

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131114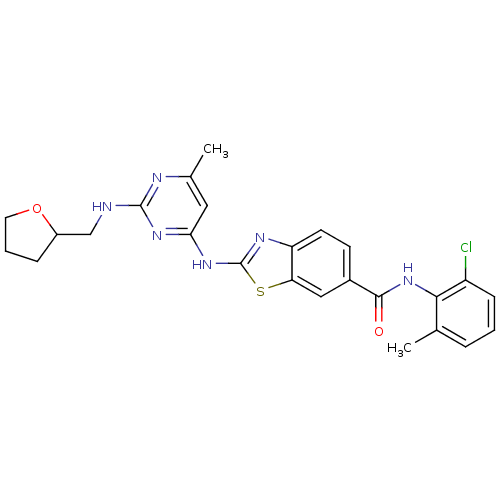 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13357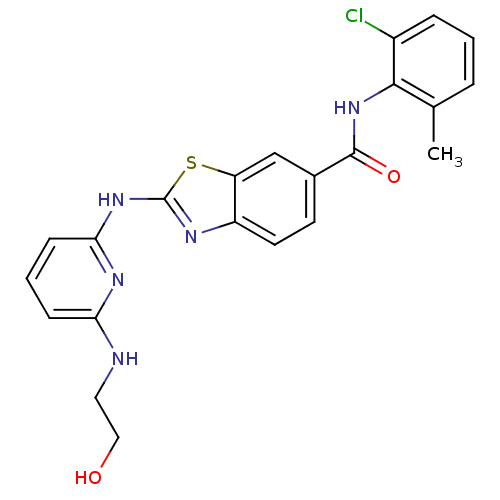 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50131114 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50131114 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50129307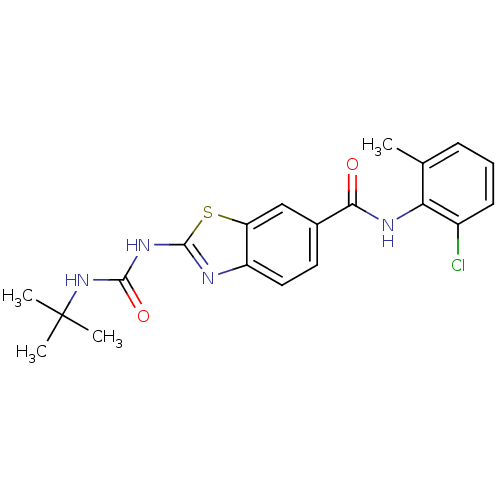 (2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131131 (2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50131131 (2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50424585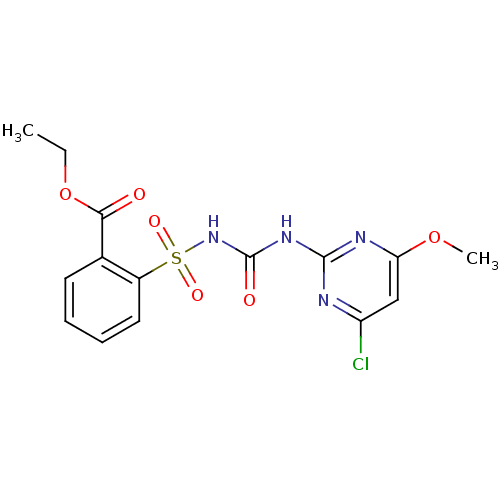 (CHEMBL1231791 | Chlorimuron Ethyl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana AHAS expressed in Escherichia coli strain BL21 (DE3) by colorimetric assay | Proc Natl Acad Sci U S A 103: 569-73 (2006) Article DOI: 10.1073/pnas.0508701103 BindingDB Entry DOI: 10.7270/Q28W3H5N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50131131 (2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50129307 (2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50424586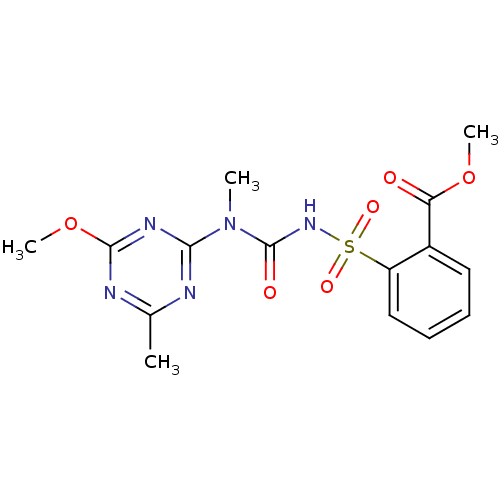 (CHEMBL1229780 | Tribenuron Methyl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana AHAS expressed in Escherichia coli strain BL21 (DE3) by colorimetric assay | Proc Natl Acad Sci U S A 103: 569-73 (2006) Article DOI: 10.1073/pnas.0508701103 BindingDB Entry DOI: 10.7270/Q28W3H5N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50129307 (2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1A1 (Rattus norvegicus) | BDBM50390979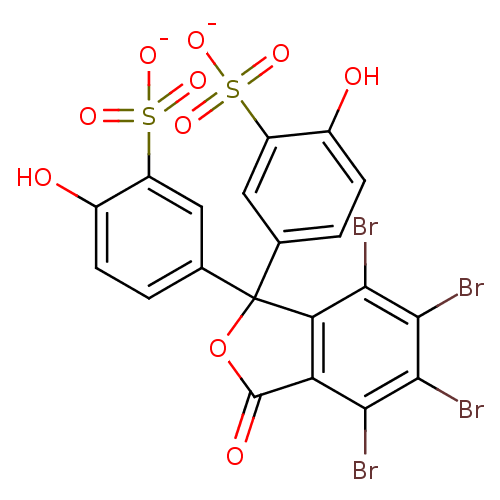 (BROMOSULFOPHTHALEIN) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Enalapril uptake in Oatp1-expressing HeLa cells | Hepatology 28: 1341-6 (1998) Article DOI: 10.1002/hep.510280524 BindingDB Entry DOI: 10.7270/Q2K64MZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50487032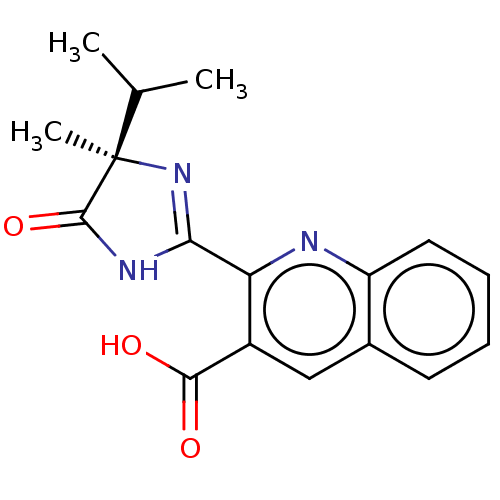 ((R)-IMAZAQUIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana AHAS expressed in Escherichia coli strain BL21 (DE3) by colorimetric assay | Proc Natl Acad Sci U S A 103: 569-73 (2006) Article DOI: 10.1073/pnas.0508701103 BindingDB Entry DOI: 10.7270/Q28W3H5N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Solute carrier organic anion transporter family member 1A1 (Rattus norvegicus) | BDBM50375594 (TAUROCHOLATE) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Enalapril uptake in Oatp1-expressing HeLa cells | Hepatology 28: 1341-6 (1998) Article DOI: 10.1002/hep.510280524 BindingDB Entry DOI: 10.7270/Q2K64MZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258790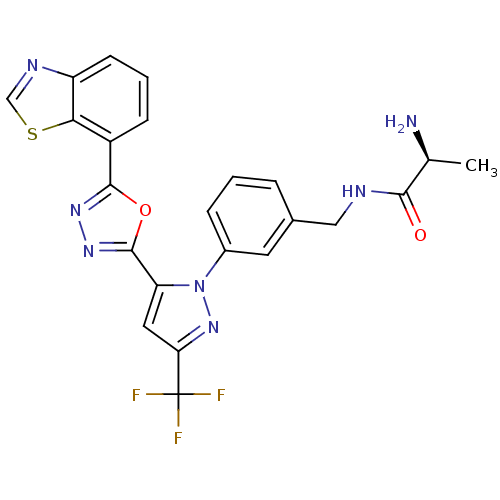 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50301077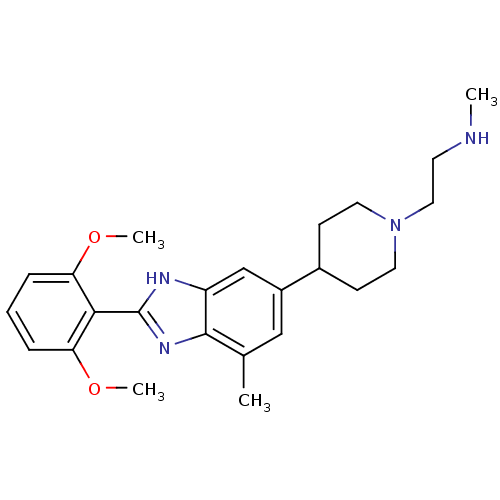 (2-(4-(2-(2,6-dimethoxyphenyl)-7-methyl-1H-benzo[d]...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50301079 (2-(4-(2-(2-fluoro-6-methoxyphenyl)-7-methyl-1H-ben...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13277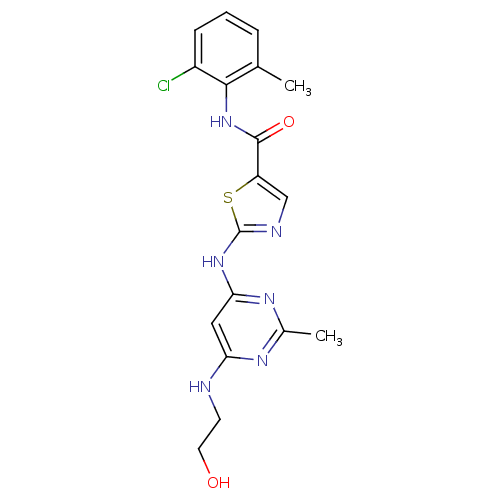 (BMS-354825 2-Heteroarylamino-thiazole Analog 12v |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13271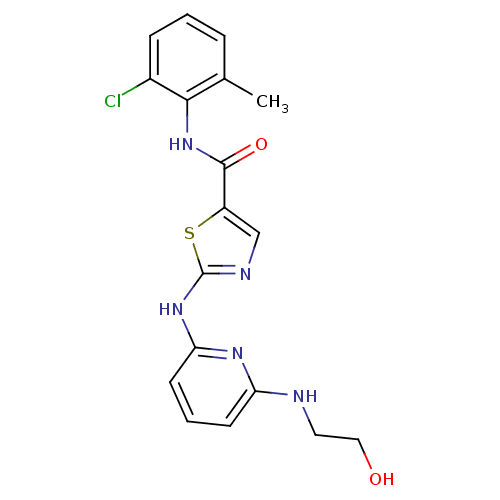 (BMS-354825 2-Heteroarylamino-thiazole Analog 12p |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50301078 (2-(4-(2-(4-fluoro-2-methoxyphenyl)-7-methyl-1H-ben...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13262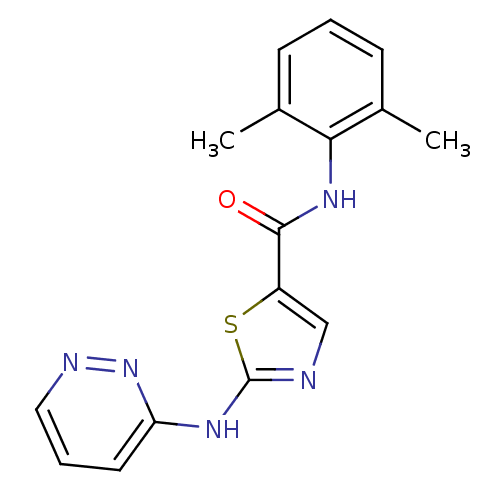 (BMS-354825 2-Heteroarylamino-thiazole Analog 12g |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of lck inase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50301076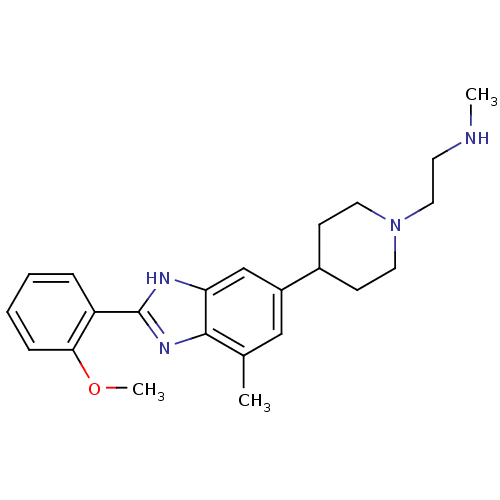 (2-(4-(2-(2-methoxyphenyl)-4-methyl-1H-benzo[d]imid...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13274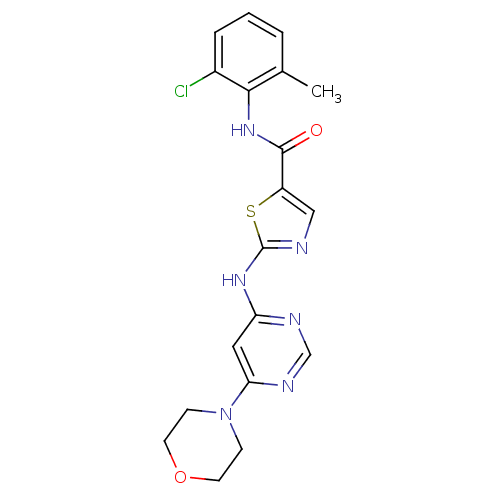 (BMS-354825 2-Heteroarylamino-thiazole Analog 12s |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13273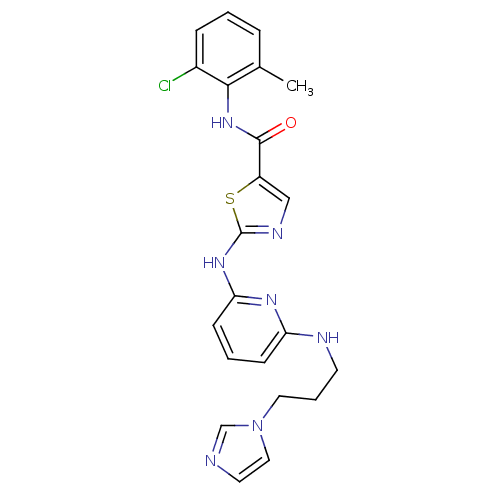 (2-(6-(3-(1H-Imidazol-1-yl)propylamino)pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13272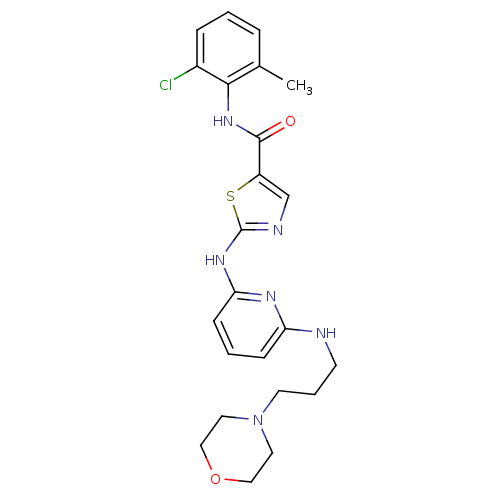 (BMS-354825 2-Heteroarylamino-thiazole Analog 12q |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13266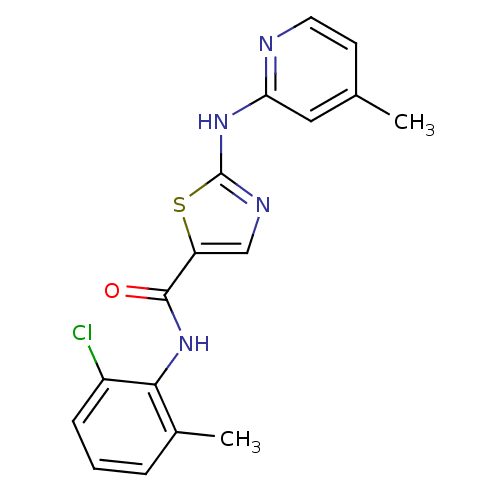 (BMS-354825 2-Heteroarylamino-thiazole Analog 12k |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of src kinase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50151366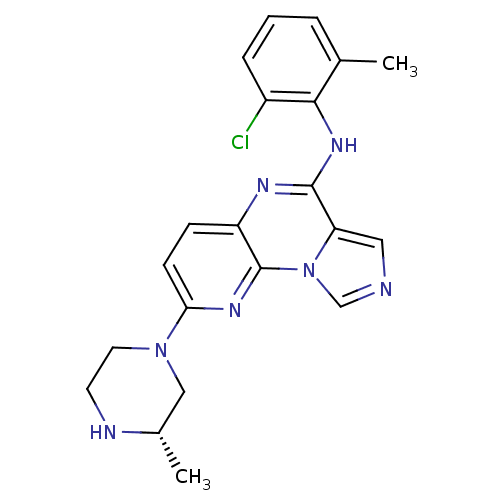 ((2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Lyn kinase | J Med Chem 47: 4517-29 (2004) Article DOI: 10.1021/jm030217e BindingDB Entry DOI: 10.7270/Q2R210VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of yes kinase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13265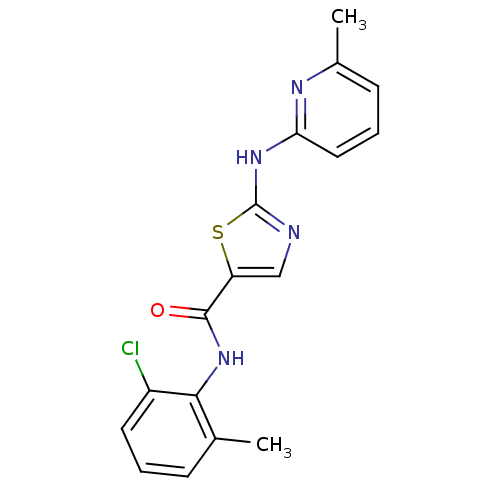 (BMS-354825 2-Heteroarylamino-thiazole Analog 12j |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13278 (BMS-354825 2-Heteroarylamino-thiazole Analog 12w |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13276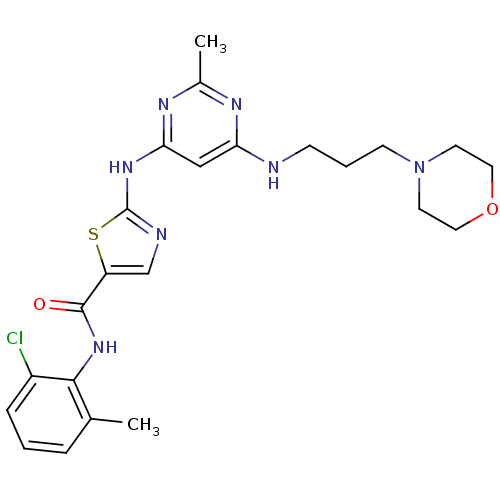 (BMS-354825 2-Heteroarylamino-thiazole Analog 12u |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50301075 (2-(4-(2-(2-methoxypyridin-3-yl)-3H-imidazo[4,5-b]p...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50301069 (2-(4-(2-(2-methoxypyridin-3-yl)-4-methyl-1H-benzo[...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131114 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fgr (Homo sapiens (Human)) | BDBM13268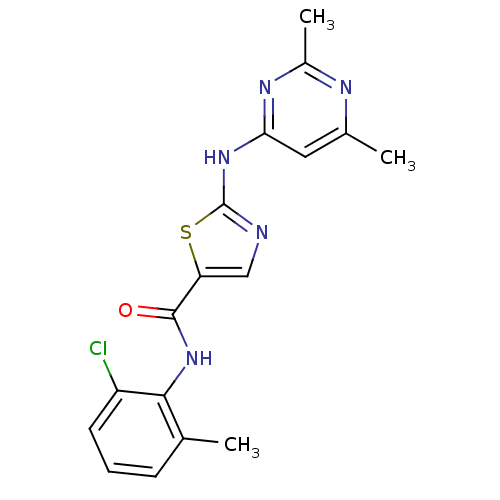 (BMS-354825 2-Heteroarylamino-thiazole Analog 12m |...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Fgr kinase | Bioorg Med Chem Lett 14: 6061-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.093 BindingDB Entry DOI: 10.7270/Q2FQ9W3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131157 (2-[4-(4-Methylamino-piperazin-1-ylmethyl)-pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM13268 (BMS-354825 2-Heteroarylamino-thiazole Analog 12m |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description inhibitory activity against Fyn protein kinase | Bioorg Med Chem Lett 14: 6061-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.093 BindingDB Entry DOI: 10.7270/Q2FQ9W3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131170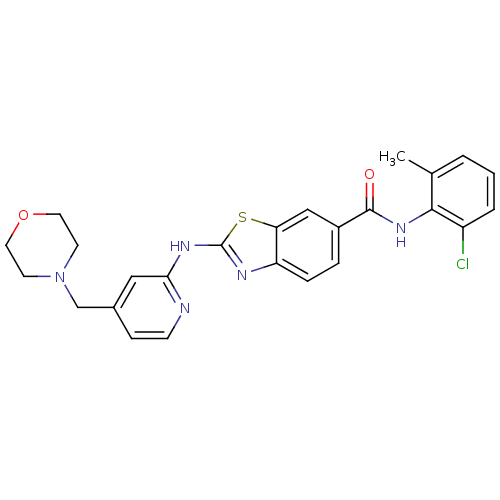 (2-(4-Morpholin-4-ylmethyl-pyridin-2-ylamino)-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13268 (BMS-354825 2-Heteroarylamino-thiazole Analog 12m |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120101 ((2-Chloro-6-methyl-phenyl)-[8-(4-methyl-piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Breakpoint cluster region protein/Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Bcr-Abl kinase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM13268 (BMS-354825 2-Heteroarylamino-thiazole Analog 12m |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Src tyrosine kinase | Bioorg Med Chem Lett 14: 6061-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.093 BindingDB Entry DOI: 10.7270/Q2FQ9W3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 635 total ) | Next | Last >> |