

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240715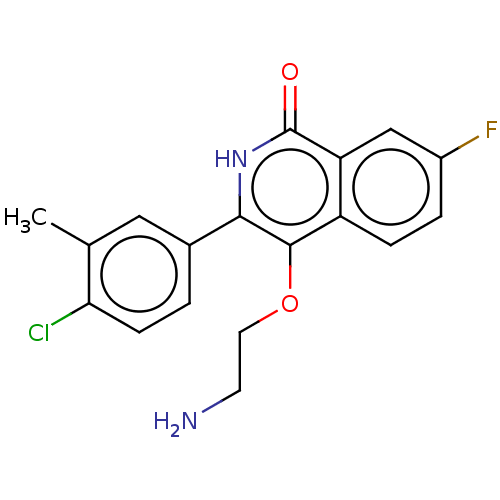 (US9422243, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50162074 (CHEMBL3792684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate | J Med Chem 59: 2343-5 (2016) Article DOI: 10.1021/acs.jmedchem.6b00283 BindingDB Entry DOI: 10.7270/Q2VX0JDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50162075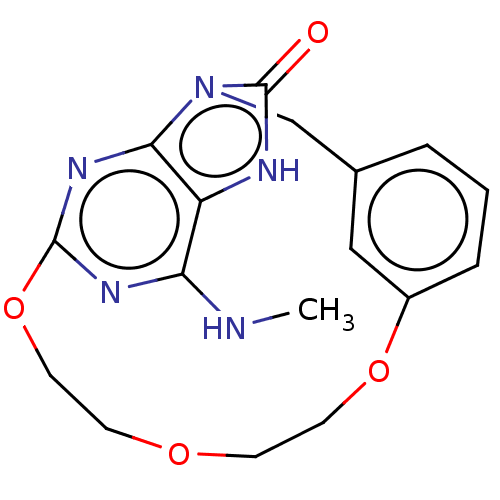 (CHEMBL3794167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate | J Med Chem 59: 2343-5 (2016) Article DOI: 10.1021/acs.jmedchem.6b00283 BindingDB Entry DOI: 10.7270/Q2VX0JDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152125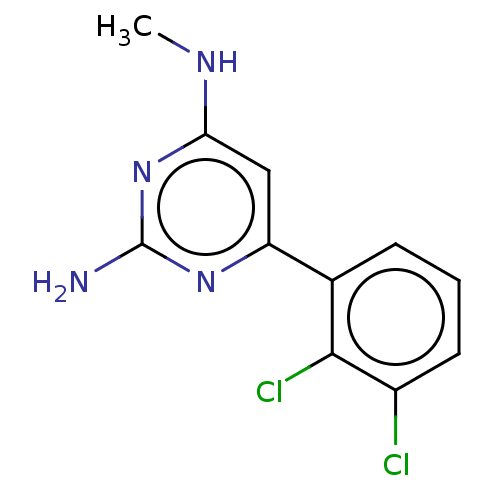 (CHEMBL3781316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate | J Med Chem 59: 2343-5 (2016) Article DOI: 10.1021/acs.jmedchem.6b00283 BindingDB Entry DOI: 10.7270/Q2VX0JDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240716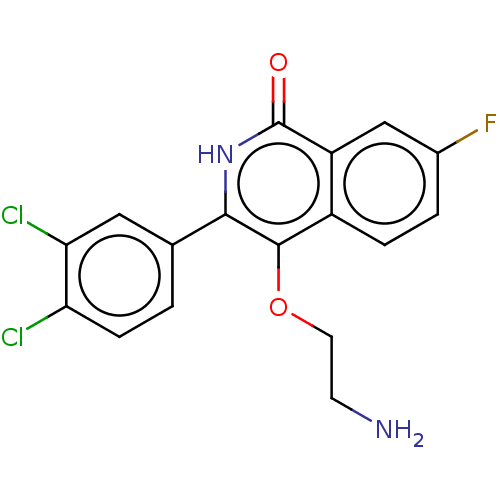 (US9422243, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021624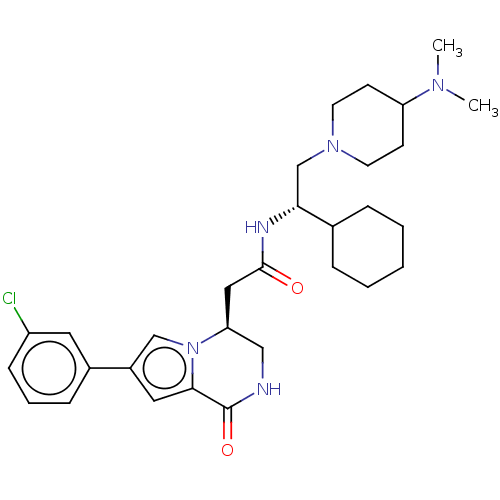 (CHEMBL3297766 | US9145418, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021620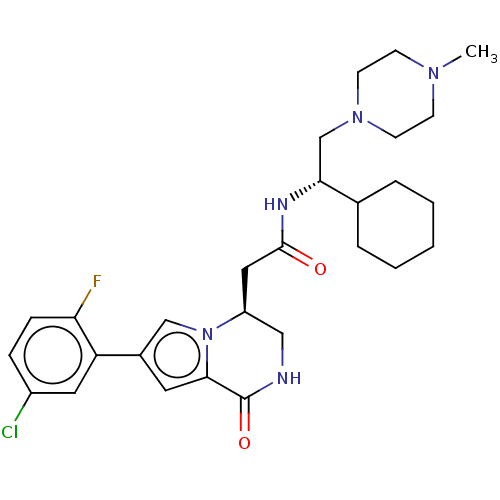 (CHEMBL3297764 | US9145418, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021622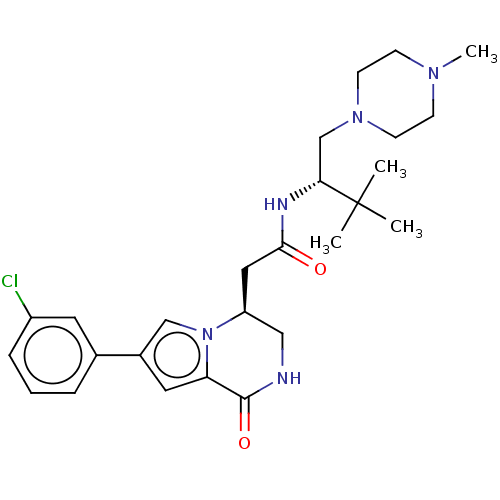 (CHEMBL3297767) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240721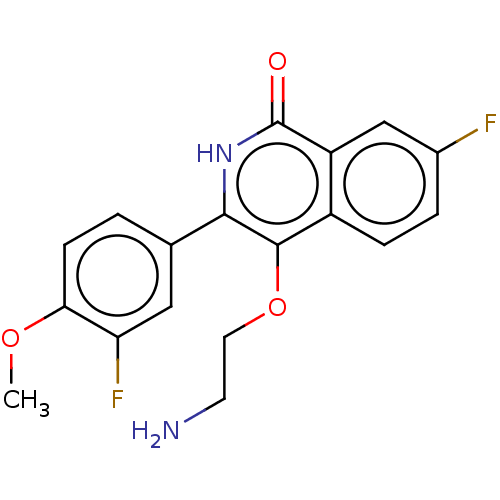 (US9422243, 21) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152124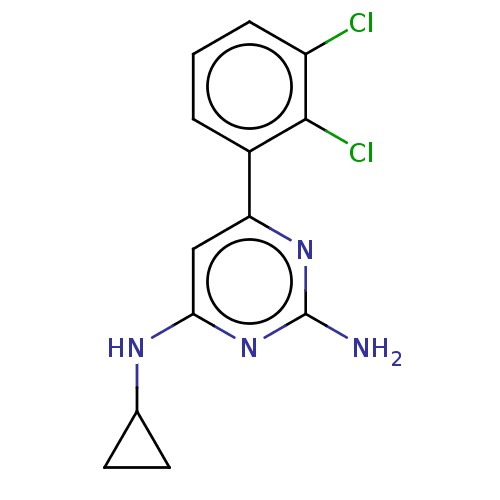 (CHEMBL3782004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate | J Med Chem 59: 2343-5 (2016) Article DOI: 10.1021/acs.jmedchem.6b00283 BindingDB Entry DOI: 10.7270/Q2VX0JDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50162072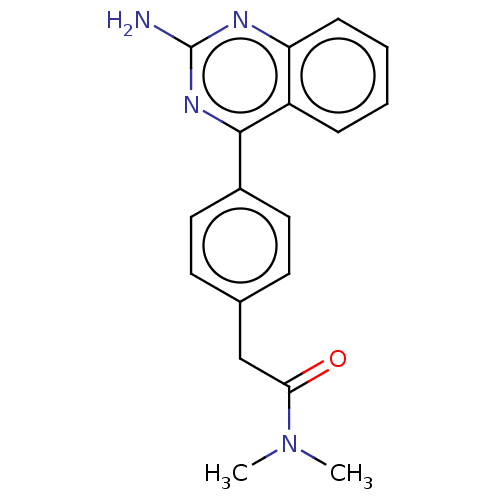 (CHEMBL3794132) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate | J Med Chem 59: 2343-5 (2016) Article DOI: 10.1021/acs.jmedchem.6b00283 BindingDB Entry DOI: 10.7270/Q2VX0JDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021617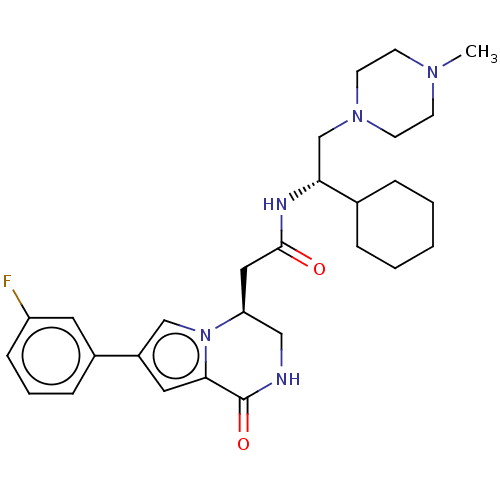 (CHEMBL3297761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240704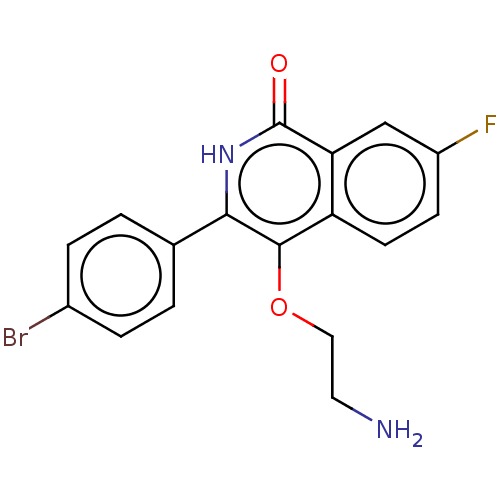 (US9422243, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138348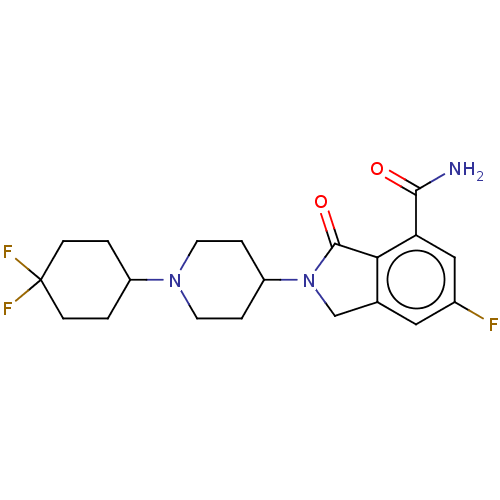 (US8877944, 99) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240713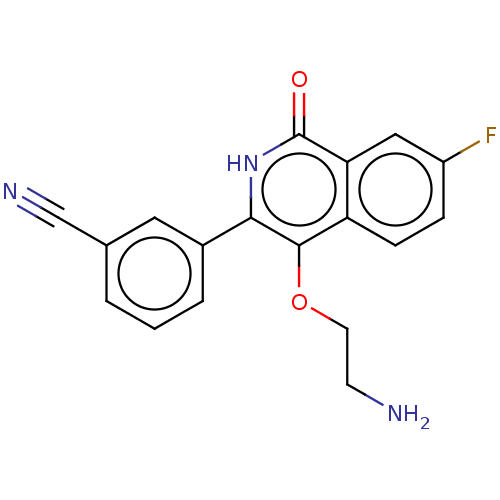 (US9422243, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240711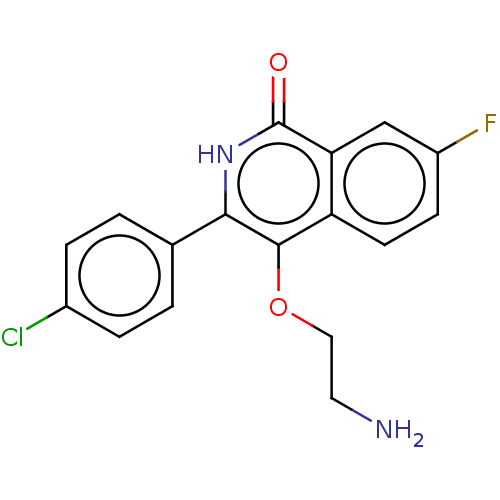 (US9422243, 8) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021649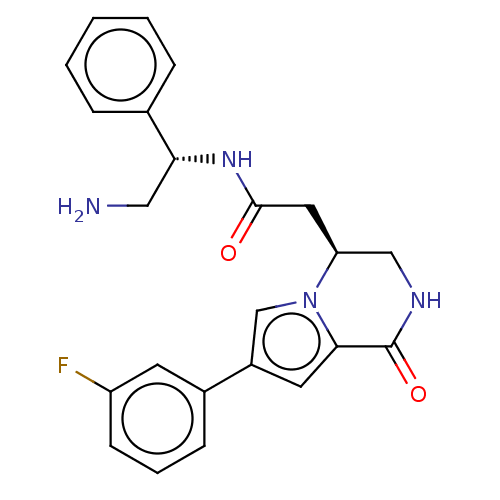 (CHEMBL3298891) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021653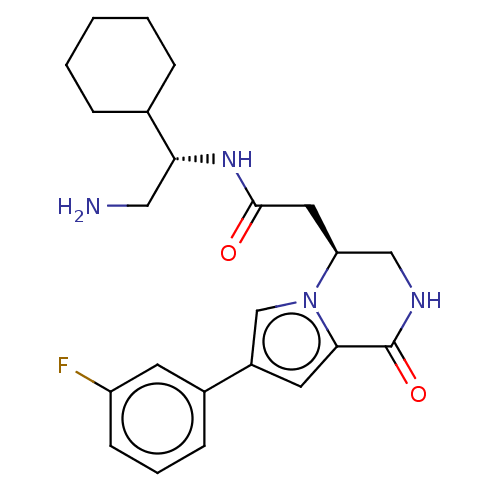 (CHEMBL3297759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50021618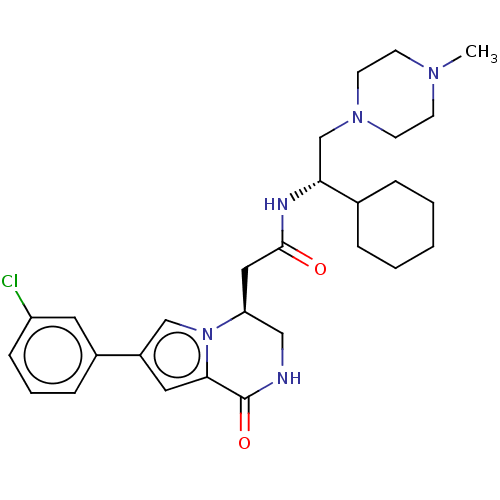 (CHEMBL3297762 | US9145418, 2) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM3 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021621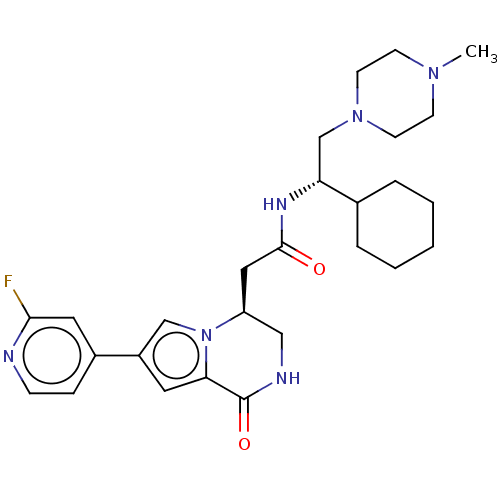 (CHEMBL3297765) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021647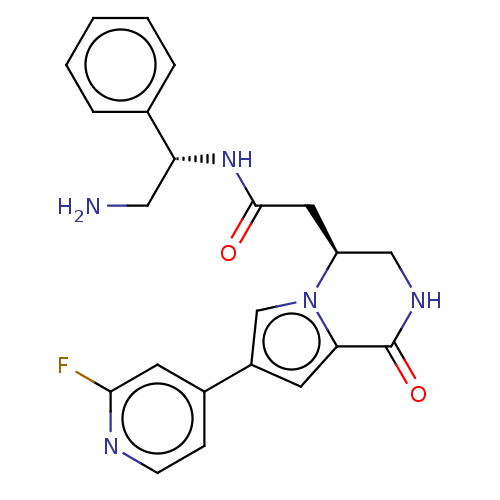 (CHEMBL3298889) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167422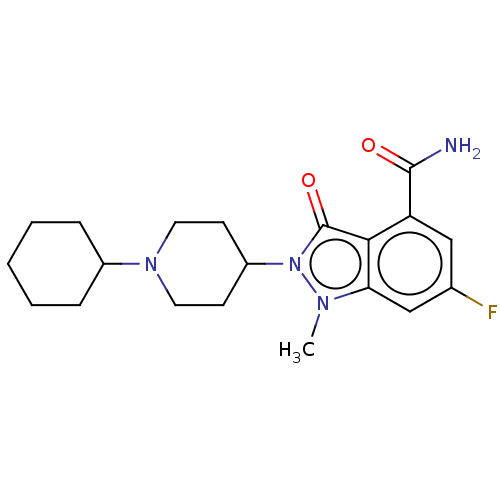 (US9073893, 22) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021650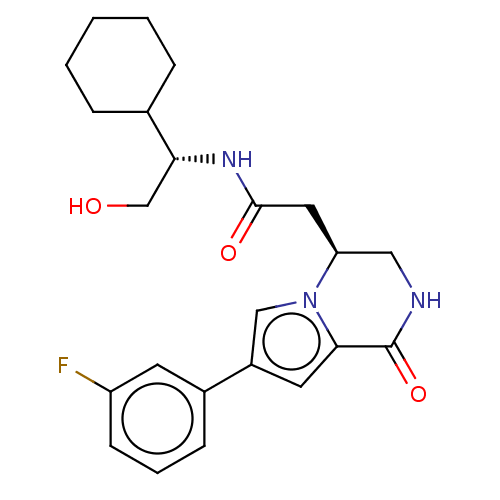 (CHEMBL3298892) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240707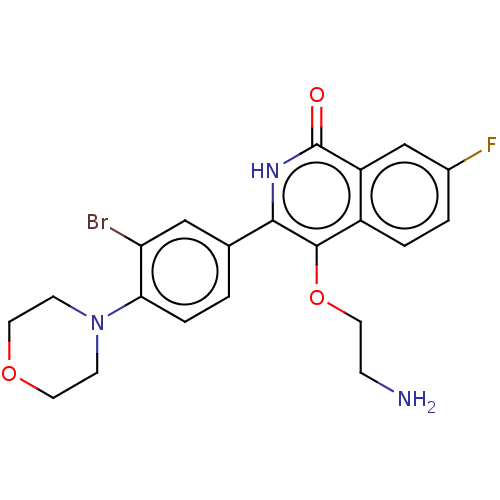 (US9422243, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240714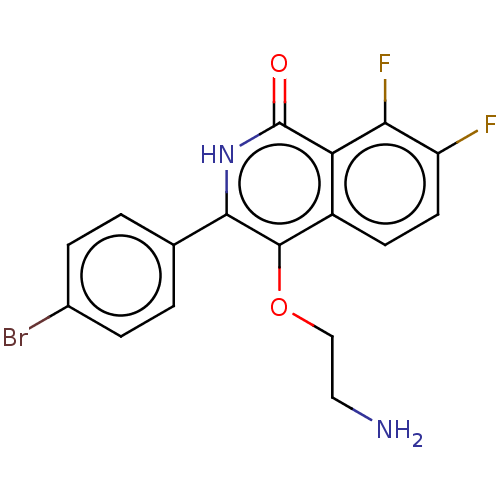 (US9422243, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167419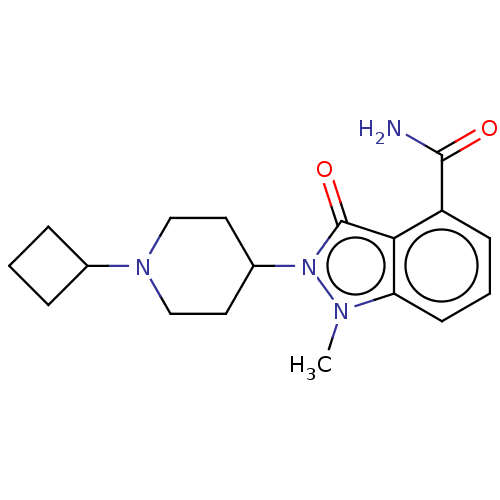 (US9073893, 18) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021619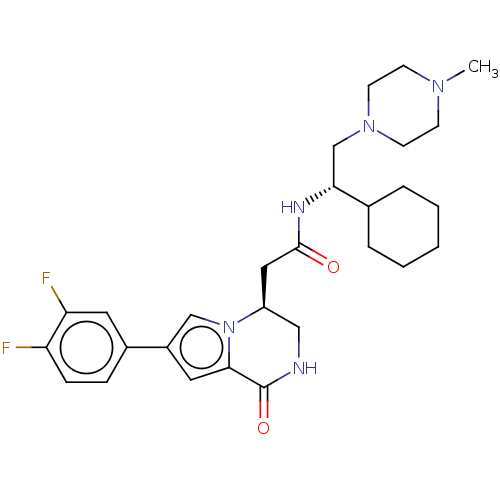 (CHEMBL3297763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167412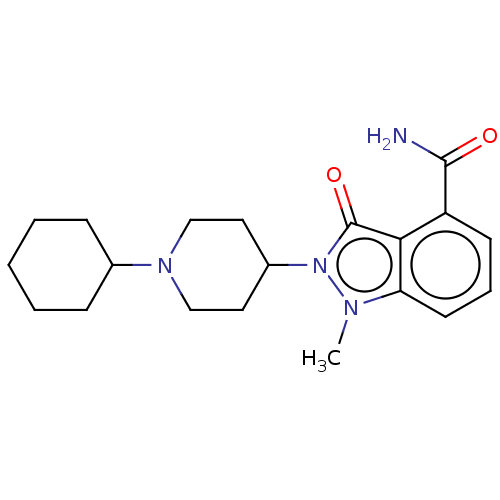 (US9073893, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM124951 (US8765972, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction in H2O2-induced PAR formation incubated for 30 mins followed by H2O2 addition for 15 mi... | ACS Med Chem Lett 10: 534-538 (2019) Article DOI: 10.1021/acsmedchemlett.8b00569 BindingDB Entry DOI: 10.7270/Q2VQ365G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021618 (CHEMBL3297762 | US9145418, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240705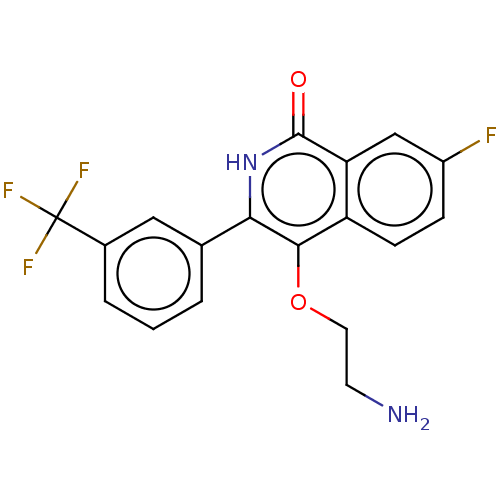 (US9422243, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021641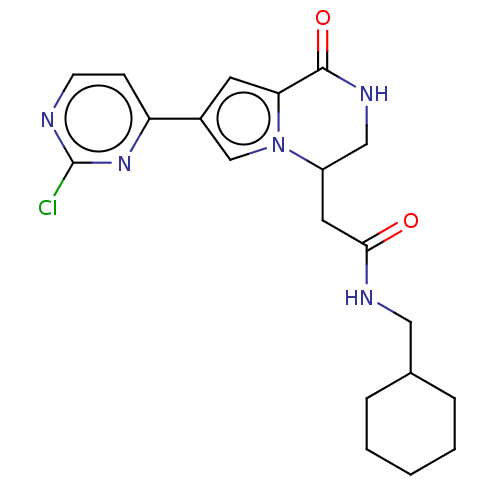 (CHEMBL3298822) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50021624 (CHEMBL3297766 | US9145418, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM2 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM138348 (US8877944, 99) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM138344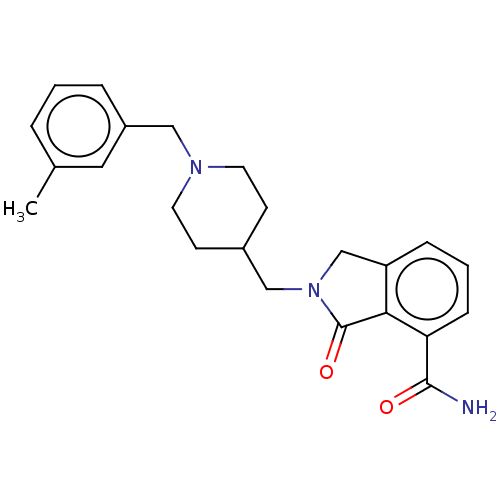 (US8877944, 49) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138344 (US8877944, 49) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021643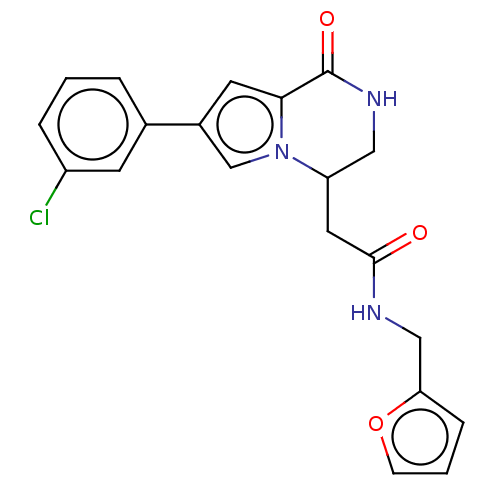 (CHEMBL3298886) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167427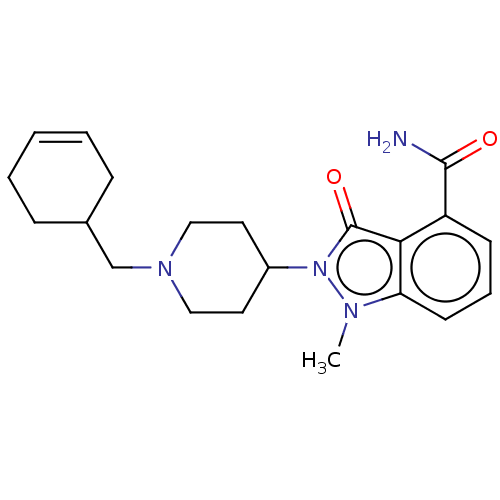 (US9073893, 65) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167416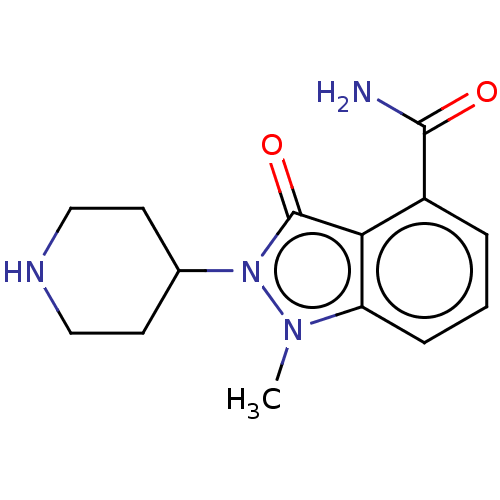 (US9073893, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM124950 (US8765972, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction in H2O2-induced PAR formation incubated for 30 mins followed by H2O2 addition for 15 mi... | ACS Med Chem Lett 10: 534-538 (2019) Article DOI: 10.1021/acsmedchemlett.8b00569 BindingDB Entry DOI: 10.7270/Q2VQ365G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240712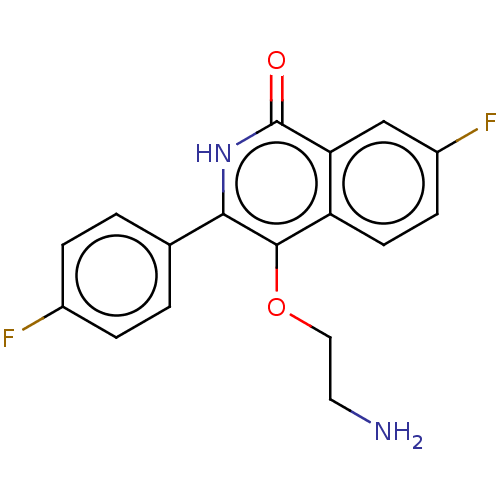 (US9422243, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50021625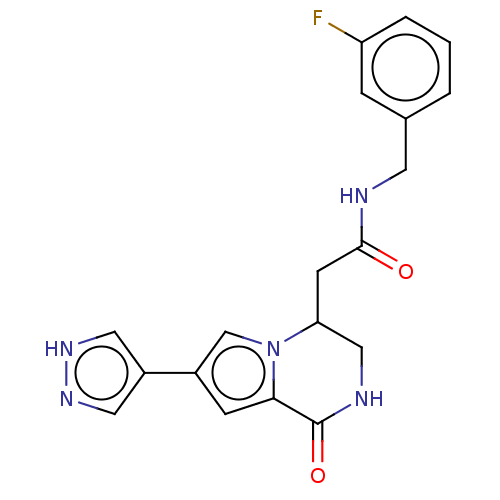 (CHEMBL3298823) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of CDK2 (unknown origin) | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240719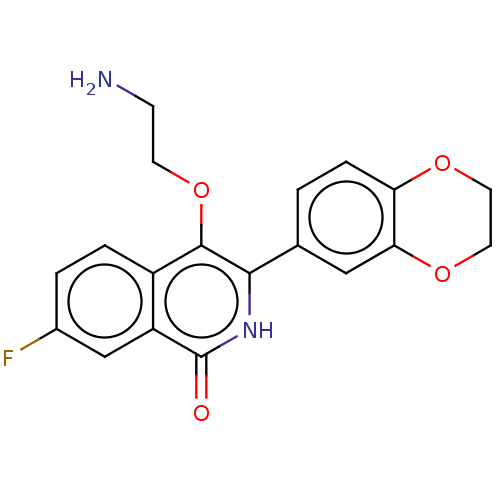 (US9422243, 19) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167426 (US9073893, 62) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50021639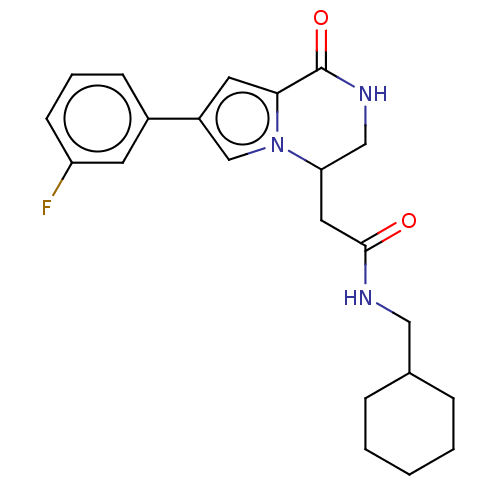 (CHEMBL3298820) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50021618 (CHEMBL3297762 | US9145418, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human PIM2 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138342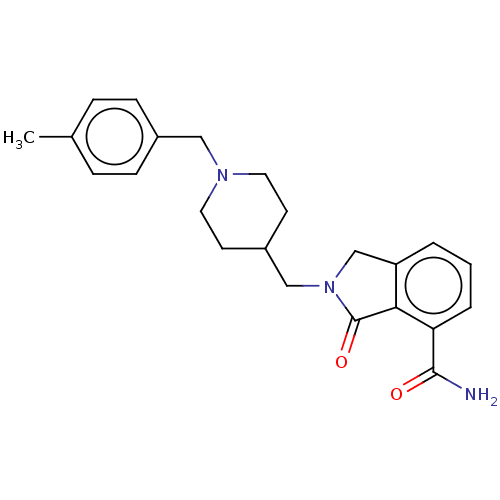 (US8877944, 47) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138354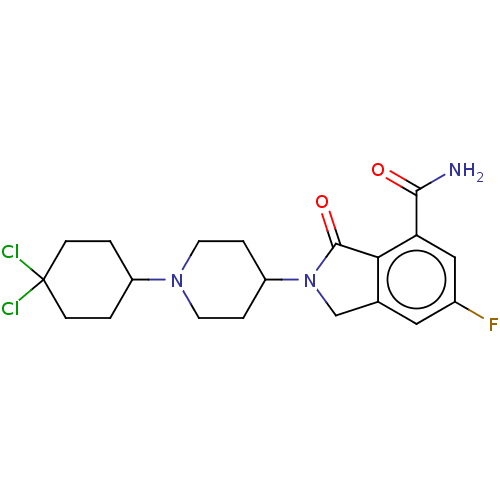 (US8877944, 105) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM240720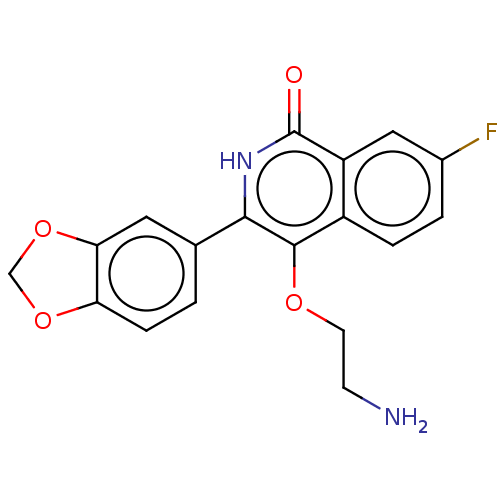 (US9422243, 20) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. a corporation US Patent | Assay Description Studies were performed as follows: 6000 cells/well were seeded in 96 well plates (Perkin Elmer) in MEM/10% FCS and incubated for 24 hs at 37° C.,... | US Patent US9422243 (2016) BindingDB Entry DOI: 10.7270/Q2T72GBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138349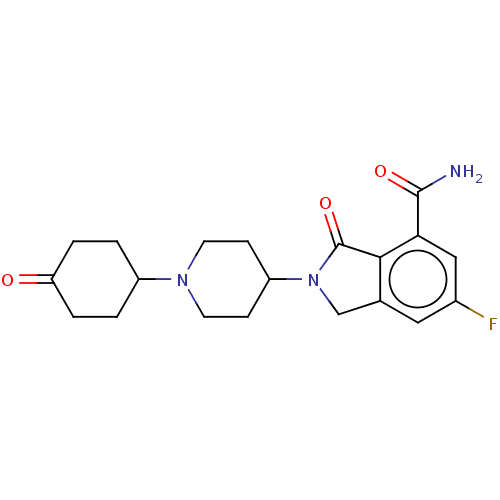 (US8877944, 103) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 698 total ) | Next | Last >> |