 Found 290 hits with Last Name = 'parang' and Initial = 'k'
Found 290 hits with Last Name = 'parang' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370722
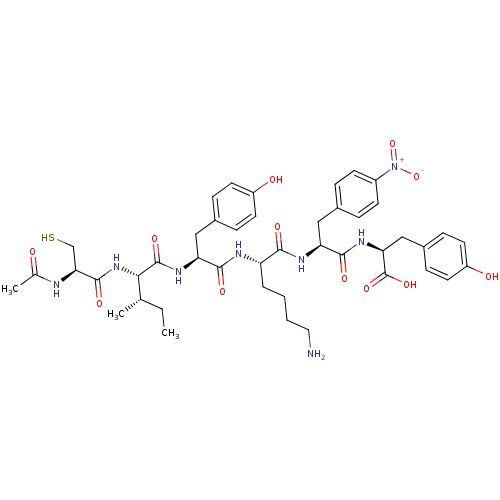
(CHEMBL1791358)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C44H58N8O12S/c1-4-25(2)38(51-42(59)37(24-65)46-26(3)53)43(60)49-35(22-28-10-16-31(54)17-11-28)40(57)47-33(7-5-6-20-45)39(56)48-34(21-27-8-14-30(15-9-27)52(63)64)41(58)50-36(44(61)62)23-29-12-18-32(55)19-13-29/h8-19,25,33-38,54-55,65H,4-7,20-24,45H2,1-3H3,(H,46,53)(H,47,57)(H,48,56)(H,49,60)(H,50,58)(H,51,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC in presence of ATP |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50200200

((S)-2-amino-N-hydroxy-3-(4-hydroxyphenyl)propanami...)Show InChI InChI=1S/C9H12N2O3/c10-8(9(13)11-14)5-6-1-3-7(12)4-2-6/h1-4,8,12,14H,5,10H2,(H,11,13)/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50187738

(Ac-Cys-Ile-cyclo[Phe-Lys]-Tyr-Tyr | CHEMBL428631)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CS)NC(C)=O)C(=O)N[C@H]1Cc2ccc(NC(=O)CCCCCCC(=O)NCCCC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)cc2 Show InChI InChI=1S/C52H70N8O12S/c1-4-31(2)46(60-50(69)43(30-73)54-32(3)61)51(70)58-41-27-33-14-20-36(21-15-33)55-45(65)13-8-6-5-7-12-44(64)53-26-10-9-11-39(56-48(41)67)47(66)57-40(28-34-16-22-37(62)23-17-34)49(68)59-42(52(71)72)29-35-18-24-38(63)25-19-35/h14-25,31,39-43,46,62-63,73H,4-13,26-30H2,1-3H3,(H,53,64)(H,54,61)(H,55,65)(H,56,67)(H,57,66)(H,58,70)(H,59,68)(H,60,69)(H,71,72)/t31-,39-,40-,41-,42-,43+,46-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of GST-fussed c-SRC after 30 mins |
Bioorg Med Chem Lett 21: 1342-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.047
BindingDB Entry DOI: 10.7270/Q2P84C6B |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of c-Src after 60 mins |
Bioorg Med Chem Lett 21: 449-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.121
BindingDB Entry DOI: 10.7270/Q23B60DN |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged c-Src preincubated for 30 mins measured after 60 mins |
Bioorg Med Chem 20: 6821-30 (2012)
Article DOI: 10.1016/j.bmc.2012.09.057
BindingDB Entry DOI: 10.7270/Q23B619Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50142887
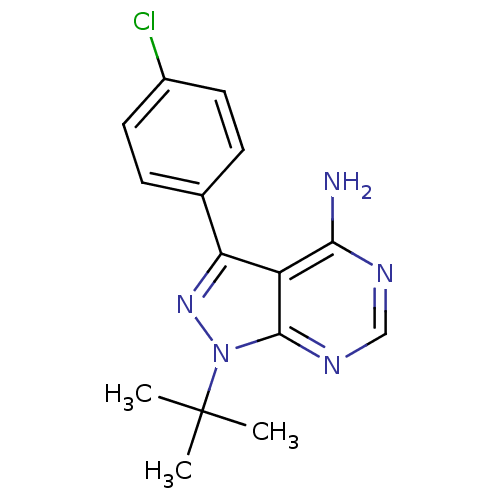
(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India
| Assay Description
The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... |
Bioorg Chem 53: 75-82 (2014)
Article DOI: 10.1016/j.bioorg.2014.02.001
BindingDB Entry DOI: 10.7270/Q2HX1BBK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged c-Src kinase domain using AEEEIYGEFEAKKKK as substrate pre-incubated for 10 mins before substrate addition measured after 3... |
Bioorg Med Chem Lett 22: 410-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.124
BindingDB Entry DOI: 10.7270/Q23X872P |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370722

(CHEMBL1791358)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C44H58N8O12S/c1-4-25(2)38(51-42(59)37(24-65)46-26(3)53)43(60)49-35(22-28-10-16-31(54)17-11-28)40(57)47-33(7-5-6-20-45)39(56)48-34(21-27-8-14-30(15-9-27)52(63)64)41(58)50-36(44(61)62)23-29-12-18-32(55)19-13-29/h8-19,25,33-38,54-55,65H,4-7,20-24,45H2,1-3H3,(H,46,53)(H,47,57)(H,48,56)(H,49,60)(H,50,58)(H,51,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged c-Src kinase domain using AEEEIYGEFEAKKKK as substrate pre-incubated for 10 mins before substrate addition measured after 3... |
Bioorg Med Chem Lett 22: 410-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.124
BindingDB Entry DOI: 10.7270/Q23X872P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India
| Assay Description
The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... |
Bioorg Chem 53: 75-82 (2014)
Article DOI: 10.1016/j.bioorg.2014.02.001
BindingDB Entry DOI: 10.7270/Q2HX1BBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370720

(CHEMBL1791362)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(I)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C44H58IN7O10S/c1-4-25(2)38(52-42(59)37(24-63)47-26(3)53)43(60)50-35(22-28-10-16-31(54)17-11-28)40(57)48-33(7-5-6-20-46)39(56)49-34(21-27-8-14-30(45)15-9-27)41(58)51-36(44(61)62)23-29-12-18-32(55)19-13-29/h8-19,25,33-38,54-55,63H,4-7,20-24,46H2,1-3H3,(H,47,53)(H,48,57)(H,49,56)(H,50,60)(H,51,58)(H,52,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50433806
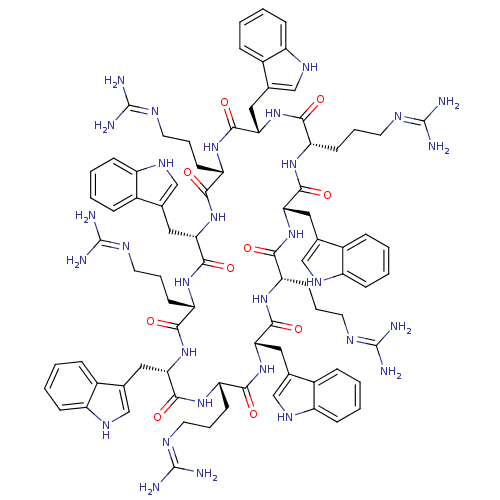
(CHEMBL2382016)Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r,wU:100.108,111.119,7.6,11.11,25.27,36.38,50.54,86.92,75.81,61.65,(13.76,10.06,;12.41,9.29,;12.41,7.74,;11.08,10.06,;9.74,9.29,;9.74,7.82,;8.46,7.09,;8.46,5.61,;7.19,4.87,;7.19,3.4,;8.46,2.66,;5.91,2.66,;4.63,3.4,;4.63,4.94,;5.88,5.86,;5.4,7.32,;3.86,7.32,;2.83,8.45,;1.32,8.13,;.85,6.67,;1.89,5.54,;3.38,5.86,;5.91,1.19,;4.63,.45,;3.37,1.19,;4.63,-1.02,;3.37,-1.77,;2.08,-1.02,;.81,-1.77,;-.55,-1,;-.55,.55,;-1.89,1.33,;.79,1.33,;5.91,-1.77,;5.9,-3.23,;7.18,-3.98,;4.62,-3.97,;3.35,-3.22,;2,-3.99,;1.83,-5.53,;.32,-5.84,;-.45,-4.5,;-1.95,-4.17,;-2.42,-2.7,;-1.39,-1.56,;.12,-1.89,;.59,-3.36,;4.61,-5.44,;5.89,-6.18,;7.17,-5.45,;5.88,-7.66,;4.6,-8.39,;3.33,-7.64,;1.99,-8.41,;1.98,-9.96,;.64,-10.73,;.64,-12.28,;-.71,-9.95,;7.16,-8.4,;7.15,-9.94,;5.81,-10.71,;8.48,-10.72,;8.48,-12.27,;9.82,-13.04,;9.99,-14.58,;11.5,-14.9,;12.27,-13.56,;13.78,-13.24,;14.26,-11.78,;13.22,-10.63,;11.72,-10.95,;11.24,-12.41,;9.83,-9.95,;9.83,-8.4,;8.49,-7.63,;11.17,-7.63,;12.51,-8.41,;13.85,-7.64,;15.19,-8.42,;16.53,-7.65,;17.87,-8.42,;19.21,-7.65,;17.87,-9.97,;11.18,-6.09,;13.56,-3.23,;14.84,-3.97,;13.56,-1.77,;14.84,-1.02,;16.18,-1.79,;17.59,-1.15,;18.64,-2.3,;17.87,-3.64,;18.34,-5.1,;17.32,-6.25,;15.81,-5.93,;15.33,-4.47,;16.36,-3.32,;12.28,-1.02,;12.28,.45,;11.01,1.19,;13.56,1.19,;14.84,.45,;16.11,1.19,;17.45,.42,;18.8,1.18,;20.13,.41,;21.47,1.17,;20.12,-1.15,;13.56,2.66,;12.28,3.4,;11.01,2.66,;12.28,4.87,;13.56,5.61,;14.9,4.84,;15.07,3.29,;16.58,2.98,;17.35,4.31,;18.86,4.62,;19.34,6.09,;18.3,7.25,;16.8,6.93,;16.33,5.45,;11.01,5.61,;9.74,4.87,;9.74,3.4,)| Show InChI InChI=1S/C85H110N30O10/c86-81(87)96-31-11-26-61-71(116)111-66(36-46-41-101-56-21-6-1-16-51(46)56)76(121)106-62(27-12-32-97-82(88)89)72(117)112-68(38-48-43-103-58-23-8-3-18-53(48)58)78(123)108-64(29-14-34-99-84(92)93)74(119)114-70(40-50-45-105-60-25-10-5-20-55(50)60)80(125)110-65(30-15-35-100-85(94)95)75(120)115-69(39-49-44-104-59-24-9-4-19-54(49)59)79(124)109-63(28-13-33-98-83(90)91)73(118)113-67(77(122)107-61)37-47-42-102-57-22-7-2-17-52(47)57/h1-10,16-25,41-45,61-70,101-105H,11-15,26-40H2,(H,106,121)(H,107,122)(H,108,123)(H,109,124)(H,110,125)(H,111,116)(H,112,117)(H,113,118)(H,114,119)(H,115,120)(H4,86,87,96)(H4,88,89,97)(H4,90,91,98)(H4,92,93,99)(H4,94,95,100)/t61-,62-,63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused Csk (unknown origin) expressed in Escherichia coli using polyE4Y as substrate after 20 mins by scintillation counting analysi... |
Bioorg Med Chem Lett 23: 3230-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.124
BindingDB Entry DOI: 10.7270/Q2JW8G8Z |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370725

(CHEMBL1791373)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C53H67N9O13S/c1-4-31(2)46(61-51(70)45(30-76)55-32(3)63)52(71)59-43(28-36-17-23-39(65)24-18-36)48(67)56-40(12-8-9-25-54)47(66)57-41(26-34-13-19-37(20-14-34)62(74)75)49(68)58-42(27-35-15-21-38(64)22-16-35)50(69)60-44(53(72)73)29-33-10-6-5-7-11-33/h5-7,10-11,13-24,31,40-46,64-65,76H,4,8-9,12,25-30,54H2,1-3H3,(H,55,63)(H,56,67)(H,57,66)(H,58,68)(H,59,71)(H,60,69)(H,61,70)(H,72,73)/t31-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370343
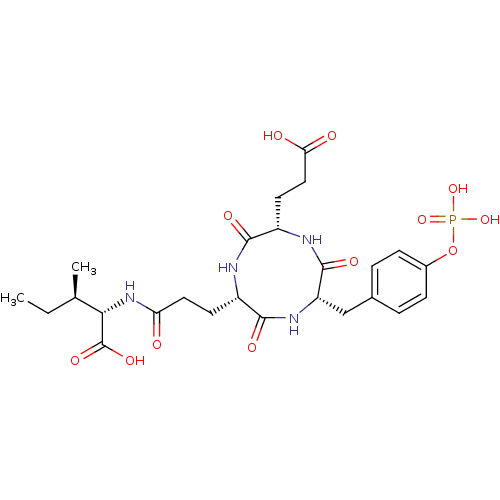
(CHEMBL1790802)Show SMILES CC[C@@H](C)[C@H](NC(=O)CC[C@@H]1NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc2ccc(OP(O)(O)=O)cc2)NC1=O)C(O)=O Show InChI InChI=1S/C25H35N4O12P/c1-3-13(2)21(25(36)37)29-19(30)10-8-16-23(34)28-18(12-14-4-6-15(7-5-14)41-42(38,39)40)24(35)27-17(22(33)26-16)9-11-20(31)32/h4-7,13,16-18,21H,3,8-12H2,1-2H3,(H,26,33)(H,27,35)(H,28,34)(H,29,30)(H,31,32)(H,36,37)(H2,38,39,40)/t13-,16+,17+,18+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Concentration of compoound required for inhibiting the binding of the fluorescent probe to the c-Src tyrosine kinase SH2 domain by 50% |
J Med Chem 47: 3131-41 (2004)
Article DOI: 10.1021/jm040008+
BindingDB Entry DOI: 10.7270/Q25B0370 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370348
(CHEMBL1790811)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CC[C@@H]1NC(=O)[C@H](Cc2ccc(OP(O)(O)=O)cc2)NC1=O)C(O)=O Show InChI InChI=1S/C25H35N4O12P/c1-3-13(2)21(25(36)37)29-23(34)16(9-11-20(31)32)26-19(30)10-8-17-22(33)28-18(24(35)27-17)12-14-4-6-15(7-5-14)41-42(38,39)40/h4-7,13,16-18,21H,3,8-12H2,1-2H3,(H,26,30)(H,27,35)(H,28,33)(H,29,34)(H,31,32)(H,36,37)(H2,38,39,40)/t13-,16+,17+,18+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Concentration of compoound required for inhibiting the binding of the fluorescent probe to the c-Src tyrosine kinase SH2 domain by 50% |
J Med Chem 47: 3131-41 (2004)
Article DOI: 10.1021/jm040008+
BindingDB Entry DOI: 10.7270/Q25B0370 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370729

(CHEMBL1791361)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C44H57N9O13S/c1-4-25(2)38(51-42(59)37(24-67)46-26(3)54)43(60)49-35(22-28-10-16-31(17-11-28)53(65)66)40(57)47-33(7-5-6-20-45)39(56)48-34(21-27-8-14-30(15-9-27)52(63)64)41(58)50-36(44(61)62)23-29-12-18-32(55)19-13-29/h8-19,25,33-38,55,67H,4-7,20-24,45H2,1-3H3,(H,46,54)(H,47,57)(H,48,56)(H,49,60)(H,50,58)(H,51,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370341
(CHEMBL1790810)Show SMILES CC[C@@H](C)[C@H](NC(=O)C[C@@H]1NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc2ccc(OP(O)(O)=O)cc2)NC1=O)C(O)=O Show InChI InChI=1S/C24H33N4O12P/c1-3-12(2)20(24(35)36)28-18(29)11-17-23(34)26-16(10-13-4-6-14(7-5-13)40-41(37,38)39)22(33)25-15(21(32)27-17)8-9-19(30)31/h4-7,12,15-17,20H,3,8-11H2,1-2H3,(H,25,33)(H,26,34)(H,27,32)(H,28,29)(H,30,31)(H,35,36)(H2,37,38,39)/t12-,15+,16+,17+,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Concentration of compoound required for inhibiting the binding of the fluorescent probe to the c-Src tyrosine kinase SH2 domain by 50% |
J Med Chem 47: 3131-41 (2004)
Article DOI: 10.1021/jm040008+
BindingDB Entry DOI: 10.7270/Q25B0370 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370735
(CHEMBL1791374)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C44H58N8O12S/c1-4-25(2)38(51-42(59)37(24-65)46-26(3)53)43(60)49-35(21-27-8-14-30(15-9-27)52(63)64)40(57)47-33(7-5-6-20-45)39(56)48-34(22-28-10-16-31(54)17-11-28)41(58)50-36(44(61)62)23-29-12-18-32(55)19-13-29/h8-19,25,33-38,54-55,65H,4-7,20-24,45H2,1-3H3,(H,46,53)(H,47,57)(H,48,56)(H,49,60)(H,50,58)(H,51,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50187732
(Ac-Cys-Ile-cyclo[Phe-Lys]-Tyr-Tyr | CHEMBL209045)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@H]1Cc2ccc(NC(=O)CCC(=O)NCCCC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)cc2 Show InChI InChI=1S/C48H62N8O12S/c1-4-27(2)42(56-46(65)39(26-69)50-28(3)57)47(66)54-37-23-29-8-14-32(15-9-29)51-41(61)21-20-40(60)49-22-6-5-7-35(52-44(37)63)43(62)53-36(24-30-10-16-33(58)17-11-30)45(64)55-38(48(67)68)25-31-12-18-34(59)19-13-31/h8-19,27,35-39,42,58-59,69H,4-7,20-26H2,1-3H3,(H,49,60)(H,50,57)(H,51,61)(H,52,63)(H,53,62)(H,54,66)(H,55,64)(H,56,65)(H,67,68)/t27-,35-,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370724

(CHEMBL1791376)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(cc1)N=[N+]=[N-])C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C44H58N10O10S/c1-4-25(2)38(52-42(61)37(24-65)47-26(3)55)43(62)50-35(22-28-10-16-31(56)17-11-28)40(59)48-33(7-5-6-20-45)39(58)49-34(21-27-8-14-30(15-9-27)53-54-46)41(60)51-36(44(63)64)23-29-12-18-32(57)19-13-29/h8-19,25,33-38,56-57,65H,4-7,20-24,45H2,1-3H3,(H,47,55)(H,48,59)(H,49,58)(H,50,62)(H,51,60)(H,52,61)(H,63,64)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200197

((S)-2-amino-N-hydroxy-3-(4-hydroxyphenyl)propaneth...)Show InChI InChI=1S/C9H12N2O2S/c10-8(9(14)11-13)5-6-1-3-7(12)4-2-6/h1-4,8,12-13H,5,10H2,(H,11,14)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged c-Src preincubated for 30 mins measured after 60 mins |
Bioorg Med Chem 20: 6821-30 (2012)
Article DOI: 10.1016/j.bmc.2012.09.057
BindingDB Entry DOI: 10.7270/Q23B619Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of GST-fussed c-SRC after 30 mins |
Bioorg Med Chem Lett 21: 1342-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.047
BindingDB Entry DOI: 10.7270/Q2P84C6B |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of c-Src after 60 mins |
Bioorg Med Chem Lett 21: 449-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.121
BindingDB Entry DOI: 10.7270/Q23B60DN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50433806

(CHEMBL2382016)Show SMILES NC(N)=NCCC[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r,wU:100.108,111.119,7.6,11.11,25.27,36.38,50.54,86.92,75.81,61.65,(13.76,10.06,;12.41,9.29,;12.41,7.74,;11.08,10.06,;9.74,9.29,;9.74,7.82,;8.46,7.09,;8.46,5.61,;7.19,4.87,;7.19,3.4,;8.46,2.66,;5.91,2.66,;4.63,3.4,;4.63,4.94,;5.88,5.86,;5.4,7.32,;3.86,7.32,;2.83,8.45,;1.32,8.13,;.85,6.67,;1.89,5.54,;3.38,5.86,;5.91,1.19,;4.63,.45,;3.37,1.19,;4.63,-1.02,;3.37,-1.77,;2.08,-1.02,;.81,-1.77,;-.55,-1,;-.55,.55,;-1.89,1.33,;.79,1.33,;5.91,-1.77,;5.9,-3.23,;7.18,-3.98,;4.62,-3.97,;3.35,-3.22,;2,-3.99,;1.83,-5.53,;.32,-5.84,;-.45,-4.5,;-1.95,-4.17,;-2.42,-2.7,;-1.39,-1.56,;.12,-1.89,;.59,-3.36,;4.61,-5.44,;5.89,-6.18,;7.17,-5.45,;5.88,-7.66,;4.6,-8.39,;3.33,-7.64,;1.99,-8.41,;1.98,-9.96,;.64,-10.73,;.64,-12.28,;-.71,-9.95,;7.16,-8.4,;7.15,-9.94,;5.81,-10.71,;8.48,-10.72,;8.48,-12.27,;9.82,-13.04,;9.99,-14.58,;11.5,-14.9,;12.27,-13.56,;13.78,-13.24,;14.26,-11.78,;13.22,-10.63,;11.72,-10.95,;11.24,-12.41,;9.83,-9.95,;9.83,-8.4,;8.49,-7.63,;11.17,-7.63,;12.51,-8.41,;13.85,-7.64,;15.19,-8.42,;16.53,-7.65,;17.87,-8.42,;19.21,-7.65,;17.87,-9.97,;11.18,-6.09,;13.56,-3.23,;14.84,-3.97,;13.56,-1.77,;14.84,-1.02,;16.18,-1.79,;17.59,-1.15,;18.64,-2.3,;17.87,-3.64,;18.34,-5.1,;17.32,-6.25,;15.81,-5.93,;15.33,-4.47,;16.36,-3.32,;12.28,-1.02,;12.28,.45,;11.01,1.19,;13.56,1.19,;14.84,.45,;16.11,1.19,;17.45,.42,;18.8,1.18,;20.13,.41,;21.47,1.17,;20.12,-1.15,;13.56,2.66,;12.28,3.4,;11.01,2.66,;12.28,4.87,;13.56,5.61,;14.9,4.84,;15.07,3.29,;16.58,2.98,;17.35,4.31,;18.86,4.62,;19.34,6.09,;18.3,7.25,;16.8,6.93,;16.33,5.45,;11.01,5.61,;9.74,4.87,;9.74,3.4,)| Show InChI InChI=1S/C85H110N30O10/c86-81(87)96-31-11-26-61-71(116)111-66(36-46-41-101-56-21-6-1-16-51(46)56)76(121)106-62(27-12-32-97-82(88)89)72(117)112-68(38-48-43-103-58-23-8-3-18-53(48)58)78(123)108-64(29-14-34-99-84(92)93)74(119)114-70(40-50-45-105-60-25-10-5-20-55(50)60)80(125)110-65(30-15-35-100-85(94)95)75(120)115-69(39-49-44-104-59-24-9-4-19-54(49)59)79(124)109-63(28-13-33-98-83(90)91)73(118)113-67(77(122)107-61)37-47-42-102-57-22-7-2-17-52(47)57/h1-10,16-25,41-45,61-70,101-105H,11-15,26-40H2,(H,106,121)(H,107,122)(H,108,123)(H,109,124)(H,110,125)(H,111,116)(H,112,117)(H,113,118)(H,114,119)(H,115,120)(H4,86,87,96)(H4,88,89,97)(H4,90,91,98)(H4,92,93,99)(H4,94,95,100)/t61-,62-,63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused Abl (unknown origin) expressed in Escherichia coli using CrkL as substrate after 20 mins by scintillation counting analysis i... |
Bioorg Med Chem Lett 23: 3230-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.124
BindingDB Entry DOI: 10.7270/Q2JW8G8Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200187

((S)-2-amino-N-hydroxy-3-(4-iodophenyl)propanamide ...)Show InChI InChI=1S/C9H11IN2O2/c10-7-3-1-6(2-4-7)5-8(11)9(13)12-14/h1-4,8,14H,5,11H2,(H,12,13)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200208

((S)-2-amino-N-hydroxy-3-(4-isopropylphenyl)propana...)Show InChI InChI=1S/C12H18N2O2/c1-8(2)10-5-3-9(4-6-10)7-11(13)12(15)14-16/h3-6,8,11,16H,7,13H2,1-2H3,(H,14,15)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370351
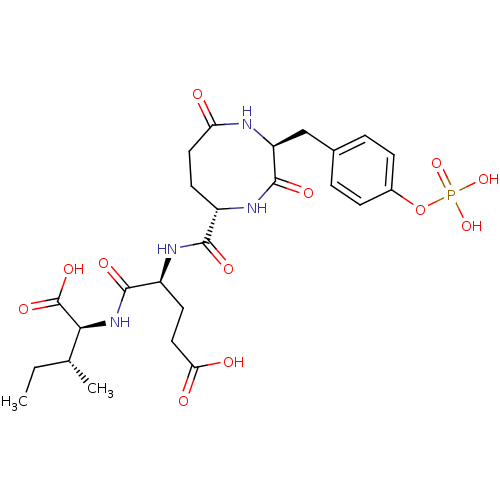
(CHEMBL1790806)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCC(=O)N[C@@H](Cc2ccc(OP(O)(O)=O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C25H35N4O12P/c1-3-13(2)21(25(36)37)29-23(34)17(9-11-20(31)32)27-22(33)16-8-10-19(30)26-18(24(35)28-16)12-14-4-6-15(7-5-14)41-42(38,39)40/h4-7,13,16-18,21H,3,8-12H2,1-2H3,(H,26,30)(H,27,33)(H,28,35)(H,29,34)(H,31,32)(H,36,37)(H2,38,39,40)/t13-,16+,17+,18+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Concentration of compoound required for inhibiting the binding of the fluorescent probe to the c-Src tyrosine kinase SH2 domain by 50% |
J Med Chem 47: 3131-41 (2004)
Article DOI: 10.1021/jm040008+
BindingDB Entry DOI: 10.7270/Q25B0370 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200198

((S)-2-amino-N-hydroxy-3-(4-hydroxy-3,5-diiodopheny...)Show InChI InChI=1S/C9H10I2N2O3/c10-5-1-4(2-6(11)8(5)14)3-7(12)9(15)13-16/h1-2,7,14,16H,3,12H2,(H,13,15)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370730

(CHEMBL1791372)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C44H57N9O13S/c1-4-25(2)38(51-42(59)37(24-67)46-26(3)54)43(60)49-35(21-27-8-14-30(15-9-27)52(63)64)40(57)47-33(7-5-6-20-45)39(56)48-34(22-29-12-18-32(55)19-13-29)41(58)50-36(44(61)62)23-28-10-16-31(17-11-28)53(65)66/h8-19,25,33-38,55,67H,4-7,20-24,45H2,1-3H3,(H,46,54)(H,47,57)(H,48,56)(H,49,60)(H,50,58)(H,51,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200190

((S)-2-amino-N-hydroxy-3-(2-iodophenyl)propanamide ...)Show InChI InChI=1S/C9H11IN2O2/c10-7-4-2-1-3-6(7)5-8(11)9(13)12-14/h1-4,8,14H,5,11H2,(H,12,13)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200189
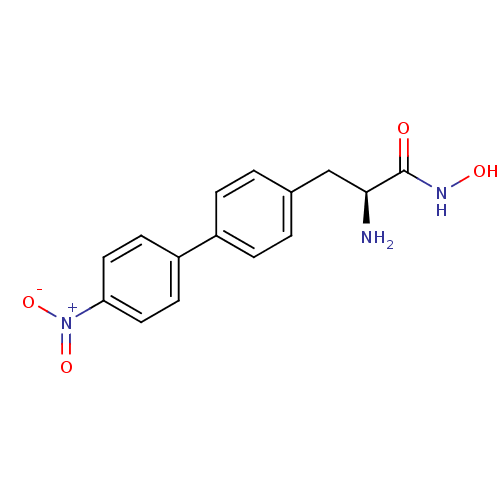
((S)-2-amino-N-hydroxy-3-[4-(nitrophenyl)phenyl]pro...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)[N+]([O-])=O)C(=O)NO |r| Show InChI InChI=1S/C15H15N3O4/c16-14(15(19)17-20)9-10-1-3-11(4-2-10)12-5-7-13(8-6-12)18(21)22/h1-8,14,20H,9,16H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370728

(CHEMBL1791375)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C44H57N9O13S/c1-4-25(2)38(51-42(59)37(24-67)46-26(3)54)43(60)49-35(22-29-12-18-32(55)19-13-29)40(57)47-33(7-5-6-20-45)39(56)48-34(21-27-8-14-30(15-9-27)52(63)64)41(58)50-36(44(61)62)23-28-10-16-31(17-11-28)53(65)66/h8-19,25,33-38,55,67H,4-7,20-24,45H2,1-3H3,(H,46,54)(H,47,57)(H,48,56)(H,49,60)(H,50,58)(H,51,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200196
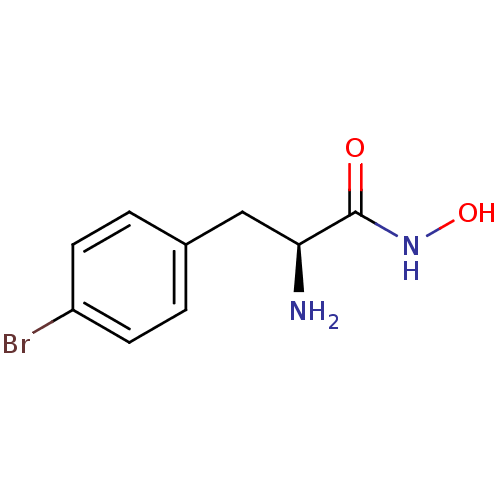
((S)-2-amino-3-(4-bromophenyl)-N-hydroxypropionamid...)Show InChI InChI=1S/C9H11BrN2O2/c10-7-3-1-6(2-4-7)5-8(11)9(13)12-14/h1-4,8,14H,5,11H2,(H,12,13)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50337691
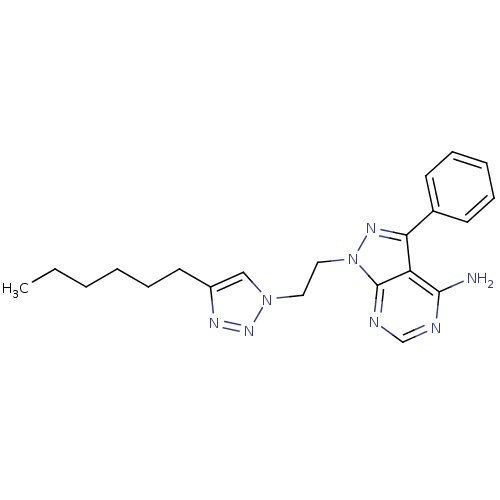
(1-(2-(4-Hexyl-1H-1,2,3-triazol-1-yl)ethyl)-3-pheny...)Show SMILES CCCCCCc1cn(CCn2nc(-c3ccccc3)c3c(N)ncnc23)nn1 Show InChI InChI=1S/C21H26N8/c1-2-3-4-8-11-17-14-28(27-25-17)12-13-29-21-18(20(22)23-15-24-21)19(26-29)16-9-6-5-7-10-16/h5-7,9-10,14-15H,2-4,8,11-13H2,1H3,(H2,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of GST-fussed c-SRC after 30 mins |
Bioorg Med Chem Lett 21: 1342-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.047
BindingDB Entry DOI: 10.7270/Q2P84C6B |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370352
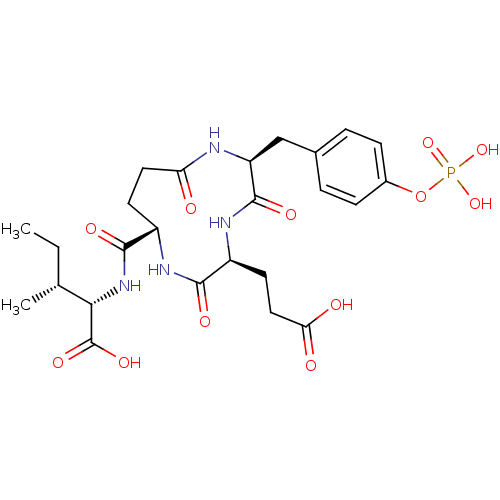
(CHEMBL1790807)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCC(=O)N[C@@H](Cc2ccc(OP(O)(O)=O)cc2)C(=O)N[C@@H](CCC(O)=O)C(=O)N1)C(O)=O Show InChI InChI=1S/C25H35N4O12P/c1-3-13(2)21(25(36)37)29-23(34)16-8-10-19(30)26-18(12-14-4-6-15(7-5-14)41-42(38,39)40)24(35)28-17(22(33)27-16)9-11-20(31)32/h4-7,13,16-18,21H,3,8-12H2,1-2H3,(H,26,30)(H,27,33)(H,28,35)(H,29,34)(H,31,32)(H,36,37)(H2,38,39,40)/t13-,16+,17+,18+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Concentration of compoound required for inhibiting the binding of the fluorescent probe to the c-Src tyrosine kinase SH2 domain by 50% |
J Med Chem 47: 3131-41 (2004)
Article DOI: 10.1021/jm040008+
BindingDB Entry DOI: 10.7270/Q25B0370 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370731

(CHEMBL1791368)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(cc1)C#N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C45H58N8O10S/c1-4-26(2)39(53-43(60)38(25-64)48-27(3)54)44(61)51-36(22-29-12-16-32(55)17-13-29)41(58)49-34(7-5-6-20-46)40(57)50-35(21-28-8-10-31(24-47)11-9-28)42(59)52-37(45(62)63)23-30-14-18-33(56)19-15-30/h8-19,26,34-39,55-56,64H,4-7,20-23,25,46H2,1-3H3,(H,48,54)(H,49,58)(H,50,57)(H,51,61)(H,52,59)(H,53,60)(H,62,63)/t26-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200201
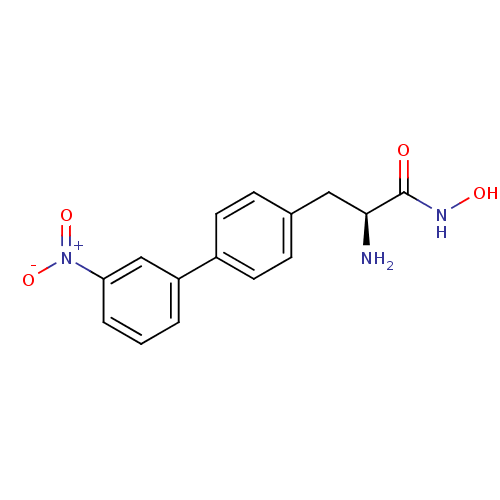
((S)-2-amino-N-hydroxy-3-[3-(nitrophenyl)phenyl]pro...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1cccc(c1)[N+]([O-])=O)C(=O)NO |r| Show InChI InChI=1S/C15H15N3O4/c16-14(15(19)17-20)8-10-4-6-11(7-5-10)12-2-1-3-13(9-12)18(21)22/h1-7,9,14,20H,8,16H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50337693

(1-(2-Azidoethyl)-3-phenyl-1H-pyrazolo[3,4-d]pyrimi...)Show InChI InChI=1S/C13H12N8/c14-12-10-11(9-4-2-1-3-5-9)19-21(7-6-18-20-15)13(10)17-8-16-12/h1-5,8H,6-7H2,(H2,14,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of GST-fussed c-SRC after 30 mins |
Bioorg Med Chem Lett 21: 1342-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.047
BindingDB Entry DOI: 10.7270/Q2P84C6B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
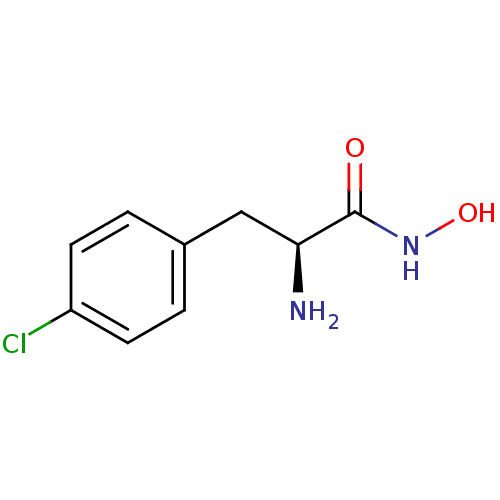
(Homo sapiens (Human)) | BDBM50200188
((S)-2-amino-3-(4-chlorophenyl)-N-hydroxypropanamid...)Show InChI InChI=1S/C9H11ClN2O2/c10-7-3-1-6(2-4-7)5-8(11)9(13)12-14/h1-4,8,14H,5,11H2,(H,12,13)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50187729

(CHEMBL429525 | Cyclo[Ac-Cys-Ile-Tyr-Lys-Tyr-Tyr])Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CS)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O Show InChI InChI=1S/C42H55N7O9S/c1-3-24(2)36-42(58)47-34(22-27-11-17-30(52)18-12-27)38(54)44-31(6-4-5-19-43)37(53)45-32(20-25-7-13-28(50)14-8-25)39(55)46-33(21-26-9-15-29(51)16-10-26)40(56)48-35(23-59)41(57)49-36/h7-18,24,31-36,50-52,59H,3-6,19-23,43H2,1-2H3,(H,44,54)(H,45,53)(H,46,55)(H,47,58)(H,48,56)(H,49,57)/t24-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50101077
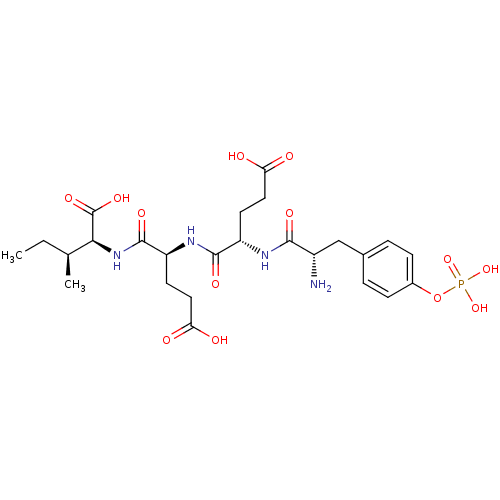
((1S,4S)-2-((S)-2-{(S)-2-[2-Amino-3-(4-phosphonooxy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)Cc1ccc(OP(O)(O)=O)cc1)C(O)=O Show InChI InChI=1S/C25H37N4O13P/c1-3-13(2)21(25(37)38)29-24(36)18(9-11-20(32)33)28-23(35)17(8-10-19(30)31)27-22(34)16(26)12-14-4-6-15(7-5-14)42-43(39,40)41/h4-7,13,16-18,21H,3,8-12,26H2,1-2H3,(H,27,34)(H,28,35)(H,29,36)(H,30,31)(H,32,33)(H,37,38)(H2,39,40,41)/t13-,16-,17-,18-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Src protein tryrosine kinase SH2 domain |
Bioorg Med Chem Lett 15: 4994-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.005
BindingDB Entry DOI: 10.7270/Q2W37VWC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50074242
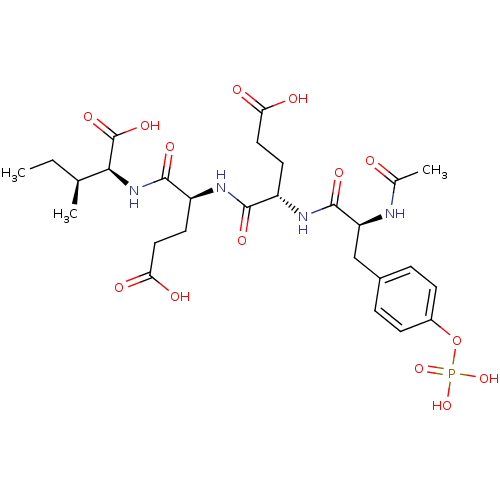
((2S,3S)-2-((S)-2-{(S)-2-[(S)-2-Acetylamino-3-(4-ph...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(O)=O Show InChI InChI=1S/C27H39N4O14P/c1-4-14(2)23(27(40)41)31-25(38)19(10-12-22(35)36)29-24(37)18(9-11-21(33)34)30-26(39)20(28-15(3)32)13-16-5-7-17(8-6-16)45-46(42,43)44/h5-8,14,18-20,23H,4,9-13H2,1-3H3,(H,28,32)(H,29,37)(H,30,39)(H,31,38)(H,33,34)(H,35,36)(H,40,41)(H2,42,43,44)/t14-,18-,19-,20-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Concentration of compoound required for inhibiting the binding of the fluorescent probe to the c-Src tyrosine kinase SH2 domain by 50% |
J Med Chem 47: 3131-41 (2004)
Article DOI: 10.1021/jm040008+
BindingDB Entry DOI: 10.7270/Q25B0370 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200206
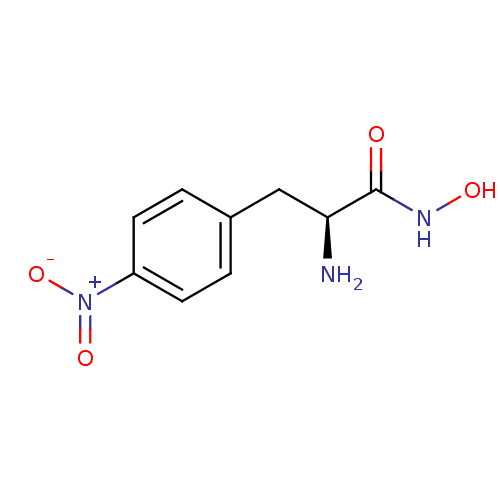
((S)-2-amino-N-hydroxy-3-(4-nitrophenyl)propanamide...)Show SMILES N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)NO |r| Show InChI InChI=1S/C9H11N3O4/c10-8(9(13)11-14)5-6-1-3-7(4-2-6)12(15)16/h1-4,8,14H,5,10H2,(H,11,13)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370737
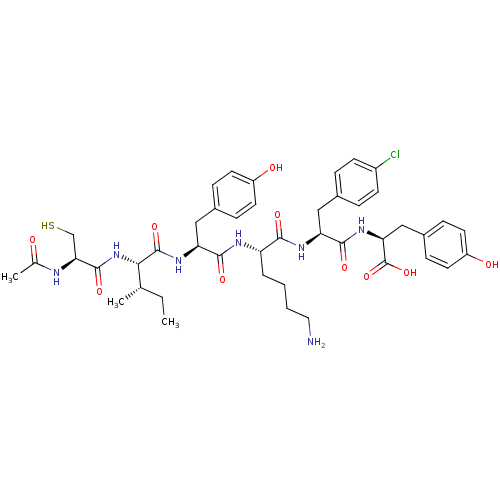
(CHEMBL1791356)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C44H58ClN7O10S/c1-4-25(2)38(52-42(59)37(24-63)47-26(3)53)43(60)50-35(22-28-10-16-31(54)17-11-28)40(57)48-33(7-5-6-20-46)39(56)49-34(21-27-8-14-30(45)15-9-27)41(58)51-36(44(61)62)23-29-12-18-32(55)19-13-29/h8-19,25,33-38,54-55,63H,4-7,20-24,46H2,1-3H3,(H,47,53)(H,48,57)(H,49,56)(H,50,60)(H,51,58)(H,52,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50337678

(3-(4-((4-Amino-3-phenyl-1H-pyrazolo[3,4-d]pyrimidi...)Show SMILES Nc1ncnc2n(Cc3cn(CC(=O)CCCO)nn3)nc(-c3ccccc3)c12 Show InChI InChI=1S/C19H20N8O2/c20-18-16-17(13-5-2-1-3-6-13)24-27(19(16)22-12-21-18)10-14-9-26(25-23-14)11-15(29)7-4-8-28/h1-3,5-6,9,12,28H,4,7-8,10-11H2,(H2,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of GST-fussed c-SRC after 30 mins |
Bioorg Med Chem Lett 21: 1342-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.047
BindingDB Entry DOI: 10.7270/Q2P84C6B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200200

((S)-2-amino-N-hydroxy-3-(4-hydroxyphenyl)propanami...)Show InChI InChI=1S/C9H12N2O3/c10-8(9(13)11-14)5-6-1-3-7(12)4-2-6/h1-4,8,12,14H,5,10H2,(H,11,13)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50200203

((S)-2-amino-N-hydroxy-3-p-tolylpropionamide | CHEM...)Show InChI InChI=1S/C10H14N2O2/c1-7-2-4-8(5-3-7)6-9(11)10(13)12-14/h2-5,9,14H,6,11H2,1H3,(H,12,13)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of CSK measured as poly-E4Y phosphorylation by acid precipitation assay in presence of 0.2 mM CoCl2 |
J Med Chem 49: 7532-9 (2006)
Article DOI: 10.1021/jm061058c
BindingDB Entry DOI: 10.7270/Q2SQ9029 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50370718

(CHEMBL1791371)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C44H58N8O12S/c1-4-25(2)38(51-42(59)37(24-65)46-26(3)53)43(60)49-35(22-29-12-18-32(55)19-13-29)40(57)47-33(7-5-6-20-45)39(56)48-34(21-28-10-16-31(54)17-11-28)41(58)50-36(44(61)62)23-27-8-14-30(15-9-27)52(63)64/h8-19,25,33-38,54-55,65H,4-7,20-24,45H2,1-3H3,(H,46,53)(H,47,57)(H,48,56)(H,49,60)(H,50,58)(H,51,59)(H,61,62)/t25-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Inhibition of SRC using polyE4Y as substrate |
J Med Chem 49: 3395-401 (2006)
Article DOI: 10.1021/jm060334k
BindingDB Entry DOI: 10.7270/Q22F7P7S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data