 Found 2643 hits with Last Name = 'pang' and Initial = 'j'
Found 2643 hits with Last Name = 'pang' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602306
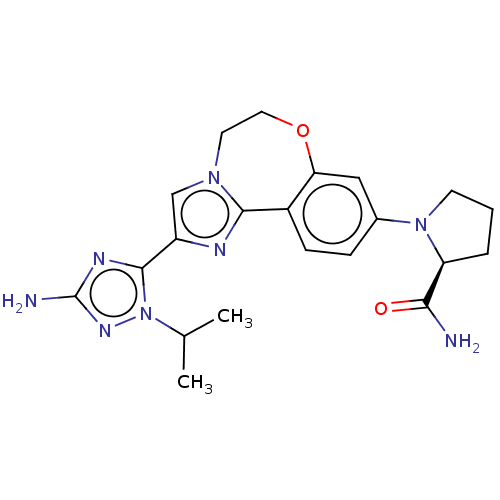
(CHEMBL5208487)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149477
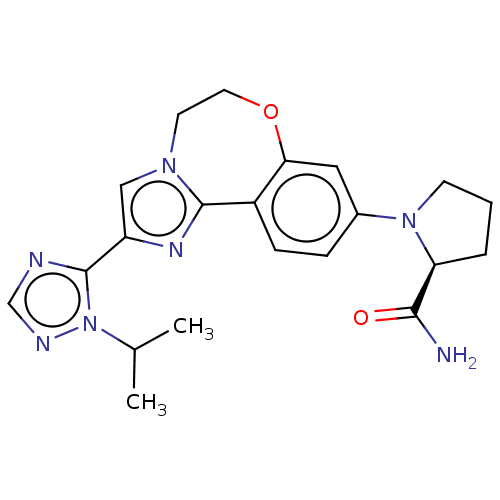
(CHEMBL3770993 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H25N7O2/c1-13(2)28-21(23-12-24-28)16-11-26-8-9-30-18-10-14(5-6-15(18)20(26)25-16)27-7-3-4-17(27)19(22)29/h5-6,10-13,17H,3-4,7-9H2,1-2H3,(H2,22,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295665
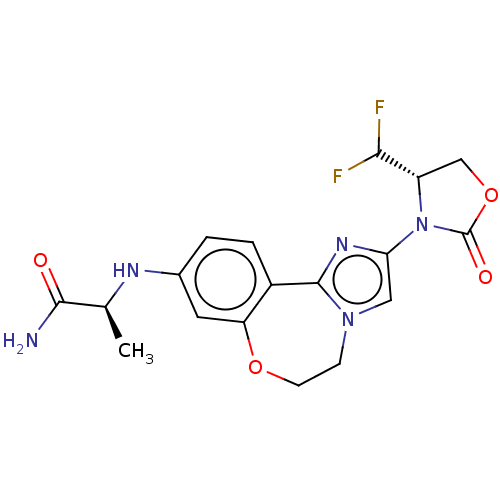
((S)-2-((2-((S)-4-(difluoromethyl)- 2-oxooxazolidin...)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C(N)=O |r| Show InChI InChI=1S/C18H19F2N5O4/c1-9(16(21)26)22-10-2-3-11-13(6-10)28-5-4-24-7-14(23-17(11)24)25-12(15(19)20)8-29-18(25)27/h2-3,6-7,9,12,15,22H,4-5,8H2,1H3,(H2,21,26)/t9-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433530
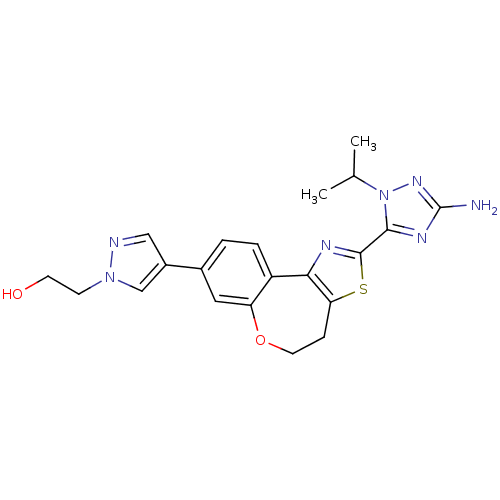
(CHEMBL2381382)Show SMILES CC(C)n1nc(N)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H23N7O2S/c1-12(2)28-19(25-21(22)26-28)20-24-18-15-4-3-13(14-10-23-27(11-14)6-7-29)9-16(15)30-8-5-17(18)31-20/h3-4,9-12,29H,5-8H2,1-2H3,(H2,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602320
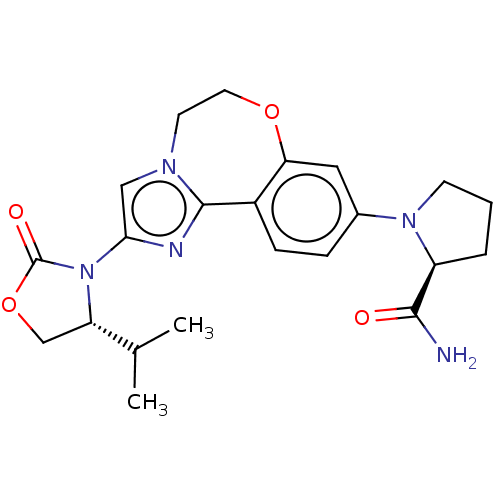
(CHEMBL5199631)Show SMILES CC(C)[C@@H]1COC(=O)N1c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602324
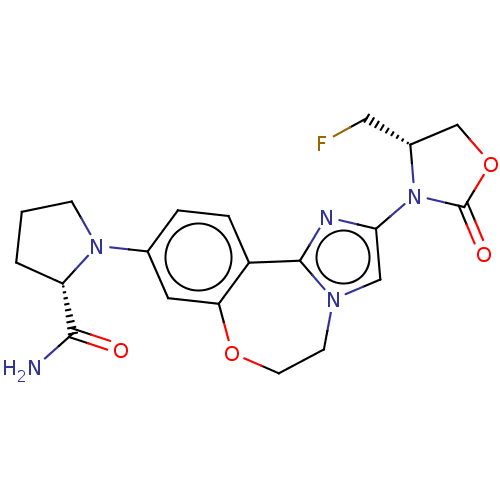
(CHEMBL5198796)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295669
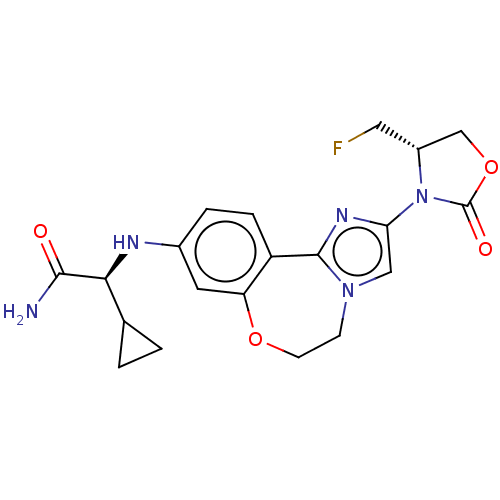
((S)-2-cyclopropyl-2-((2-((S)-4- (fluoromethyl)-2-o...)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O)C1CC1 |r| Show InChI InChI=1S/C20H22FN5O4/c21-8-13-10-30-20(28)26(13)16-9-25-5-6-29-15-7-12(3-4-14(15)19(25)24-16)23-17(18(22)27)11-1-2-11/h3-4,7,9,11,13,17,23H,1-2,5-6,8,10H2,(H2,22,27)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602323
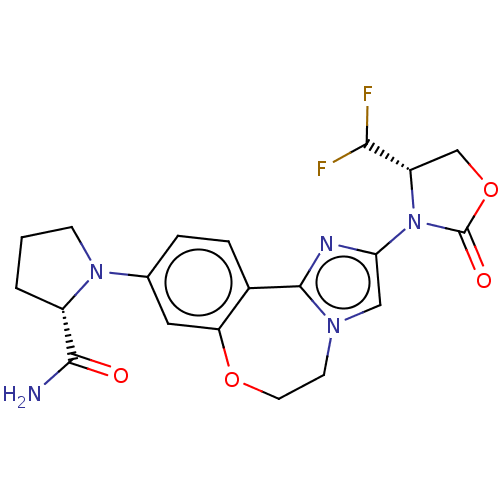
(CHEMBL5209168)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433534
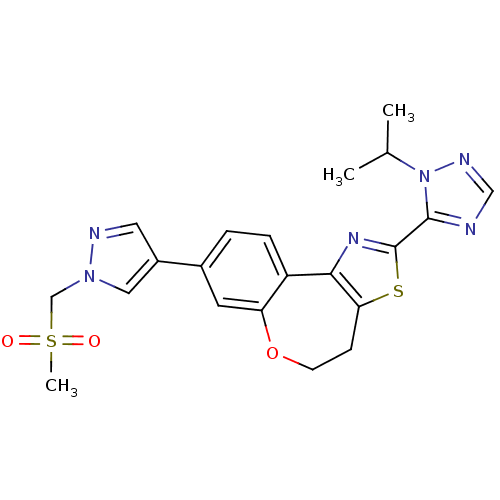
(CHEMBL2381375)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CS(C)(=O)=O)c2)s1 Show InChI InChI=1S/C21H22N6O3S2/c1-13(2)27-20(22-11-24-27)21-25-19-16-5-4-14(8-17(16)30-7-6-18(19)31-21)15-9-23-26(10-15)12-32(3,28)29/h4-5,8-11,13H,6-7,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM475607
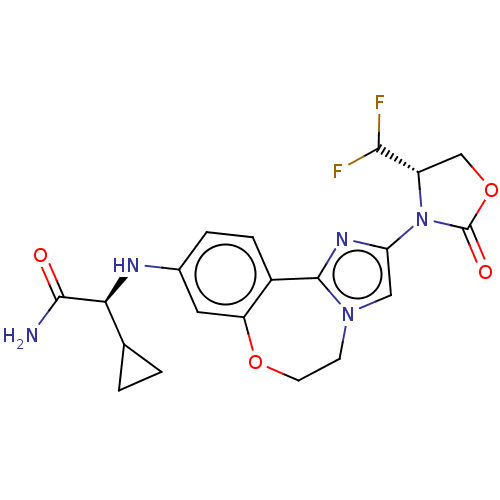
(US10851091, Compound 103)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C1CC1 |r| Show InChI InChI=1S/C20H21F2N5O4/c21-17(22)13-9-31-20(29)27(13)15-8-26-5-6-30-14-7-11(3-4-12(14)19(26)25-15)24-16(18(23)28)10-1-2-10/h3-4,7-8,10,13,16-17,24H,1-2,5-6,9H2,(H2,23,28)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433533
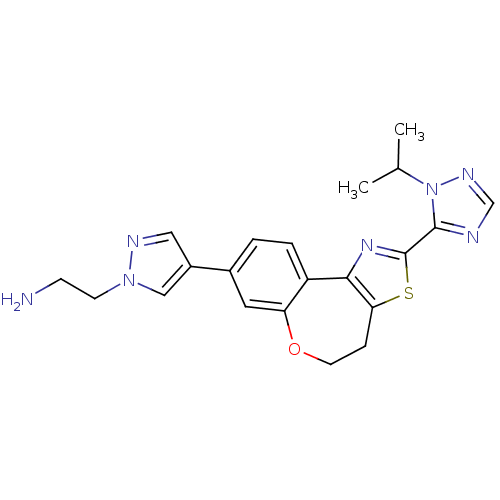
(CHEMBL2381376)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCN)c2)s1 Show InChI InChI=1S/C21H23N7OS/c1-13(2)28-20(23-12-25-28)21-26-19-16-4-3-14(15-10-24-27(11-15)7-6-22)9-17(16)29-8-5-18(19)30-21/h3-4,9-13H,5-8,22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602305
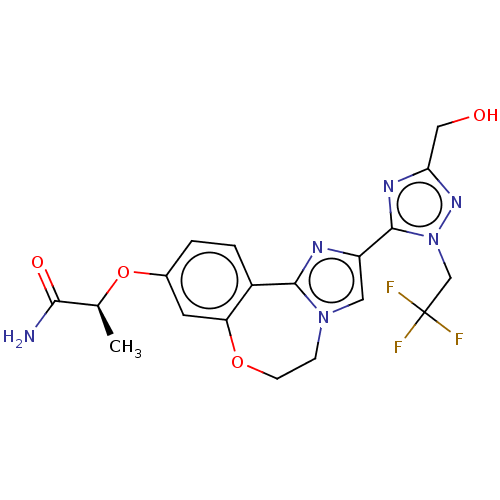
(CHEMBL5209048)Show SMILES C[C@H](Oc1ccc2-c3nc(cn3CCOc2c1)-c1nc(CO)nn1CC(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806
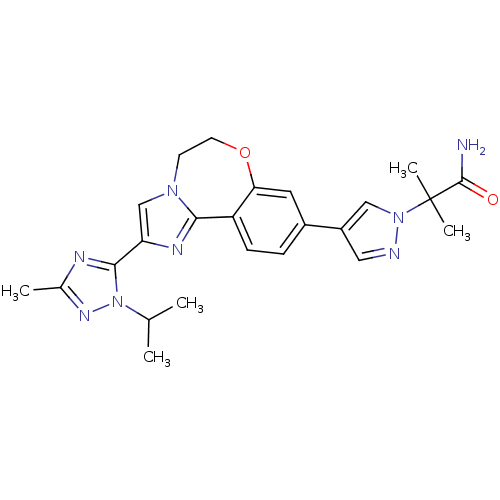
(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602328
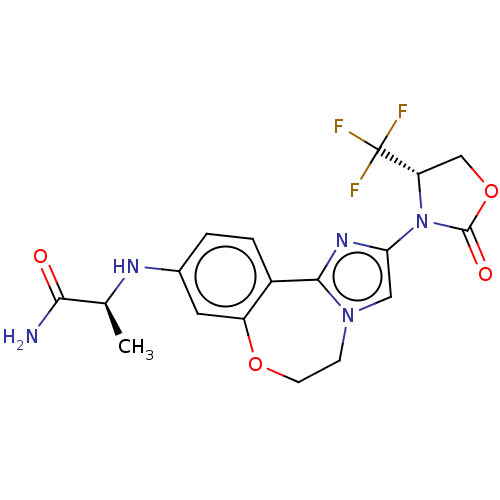
(CHEMBL5205438)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602326
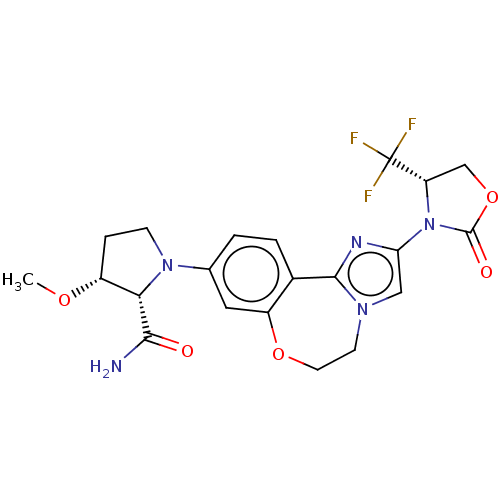
(CHEMBL5181348)Show SMILES CO[C@@H]1CCN([C@@H]1C(N)=O)c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149482
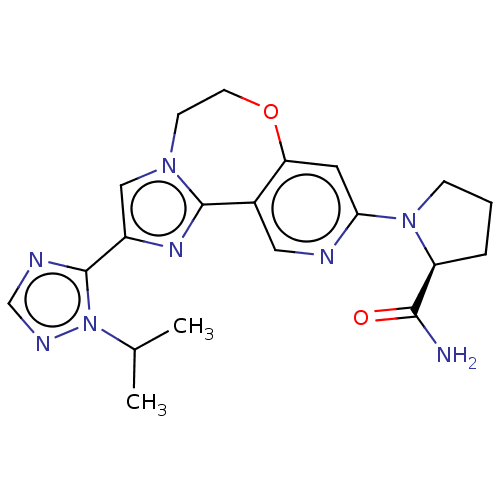
(CHEMBL3770709)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H24N8O2/c1-12(2)28-20(23-11-24-28)14-10-26-6-7-30-16-8-17(22-9-13(16)19(26)25-14)27-5-3-4-15(27)18(21)29/h8-12,15H,3-7H2,1-2H3,(H2,21,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602331
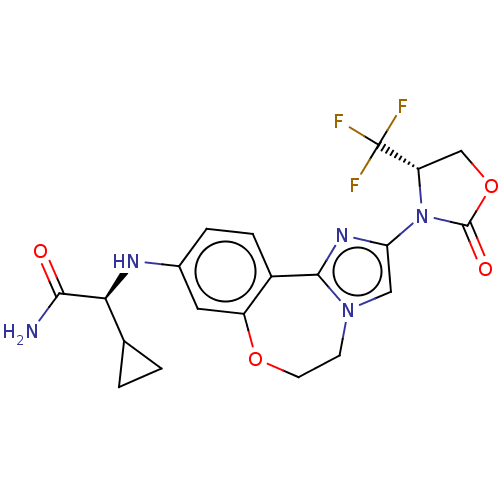
(CHEMBL5182371)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246967
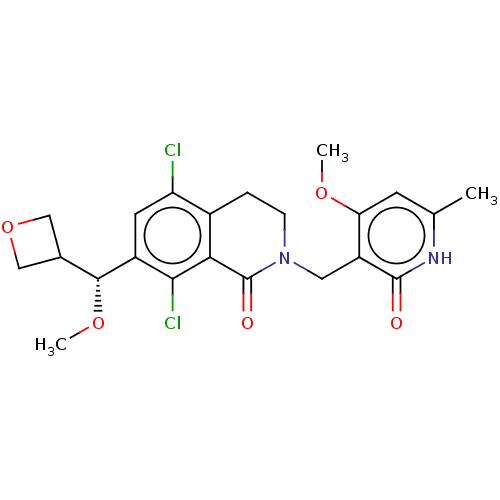
(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Binding affinity to EZH2 (unknown origin) |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149553

(CHEMBL3770306)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H23N7O2/c1-12(2)27-20(22-11-23-27)15-10-25-7-8-29-17-9-13(3-4-14(17)19(25)24-15)26-6-5-16(26)18(21)28/h3-4,9-12,16H,5-8H2,1-2H3,(H2,21,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602304
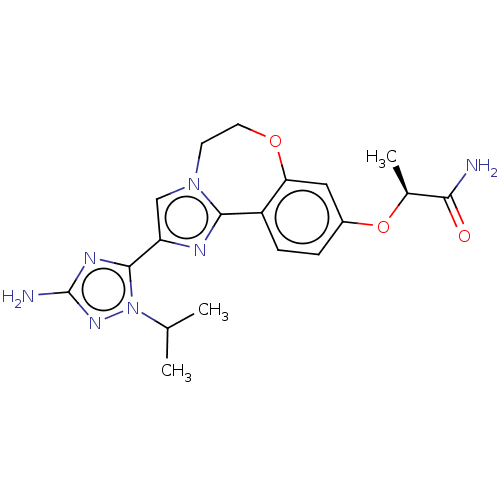
(CHEMBL5182339)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433532
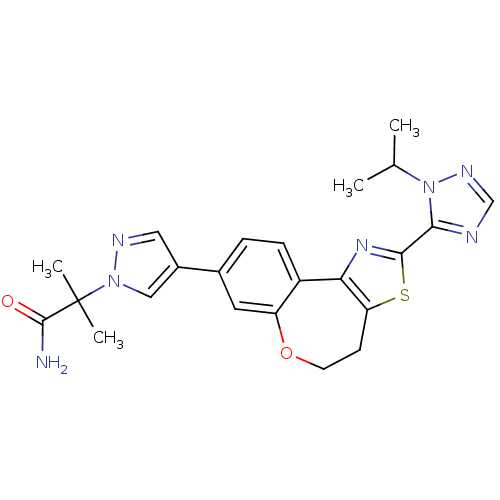
(CHEMBL2381377)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(c2)C(C)(C)C(N)=O)s1 Show InChI InChI=1S/C23H25N7O2S/c1-13(2)30-20(25-12-27-30)21-28-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-26-29(11-15)23(3,4)22(24)31/h5-6,9-13H,7-8H2,1-4H3,(H2,24,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434810
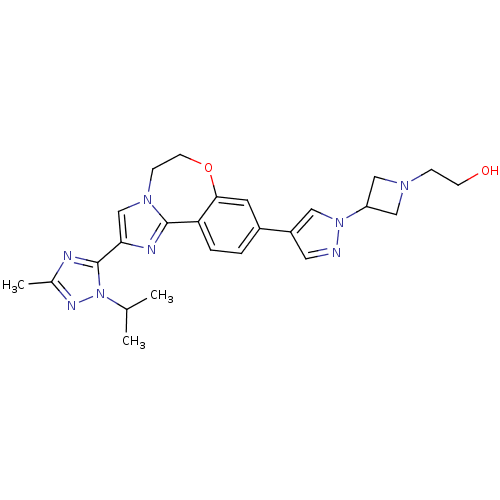
(CHEMBL2386970)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C1CN(CCO)C1 Show InChI InChI=1S/C25H30N8O2/c1-16(2)33-25(27-17(3)29-33)22-15-31-7-9-35-23-10-18(4-5-21(23)24(31)28-22)19-11-26-32(12-19)20-13-30(14-20)6-8-34/h4-5,10-12,15-16,20,34H,6-9,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433529
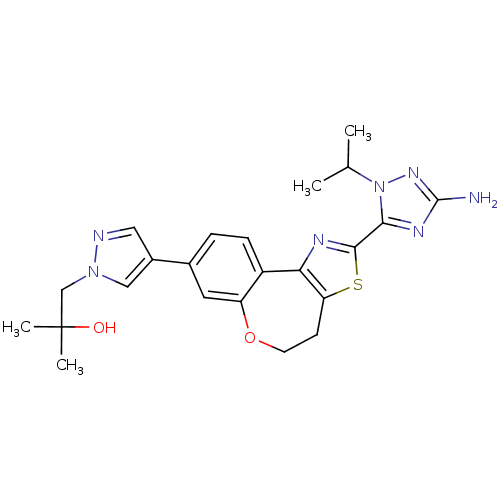
(CHEMBL2381380)Show SMILES CC(C)n1nc(N)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H27N7O2S/c1-13(2)30-20(27-22(24)28-30)21-26-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-25-29(11-15)12-23(3,4)31/h5-6,9-11,13,31H,7-8,12H2,1-4H3,(H2,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433527

(CHEMBL2381379)Show SMILES CC(C)(O)Cn1cc(cn1)-c1ccc2-c3nc(sc3CCOc2c1)-c1ncnn1CC(F)(F)F Show InChI InChI=1S/C22H21F3N6O2S/c1-21(2,32)10-30-9-14(8-27-30)13-3-4-15-16(7-13)33-6-5-17-18(15)29-20(34-17)19-26-12-28-31(19)11-22(23,24)25/h3-4,7-9,12,32H,5-6,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434807

(CHEMBL2387079)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CC(C)(C)O)c1 Show InChI InChI=1S/C23H27N7O2/c1-15(2)30-22(24-14-26-30)19-12-28-7-8-32-20-9-16(5-6-18(20)21(28)27-19)17-10-25-29(11-17)13-23(3,4)31/h5-6,9-12,14-15,31H,7-8,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433535
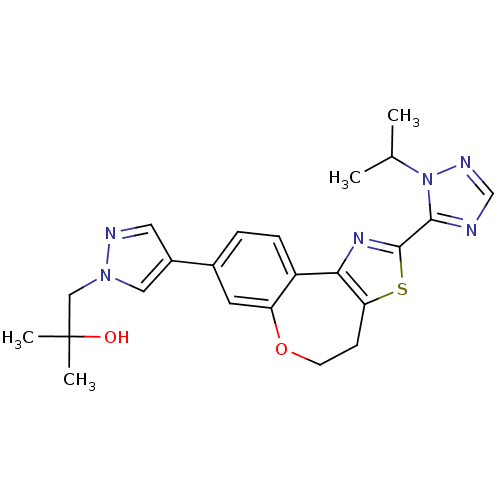
(CHEMBL2381374)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H26N6O2S/c1-14(2)29-21(24-13-26-29)22-27-20-17-6-5-15(9-18(17)31-8-7-19(20)32-22)16-10-25-28(11-16)12-23(3,4)30/h5-6,9-11,13-14,30H,7-8,12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602321
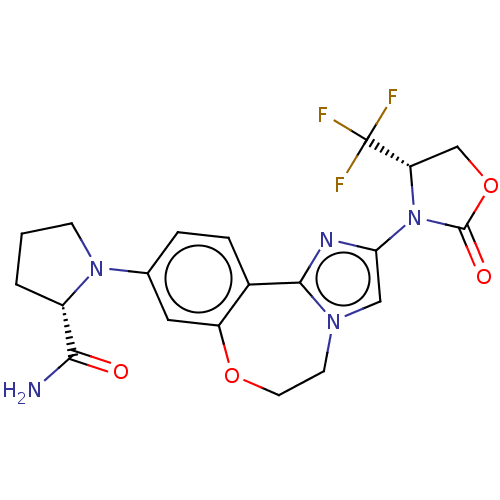
(CHEMBL5202305)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602329
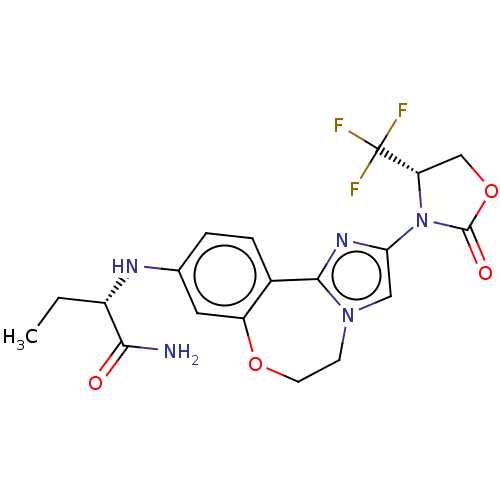
(CHEMBL5189517)Show SMILES CC[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434812

(CHEMBL2387086)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C21H23N7O2/c1-14(2)28-21(22-13-24-28)18-12-26-6-8-30-19-9-15(3-4-17(19)20(26)25-18)16-10-23-27(11-16)5-7-29/h3-4,9-14,29H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433528
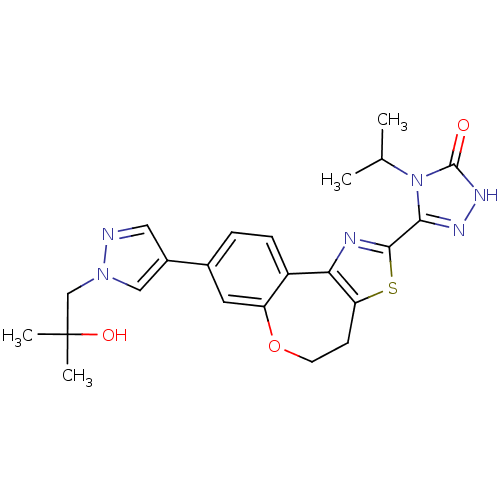
(CHEMBL2381381)Show SMILES CC(C)n1c(n[nH]c1=O)-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H26N6O3S/c1-13(2)29-20(26-27-22(29)30)21-25-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-24-28(11-15)12-23(3,4)31/h5-6,9-11,13,31H,7-8,12H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433531
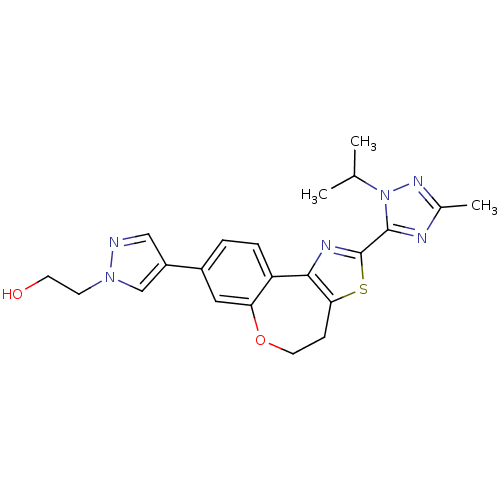
(CHEMBL2381378)Show SMILES CC(C)n1nc(C)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C22H24N6O2S/c1-13(2)28-21(24-14(3)26-28)22-25-20-17-5-4-15(16-11-23-27(12-16)7-8-29)10-18(17)30-9-6-19(20)31-22/h4-5,10-13,29H,6-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295676
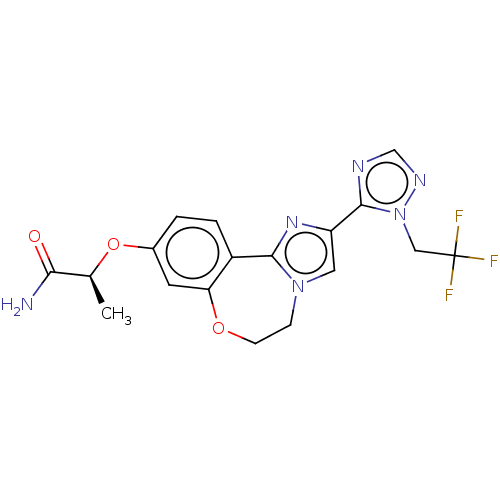
(US10851091, U.S. Pat. No. 8,242,104 No. 486 | US82...)Show SMILES C[C@H](Oc1ccc2-c3nc(cn3CCOc2c1)-c1ncnn1CC(F)(F)F)C(N)=O |r| Show InChI InChI=1S/C18H17F3N6O3/c1-10(15(22)28)30-11-2-3-12-14(6-11)29-5-4-26-7-13(25-16(12)26)17-23-9-24-27(17)8-18(19,20)21/h2-3,6-7,9-10H,4-5,8H2,1H3,(H2,22,28)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149548
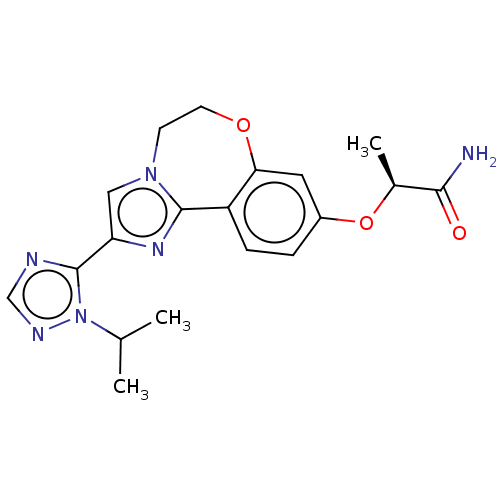
(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149483
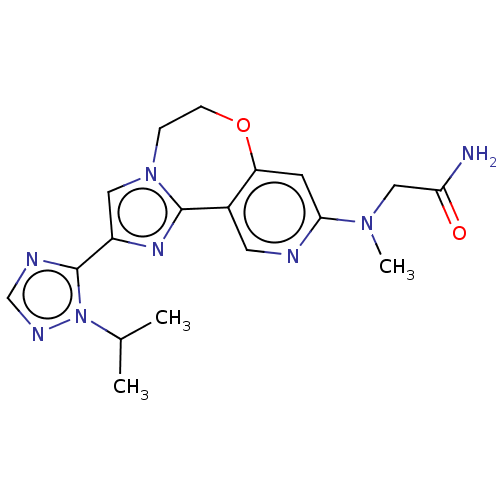
(CHEMBL3770325)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N(C)CC(N)=O Show InChI InChI=1S/C18H22N8O2/c1-11(2)26-18(21-10-22-26)13-8-25-4-5-28-14-6-16(24(3)9-15(19)27)20-7-12(14)17(25)23-13/h6-8,10-11H,4-5,9H2,1-3H3,(H2,19,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438628
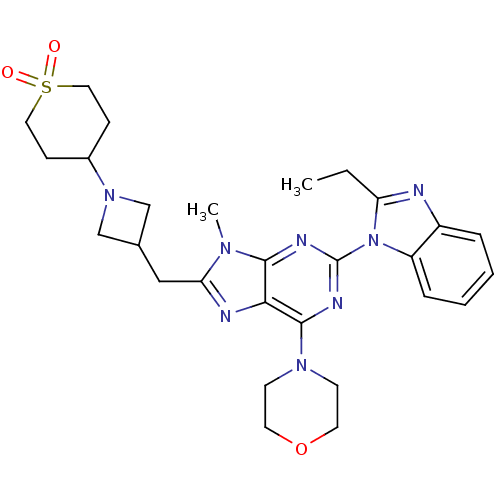
(CHEMBL2414299)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O3S/c1-3-23-29-21-6-4-5-7-22(21)36(23)28-31-26-25(27(32-28)34-10-12-39-13-11-34)30-24(33(26)2)16-19-17-35(18-19)20-8-14-40(37,38)15-9-20/h4-7,19-20H,3,8-18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433536
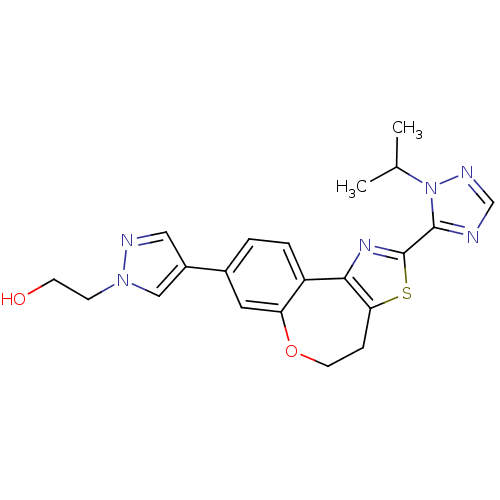
(CHEMBL2381373)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H22N6O2S/c1-13(2)27-20(22-12-24-27)21-25-19-16-4-3-14(15-10-23-26(11-15)6-7-28)9-17(16)29-8-5-18(19)30-21/h3-4,9-13,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602325
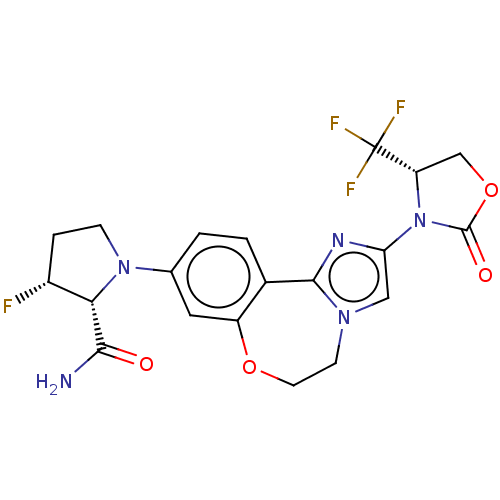
(CHEMBL5209394)Show SMILES NC(=O)[C@@H]1[C@H](F)CCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434814

(CHEMBL2387082)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(C)c1 Show InChI InChI=1S/C21H23N7O/c1-13(2)28-21(23-14(3)25-28)18-12-27-7-8-29-19-9-15(16-10-22-26(4)11-16)5-6-17(19)20(27)24-18/h5-6,9-13H,7-8H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149476
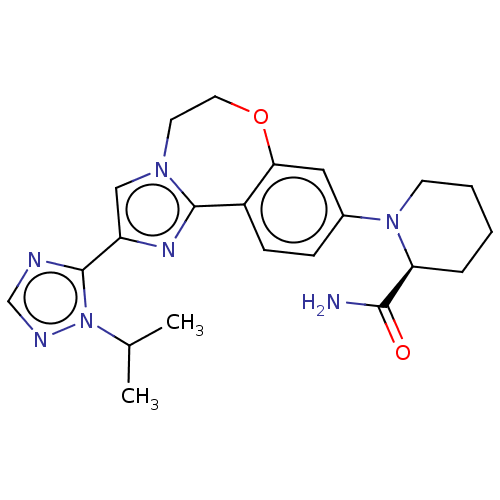
(CHEMBL3770717)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C22H27N7O2/c1-14(2)29-22(24-13-25-29)17-12-27-9-10-31-19-11-15(6-7-16(19)21(27)26-17)28-8-4-3-5-18(28)20(23)30/h6-7,11-14,18H,3-5,8-10H2,1-2H3,(H2,23,30)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149554
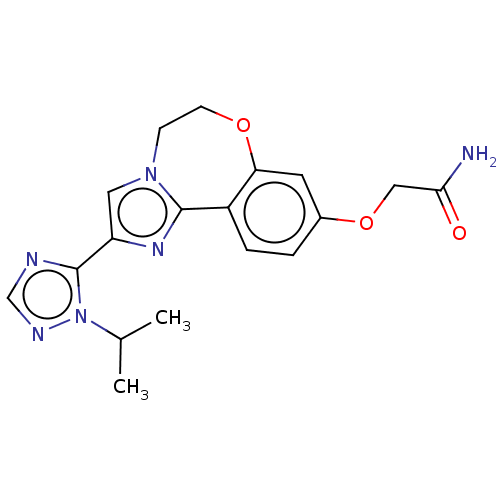
(CHEMBL3770140)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(OCC(N)=O)ccc3-c2n1 Show InChI InChI=1S/C18H20N6O3/c1-11(2)24-18(20-10-21-24)14-8-23-5-6-26-15-7-12(27-9-16(19)25)3-4-13(15)17(23)22-14/h3-4,7-8,10-11H,5-6,9H2,1-2H3,(H2,19,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521925

(CHEMBL4529532)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(CCCF)C3)C2)ncc1F |r| Show InChI InChI=1S/C26H32F2N8/c1-18-5-2-3-10-36-24(18)20(12-31-36)23-21(28)13-30-25(33-23)32-22-7-6-19(11-29-22)35-16-26(17-35)14-34(15-26)9-4-8-27/h6-7,11-13,18H,2-5,8-10,14-17H2,1H3,(H,29,30,32,33)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521938
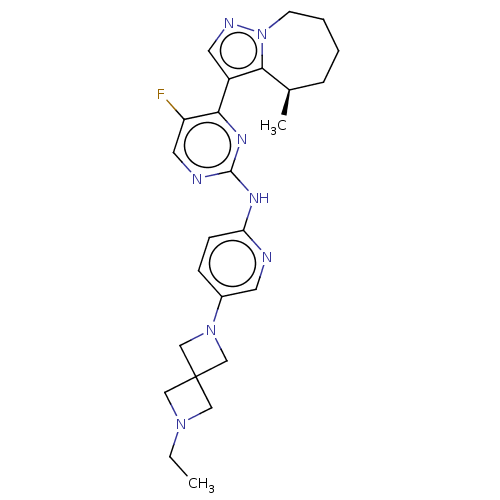
(CHEMBL4545456)Show SMILES CCN1CC2(C1)CN(C2)c1ccc(Nc2ncc(F)c(n2)-c2cnn3CCCC[C@@H](C)c23)nc1 |r| Show InChI InChI=1S/C25H31FN8/c1-3-32-13-25(14-32)15-33(16-25)18-7-8-21(27-10-18)30-24-28-12-20(26)22(31-24)19-11-29-34-9-5-4-6-17(2)23(19)34/h7-8,10-12,17H,3-6,9,13-16H2,1-2H3,(H,27,28,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
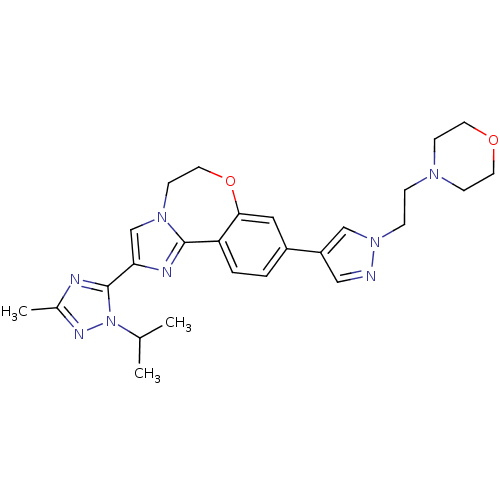
(Homo sapiens (Human)) | BDBM50434808
(CHEMBL2386972)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCN2CCOCC2)c1 Show InChI InChI=1S/C26H32N8O2/c1-18(2)34-26(28-19(3)30-34)23-17-32-10-13-36-24-14-20(4-5-22(24)25(32)29-23)21-15-27-33(16-21)7-6-31-8-11-35-12-9-31/h4-5,14-18H,6-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521933

(CHEMBL4440980)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CCN(C)CC2)ncc1F |r| Show InChI InChI=1S/C23H29FN8/c1-16-5-3-4-8-32-22(16)18(14-27-32)21-19(24)15-26-23(29-21)28-20-7-6-17(13-25-20)31-11-9-30(2)10-12-31/h6-7,13-16H,3-5,8-12H2,1-2H3,(H,25,26,28,29)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521935

(CHEMBL4589135)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(C)C3)C2)ncc1F |r| Show InChI InChI=1S/C24H29FN8/c1-16-5-3-4-8-33-22(16)18(10-28-33)21-19(25)11-27-23(30-21)29-20-7-6-17(9-26-20)32-14-24(15-32)12-31(2)13-24/h6-7,9-11,16H,3-5,8,12-15H2,1-2H3,(H,26,27,29,30)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521937

(CHEMBL4564687)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(CCF)C3)C2)ncc1F |r| Show InChI InChI=1S/C25H30F2N8/c1-17-4-2-3-8-35-23(17)19(11-30-35)22-20(27)12-29-24(32-22)31-21-6-5-18(10-28-21)34-15-25(16-34)13-33(14-25)9-7-26/h5-6,10-12,17H,2-4,7-9,13-16H2,1H3,(H,28,29,31,32)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434817

(CHEMBL2387081)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cn[nH]c1 Show InChI InChI=1S/C19H19N7O/c1-12(2)26-19(20-11-23-26)16-10-25-5-6-27-17-7-13(14-8-21-22-9-14)3-4-15(17)18(25)24-16/h3-4,7-12H,5-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data