

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015639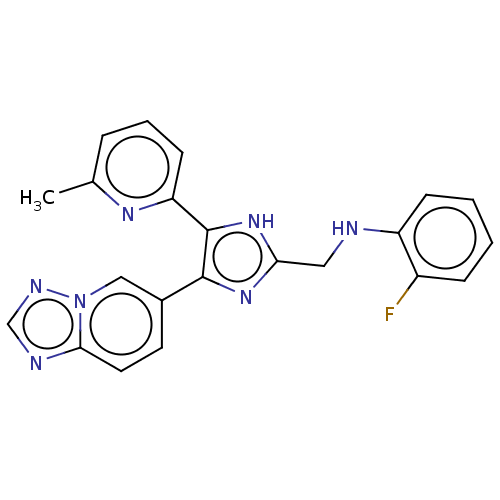 (CHEMBL3260567 | USRE47141, Example 2) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Competitive inhibition of ALK5 (unknown origin) assessed as enzyme/ATP complex by Michaelis-Menten plot analysis in presence of ATP | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015639 (CHEMBL3260567 | USRE47141, Example 2) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Competitive inhibition of ALK5 (unknown origin) by Michaelis-Menten plot analysis in presence of ATP | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50240041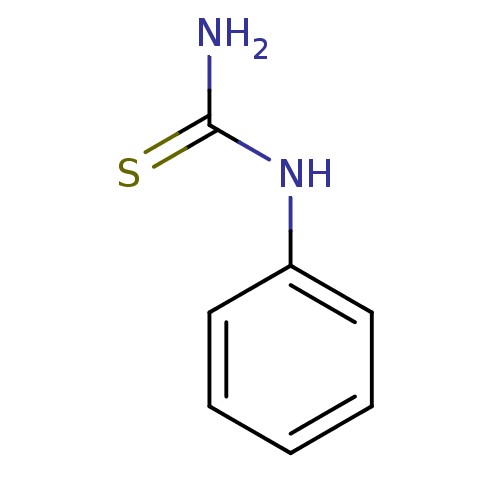 (1-phenyl-2-thiourea | 1-phenylthiourea | CHEMBL263...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Lineweaver-Burk plots analysis | Eur J Med Chem 106: 157-66 (2015) Article DOI: 10.1016/j.ejmech.2015.10.033 BindingDB Entry DOI: 10.7270/Q2514270 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50499815 (CHEMBL3739883) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Lineweaver-Burk plots analysis | Eur J Med Chem 106: 157-66 (2015) Article DOI: 10.1016/j.ejmech.2015.10.033 BindingDB Entry DOI: 10.7270/Q2514270 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50481948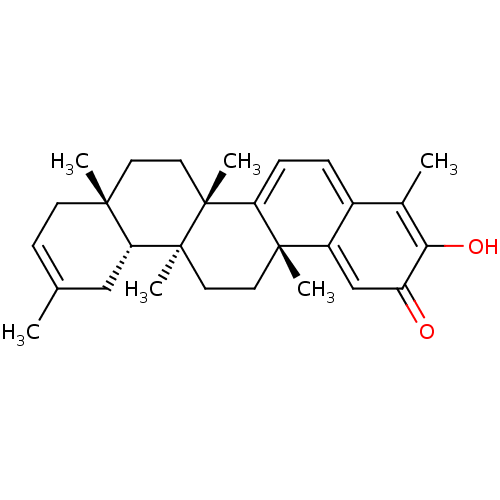 (Iguesterin | acs.jmedchem.1c00409_ST.224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot | Bioorg Med Chem Lett 20: 1873-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.152 BindingDB Entry DOI: 10.7270/Q28P63CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50499816 (CHEMBL3740596) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Lineweaver-Burk plots analysis | Eur J Med Chem 106: 157-66 (2015) Article DOI: 10.1016/j.ejmech.2015.10.033 BindingDB Entry DOI: 10.7270/Q2514270 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50239976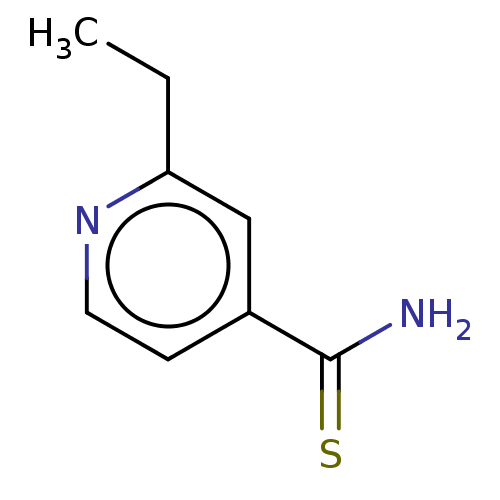 (1314 TH | CHEBI:4885 | Ethionamide | Trecator | Tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Lineweaver-Burk plots analysis | Eur J Med Chem 106: 157-66 (2015) Article DOI: 10.1016/j.ejmech.2015.10.033 BindingDB Entry DOI: 10.7270/Q2514270 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50499814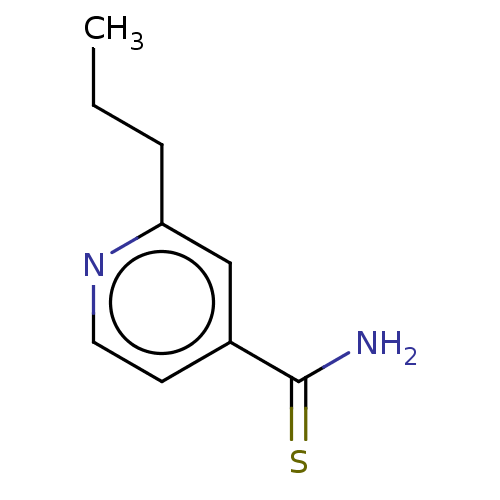 (Ektebin | Peteha | Prothionamide | Protionamide | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Lineweaver-Burk plots analysis | Eur J Med Chem 106: 157-66 (2015) Article DOI: 10.1016/j.ejmech.2015.10.033 BindingDB Entry DOI: 10.7270/Q2514270 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50499813 (CHEMBL3741839) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Lineweaver-Burk plots analysis | Eur J Med Chem 106: 157-66 (2015) Article DOI: 10.1016/j.ejmech.2015.10.033 BindingDB Entry DOI: 10.7270/Q2514270 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50481947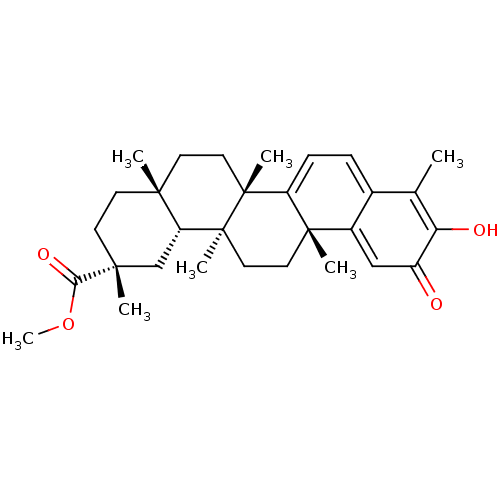 (CHEBI:8416 | GNF-Pf-476 | PRISTIMERIN | Pristimeri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot | Bioorg Med Chem Lett 20: 1873-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.152 BindingDB Entry DOI: 10.7270/Q28P63CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50071055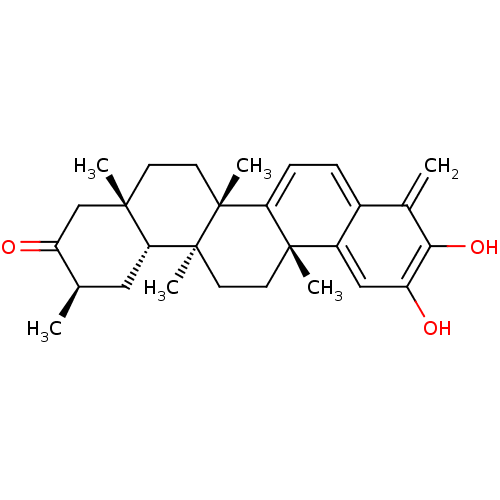 ((6bS,8aS,11R,12aR,12bS,14aR)-3-Hydroxy-4,6b,8a,11,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot | Bioorg Med Chem Lett 20: 1873-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.152 BindingDB Entry DOI: 10.7270/Q28P63CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50071058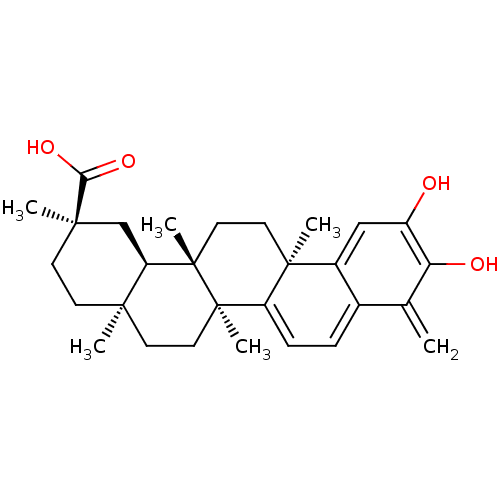 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of 3C-like protease of SARS coronavirus assessed as concentration of FRET peptide for 60 mins by dixon plot | Bioorg Med Chem Lett 20: 1873-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.152 BindingDB Entry DOI: 10.7270/Q28P63CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM60920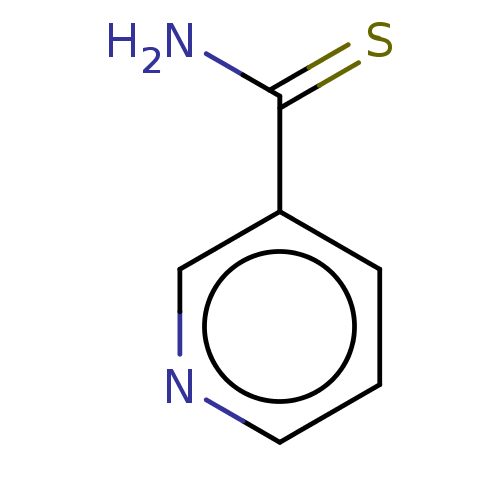 (thionicotinamide) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Lineweaver-Burk plots analysis | Eur J Med Chem 106: 157-66 (2015) Article DOI: 10.1016/j.ejmech.2015.10.033 BindingDB Entry DOI: 10.7270/Q2514270 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM53072 ((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50391431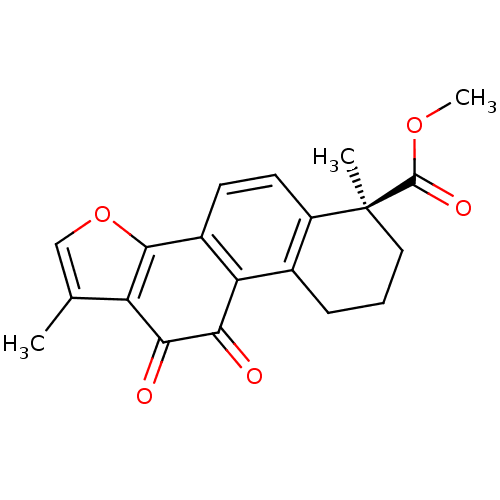 (CHEMBL2146517 | acs.jmedchem.1c00409_ST.502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50423877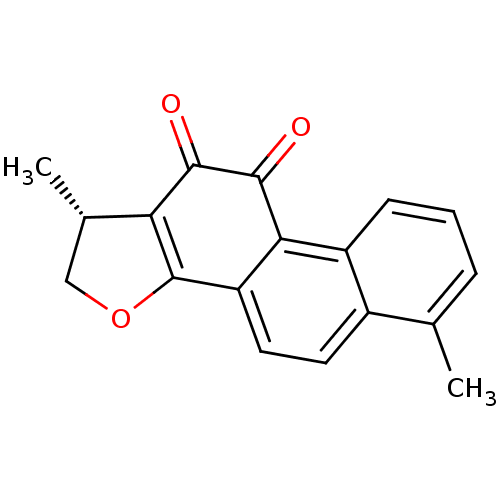 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM83922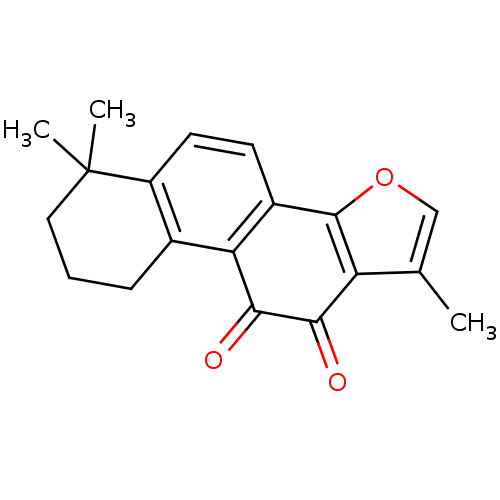 (1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM51317 (1,6-dimethylnaphtho[1,2-g][1]benzofuran-10,11-dion...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50129952 (2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis | Bioorg Med Chem 18: 7940-7 (2010) Article DOI: 10.1016/j.bmc.2010.09.035 BindingDB Entry DOI: 10.7270/Q2MG7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352810 (CHEMBL1711961) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352812 (CHEMBL1823414) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM76798 ((E)-1-[2,4-dihydroxy-3-[(2E)-6-hydroxy-3,7-dimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50009219 (2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenant...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50391429 (CHEMBL215254 | acs.jmedchem.1c00409_ST.620) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50499817 (CHEMBL2441351) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Lineweaver-Burk plots analysis | Eur J Med Chem 106: 157-66 (2015) Article DOI: 10.1016/j.ejmech.2015.10.033 BindingDB Entry DOI: 10.7270/Q2514270 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323199 (5,7-dihydroxy-8-(5-(5-hydroxy-7-methoxy-4-oxo-4H-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis | Bioorg Med Chem 18: 7940-7 (2010) Article DOI: 10.1016/j.bmc.2010.09.035 BindingDB Entry DOI: 10.7270/Q2MG7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352808 (CHEMBL1823413) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323206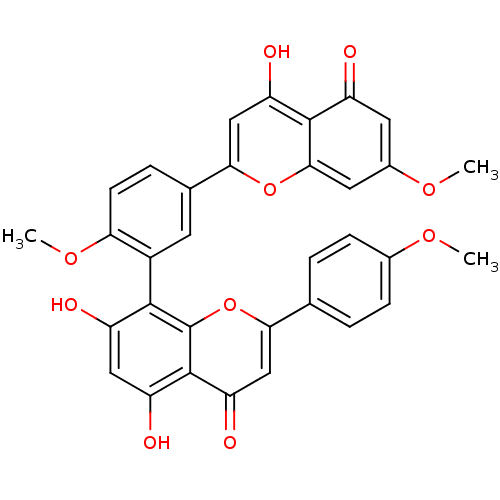 (CHEMBL208908 | sciadopitisin | sciadopitysin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis | Bioorg Med Chem 18: 7940-7 (2010) Article DOI: 10.1016/j.bmc.2010.09.035 BindingDB Entry DOI: 10.7270/Q2MG7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352809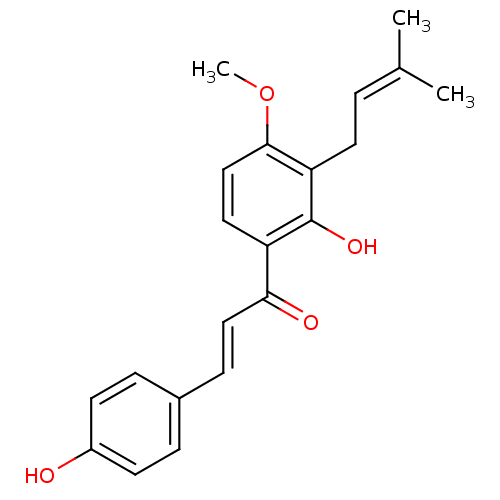 (CHEMBL458094) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50323196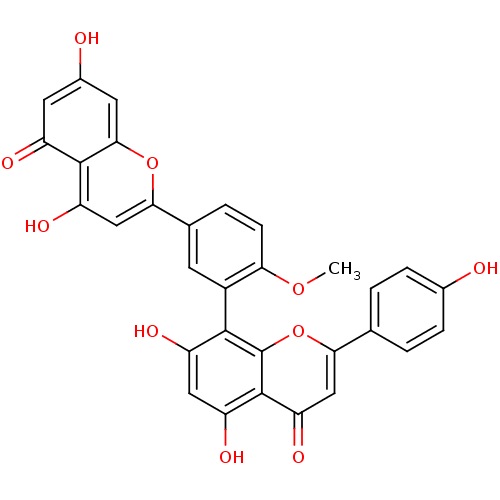 (4'-methylamentoflavone | CHEMBL378188 | bilobetin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Non-competitive inhibition of SARS coronavirus 3C-like protease by Dixon plot analysis | Bioorg Med Chem 18: 7940-7 (2010) Article DOI: 10.1016/j.bmc.2010.09.035 BindingDB Entry DOI: 10.7270/Q2MG7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Brevig Mission/1/1918 ...) | BDBM50352811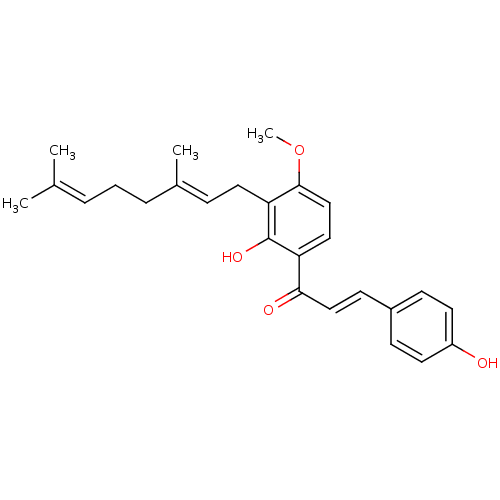 (CHEMBL1722838) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A virus (A/Brevig Mission/1/1918(H1N1)) recombinant neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetyln... | Bioorg Med Chem Lett 21: 5602-4 (2011) Article DOI: 10.1016/j.bmcl.2011.06.130 BindingDB Entry DOI: 10.7270/Q2571CDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015959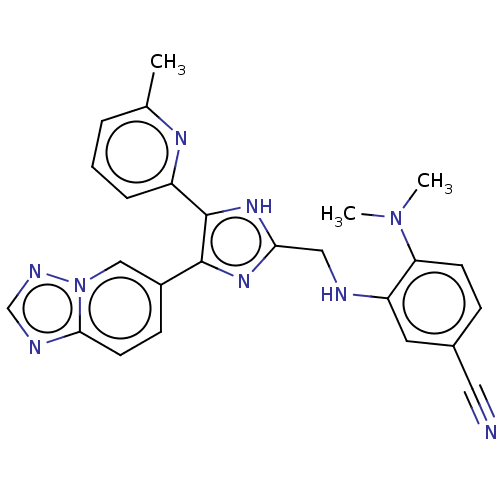 (CHEMBL3260904) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50261163 (CHEMBL4081756) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/CyclinA (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay | Bioorg Med Chem Lett 27: 4399-4404 (2017) Article DOI: 10.1016/j.bmcl.2017.08.018 BindingDB Entry DOI: 10.7270/Q2W66P60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50261164 (CHEMBL4103221) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of CDK5/p35 (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay | Bioorg Med Chem Lett 27: 4399-4404 (2017) Article DOI: 10.1016/j.bmcl.2017.08.018 BindingDB Entry DOI: 10.7270/Q2W66P60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015952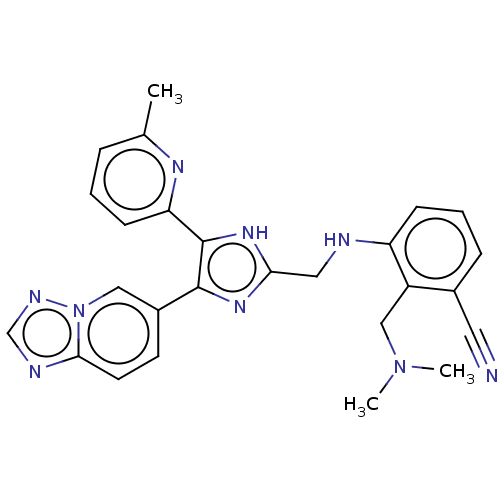 (CHEMBL3260897) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015955 (CHEMBL3260900) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015946 (CHEMBL3260627) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015960 (CHEMBL3260905) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015958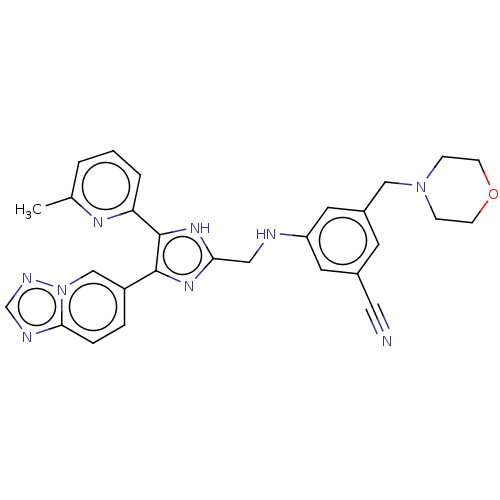 (CHEMBL3260903) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50237650 (CHEMBL4071507) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells assessed as reduction in H2O2 production using p-tyramine as... | Eur J Med Chem 130: 365-378 (2017) Article DOI: 10.1016/j.ejmech.2017.02.059 BindingDB Entry DOI: 10.7270/Q2K076J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50261163 (CHEMBL4081756) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of CDK5/p35 (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay | Bioorg Med Chem Lett 27: 4399-4404 (2017) Article DOI: 10.1016/j.bmcl.2017.08.018 BindingDB Entry DOI: 10.7270/Q2W66P60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015962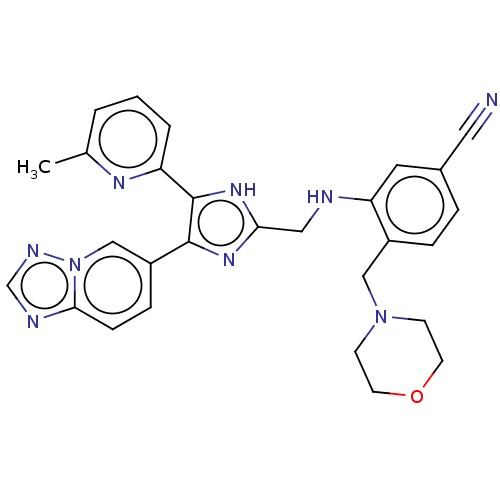 (CHEMBL3260907) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015956 (CHEMBL3260901) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015670 (CHEMBL3260583 | USRE47141, Example 20) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015679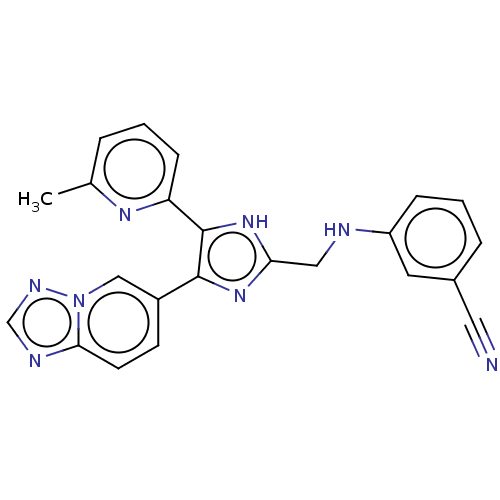 (CHEMBL3260591 | USRE47141, Example 60) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50261205 (CHEMBL4091107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/CyclinA (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay | Bioorg Med Chem Lett 27: 4399-4404 (2017) Article DOI: 10.1016/j.bmcl.2017.08.018 BindingDB Entry DOI: 10.7270/Q2W66P60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015953 (CHEMBL3260898) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19187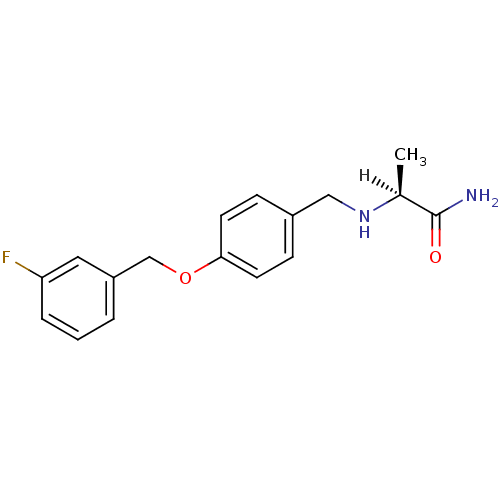 ((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells assessed as reduction in H2O2 production using p-tyramine as... | Eur J Med Chem 130: 365-378 (2017) Article DOI: 10.1016/j.ejmech.2017.02.059 BindingDB Entry DOI: 10.7270/Q2K076J6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50261165 (CHEMBL4070146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of CDK5/p35 (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay | Bioorg Med Chem Lett 27: 4399-4404 (2017) Article DOI: 10.1016/j.bmcl.2017.08.018 BindingDB Entry DOI: 10.7270/Q2W66P60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50015942 (CHEMBL3260623 | USRE47141, Example 9) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK5 expressed in insect Sf9 cells using casein as substrate by radioisotopic assay | J Med Chem 57: 4213-38 (2014) Article DOI: 10.1021/jm500115w BindingDB Entry DOI: 10.7270/Q2DV1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1883 total ) | Next | Last >> |