)c1=O Show InChI InChI=1S/C19H17N3O6S/c23-15(24)7-20-18(26)16-17(25)10-9-28-6-5-12(10)22(19(16)27)8-14-21-11-3-1-2-4-13(11)29-14/h1-4,25H,5-9H2,(H,20,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN3
(Homo sapiens (Human)) | BDBM50317547

(CHEMBL4163812)Show SMILES OC(=O)CNC(=O)c1c(O)n(C2CCCCC2)c(=O)n(C2CCCCC2)c1=O Show InChI InChI=1S/C19H27N3O6/c23-14(24)11-20-16(25)15-17(26)21(12-7-3-1-4-8-12)19(28)22(18(15)27)13-9-5-2-6-10-13/h12-13,26H,1-11H2,(H,20,25)(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of PHD3 (unknown origin) |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
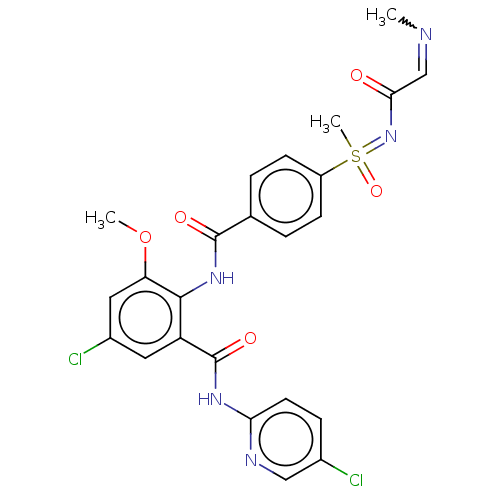
(Homo sapiens (Human)) | BDBM50392594
(CHEMBL2153391)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)S(C)(=O)=NC(=O)C=NC |w:35.38| Show InChI InChI=1S/C24H23Cl2N5O5S/c1-27-13-21(32)31-37(3,35)17-7-4-14(5-8-17)23(33)30-22-18(10-16(26)11-19(22)36-2)24(34)29-20-9-6-15(25)12-28-20/h4-13,37H,1-3H3,(H,30,33)(H,28,29,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data