 Found 2088 hits with Last Name = 'park' and Initial = 't'
Found 2088 hits with Last Name = 'park' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00525
BindingDB Entry DOI: 10.7270/Q29W0K29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM30369
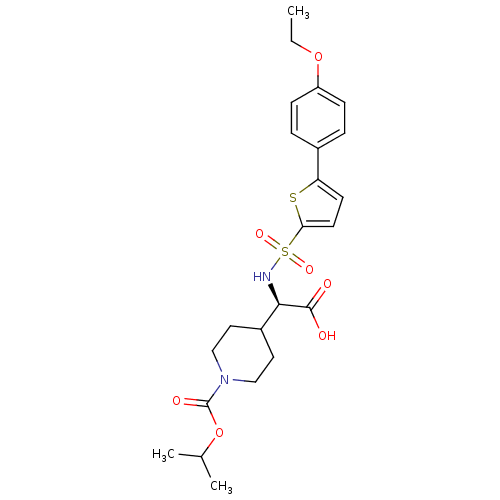
(piperidinyl glycine derivative, 24f)Show SMILES CCOc1ccc(cc1)-c1ccc(s1)S(=O)(=O)N[C@H](C1CCN(CC1)C(=O)OC(C)C)C(O)=O |r| Show InChI InChI=1S/C23H30N2O7S2/c1-4-31-18-7-5-16(6-8-18)19-9-10-20(33-19)34(29,30)24-21(22(26)27)17-11-13-25(14-12-17)23(28)32-15(2)3/h5-10,15,17,21,24H,4,11-14H2,1-3H3,(H,26,27)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.190 | -54.9 | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... |
J Med Chem 52: 3523-38 (2009)
Article DOI: 10.1021/jm801394m
BindingDB Entry DOI: 10.7270/Q2B27SN3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.280 | -54.0 | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... |
J Med Chem 52: 3523-38 (2009)
Article DOI: 10.1021/jm801394m
BindingDB Entry DOI: 10.7270/Q2B27SN3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM30344
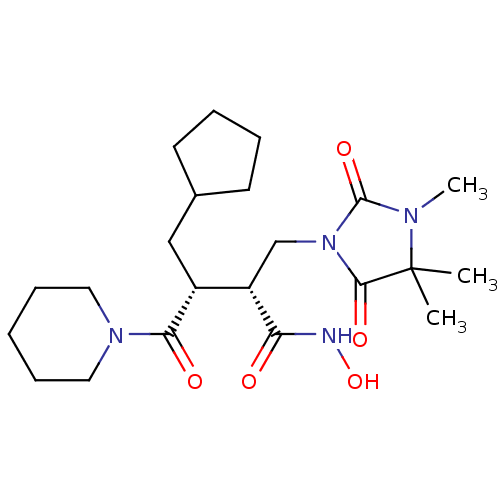
(Cipemastat | Trocade)Show SMILES CN1C(=O)N(C[C@@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | -52.4 | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... |
J Med Chem 52: 3523-38 (2009)
Article DOI: 10.1021/jm801394m
BindingDB Entry DOI: 10.7270/Q2B27SN3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180022
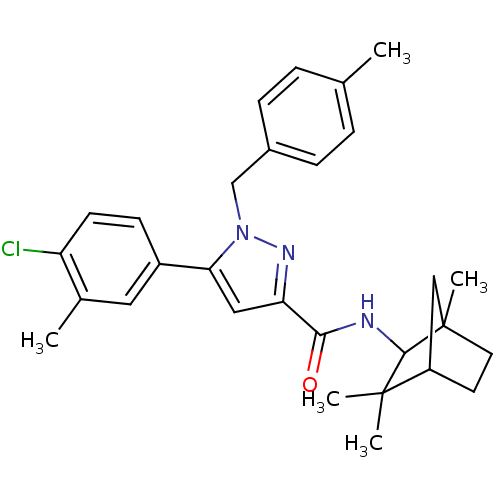
(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2C3(C)CCC(C3)C2(C)C)cc1 |THB:21:22:26.25:28| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB1 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 90: 526-36 (2015)
Article DOI: 10.1016/j.ejmech.2014.11.066
BindingDB Entry DOI: 10.7270/Q270833D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180022

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2C3(C)CCC(C3)C2(C)C)cc1 |THB:21:22:26.25:28| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082769
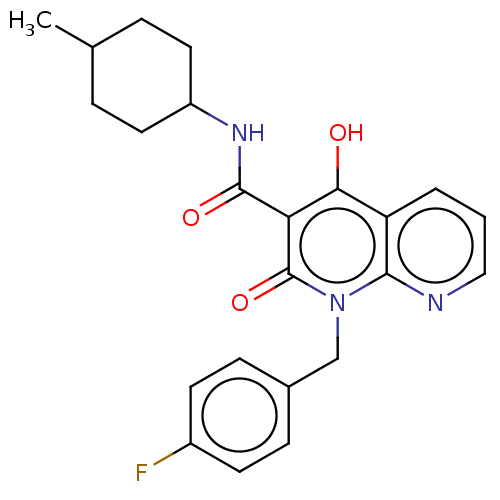
(CHEMBL3422790)Show SMILES CC1CCC(CC1)NC(=O)c1c(O)c2cccnc2n(Cc2ccc(F)cc2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.68,-5.39,;4.01,-6.15,;5.34,-5.38,;6.41,-5.99,;5.34,-3.84,;4,-3.07,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C23H24FN3O3/c1-14-4-10-17(11-5-14)26-22(29)19-20(28)18-3-2-12-25-21(18)27(23(19)30)13-15-6-8-16(24)9-7-15/h2-3,6-9,12,14,17,28H,4-5,10-11,13H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082777
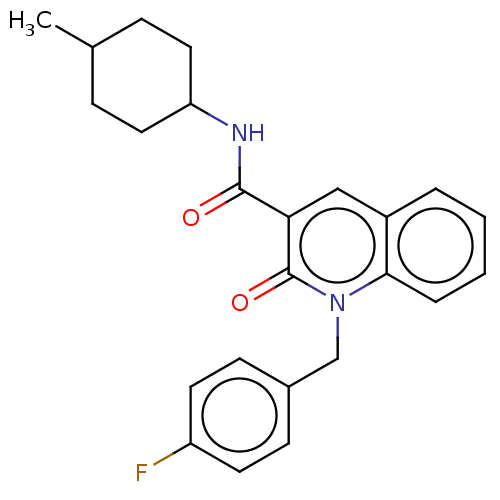
(CHEMBL3422784)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(Cc2ccc(F)cc2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.68,-5.39,;4.01,-6.15,;5.34,-5.38,;6.41,-5.99,;5.34,-3.84,;4,-3.07,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C24H25FN2O2/c1-16-6-12-20(13-7-16)26-23(28)21-14-18-4-2-3-5-22(18)27(24(21)29)15-17-8-10-19(25)11-9-17/h2-5,8-11,14,16,20H,6-7,12-13,15H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495633
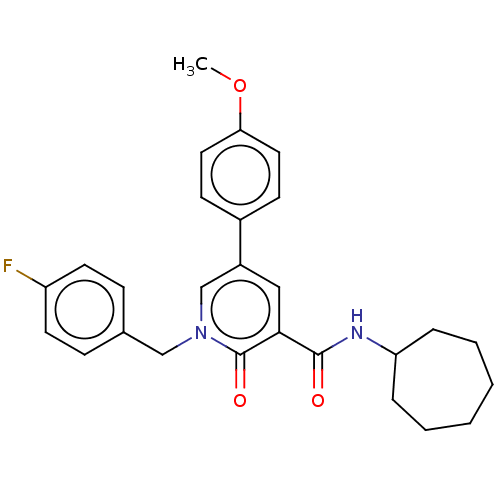
(CHEMBL3114181)Show SMILES COc1ccc(cc1)-c1cc(C(=O)NC2CCCCCC2)c(=O)n(Cc2ccc(F)cc2)c1 Show InChI InChI=1S/C27H29FN2O3/c1-33-24-14-10-20(11-15-24)21-16-25(26(31)29-23-6-4-2-3-5-7-23)27(32)30(18-21)17-19-8-12-22(28)13-9-19/h8-16,18,23H,2-7,17H2,1H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495626
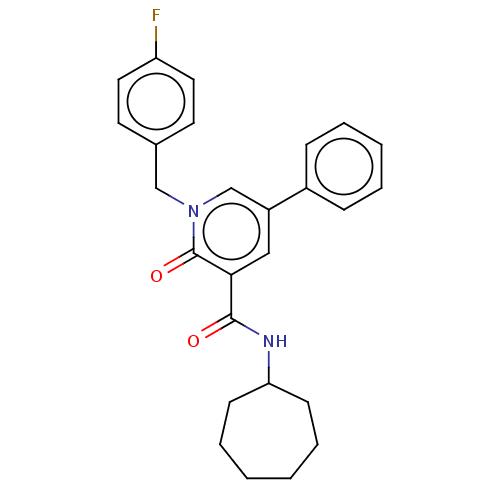
(CHEMBL3114182)Show SMILES Fc1ccc(Cn2cc(cc(C(=O)NC3CCCCCC3)c2=O)-c2ccccc2)cc1 Show InChI InChI=1S/C26H27FN2O2/c27-22-14-12-19(13-15-22)17-29-18-21(20-8-4-3-5-9-20)16-24(26(29)31)25(30)28-23-10-6-1-2-7-11-23/h3-5,8-9,12-16,18,23H,1-2,6-7,10-11,17H2,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.27 | -50.3 | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... |
J Med Chem 52: 3523-38 (2009)
Article DOI: 10.1021/jm801394m
BindingDB Entry DOI: 10.7270/Q2B27SN3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082768
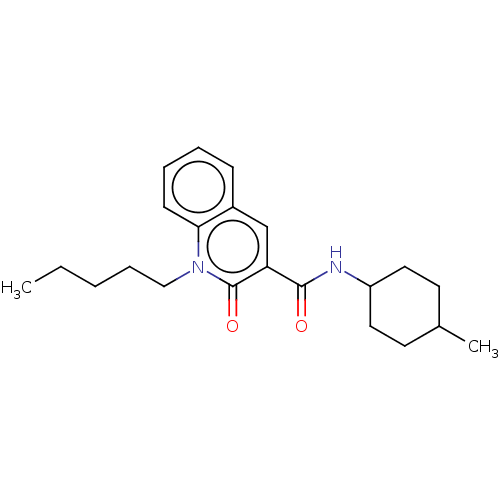
(CHEMBL3422788)Show SMILES CCCCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(4.01,-7.39,;4.01,-6.16,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C22H30N2O2/c1-3-4-7-14-24-20-9-6-5-8-17(20)15-19(22(24)26)21(25)23-18-12-10-16(2)11-13-18/h5-6,8-9,15-16,18H,3-4,7,10-14H2,1-2H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495628
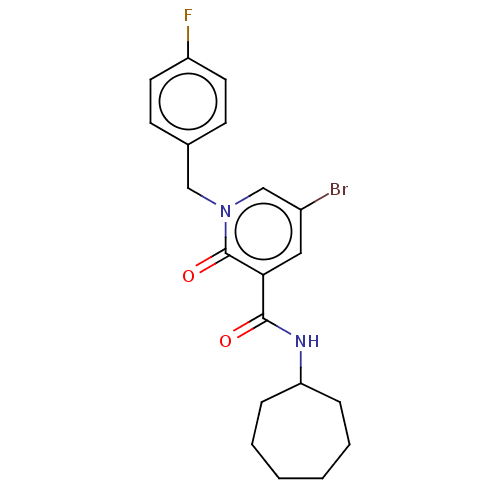
(CHEMBL3114178)Show SMILES Fc1ccc(Cn2cc(Br)cc(C(=O)NC3CCCCCC3)c2=O)cc1 Show InChI InChI=1S/C20H22BrFN2O2/c21-15-11-18(19(25)23-17-5-3-1-2-4-6-17)20(26)24(13-15)12-14-7-9-16(22)10-8-14/h7-11,13,17H,1-6,12H2,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50077661
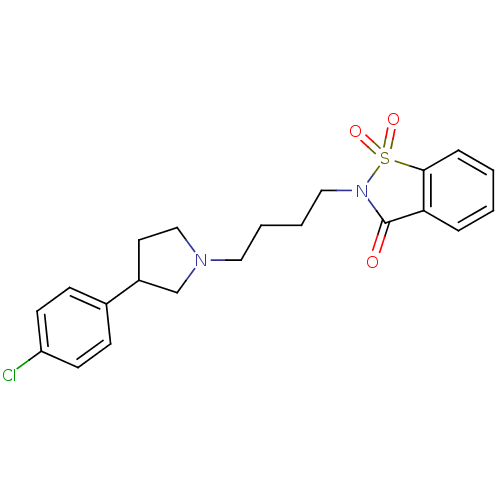
(2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...)Show SMILES Clc1ccc(cc1)C1CCN(CCCCN2C(=O)c3ccccc3S2(=O)=O)C1 Show InChI InChI=1S/C21H23ClN2O3S/c22-18-9-7-16(8-10-18)17-11-14-23(15-17)12-3-4-13-24-21(25)19-5-1-2-6-20(19)28(24,26)27/h1-2,5-10,17H,3-4,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50077658
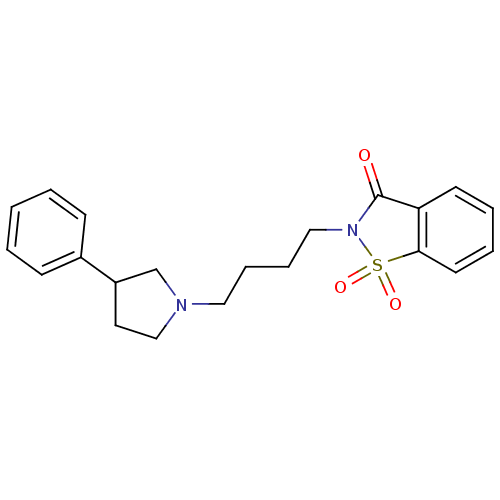
(1,1-Dioxo-2-[4-(3-phenyl-pyrrolidin-1-yl)-butyl]-1...)Show SMILES O=C1N(CCCCN2CCC(C2)c2ccccc2)S(=O)(=O)c2ccccc12 Show InChI InChI=1S/C21H24N2O3S/c24-21-19-10-4-5-11-20(19)27(25,26)23(21)14-7-6-13-22-15-12-18(16-22)17-8-2-1-3-9-17/h1-5,8-11,18H,6-7,12-16H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495632
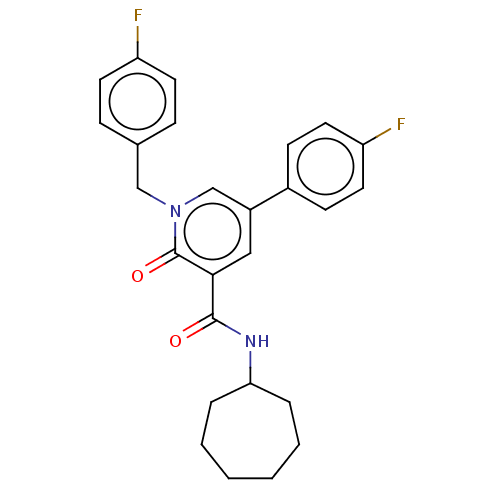
(CHEMBL3114341)Show SMILES Fc1ccc(Cn2cc(cc(C(=O)NC3CCCCCC3)c2=O)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C26H26F2N2O2/c27-21-11-7-18(8-12-21)16-30-17-20(19-9-13-22(28)14-10-19)15-24(26(30)32)25(31)29-23-5-3-1-2-4-6-23/h7-15,17,23H,1-6,16H2,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180036
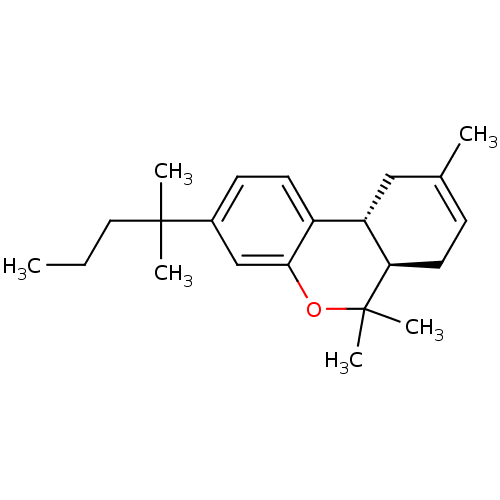
((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180036

((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180036

((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 90: 526-36 (2015)
Article DOI: 10.1016/j.ejmech.2014.11.066
BindingDB Entry DOI: 10.7270/Q270833D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495639
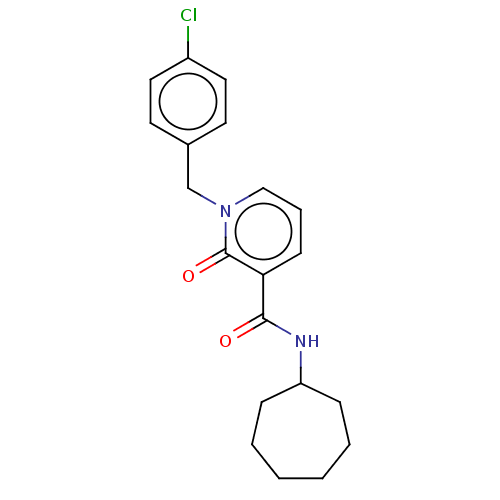
(CHEMBL3114350)Show InChI InChI=1S/C20H23ClN2O2/c21-16-11-9-15(10-12-16)14-23-13-5-8-18(20(23)25)19(24)22-17-6-3-1-2-4-7-17/h5,8-13,17H,1-4,6-7,14H2,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50077664

(2-{4-[3-(4-Fluoro-phenyl)-pyrrolidin-1-yl]-butyl}-...)Show SMILES Fc1ccc(cc1)C1CCN(CCCCN2C(=O)c3ccccc3S2(=O)=O)C1 Show InChI InChI=1S/C21H23FN2O3S/c22-18-9-7-16(8-10-18)17-11-14-23(15-17)12-3-4-13-24-21(25)19-5-1-2-6-20(19)28(24,26)27/h1-2,5-10,17H,3-4,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082776
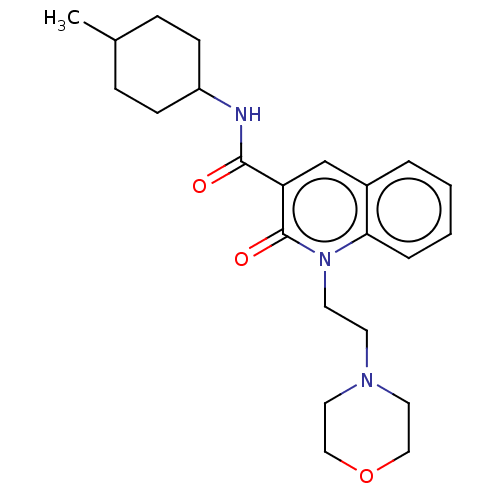
(CHEMBL3422785)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(CCN2CCOCC2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;4.01,-6.16,;4.01,-7.7,;2.67,-8.47,;1.34,-7.7,;1.34,-6.16,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C23H31N3O3/c1-17-6-8-19(9-7-17)24-22(27)20-16-18-4-2-3-5-21(18)26(23(20)28)11-10-25-12-14-29-15-13-25/h2-5,16-17,19H,6-15H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495636
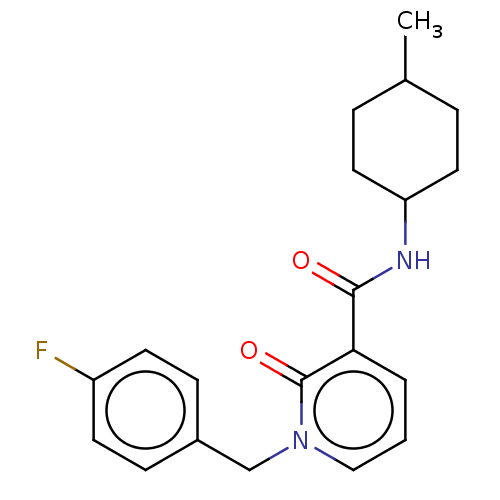
(CHEMBL3114172)Show SMILES CC1CCC(CC1)NC(=O)c1cccn(Cc2ccc(F)cc2)c1=O |(26.39,-29.35,;27.93,-29.35,;28.7,-30.69,;30.24,-30.69,;31.01,-29.35,;30.24,-28.02,;28.7,-28.02,;32.55,-29.35,;33.32,-28.02,;32.55,-26.69,;34.86,-28.02,;35.63,-26.69,;37.17,-26.69,;37.94,-28.02,;37.17,-29.35,;37.94,-30.69,;39.48,-30.69,;40.25,-29.35,;41.79,-29.35,;42.56,-30.69,;44.1,-30.69,;41.79,-32.03,;40.25,-32.03,;35.63,-29.35,;34.86,-30.69,)| Show InChI InChI=1S/C20H23FN2O2/c1-14-4-10-17(11-5-14)22-19(24)18-3-2-12-23(20(18)25)13-15-6-8-16(21)9-7-15/h2-3,6-9,12,14,17H,4-5,10-11,13H2,1H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495634
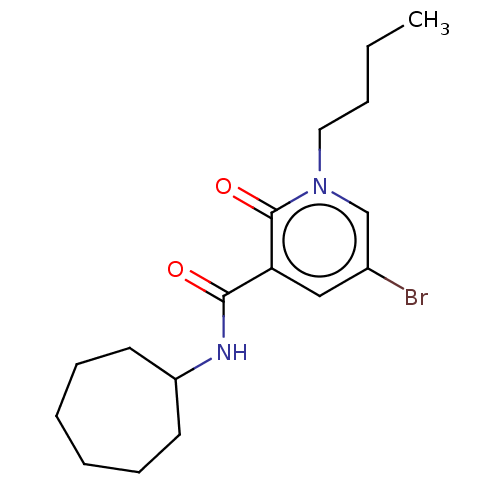
(CHEMBL3114180)Show InChI InChI=1S/C17H25BrN2O2/c1-2-3-10-20-12-13(18)11-15(17(20)22)16(21)19-14-8-6-4-5-7-9-14/h11-12,14H,2-10H2,1H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082772
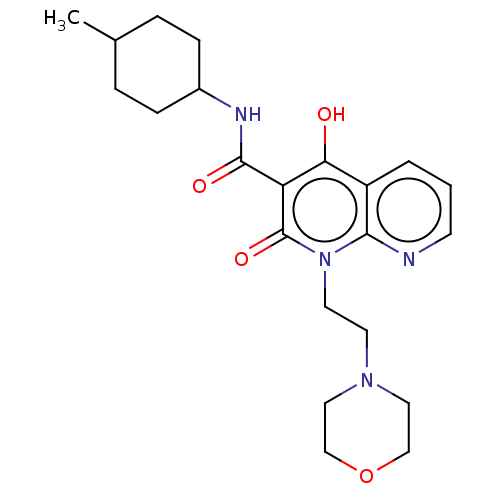
(CHEMBL3422791)Show SMILES CC1CCC(CC1)NC(=O)c1c(O)c2cccnc2n(CCN2CCOCC2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;4.01,-6.16,;4.01,-7.7,;2.67,-8.47,;1.34,-7.7,;1.34,-6.16,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C22H30N4O4/c1-15-4-6-16(7-5-15)24-21(28)18-19(27)17-3-2-8-23-20(17)26(22(18)29)10-9-25-11-13-30-14-12-25/h2-3,8,15-16,27H,4-7,9-14H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50381880
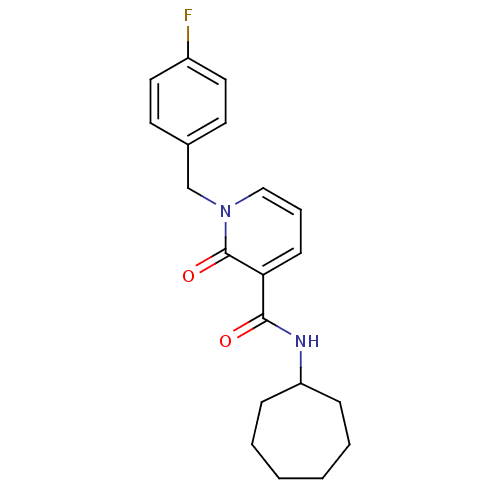
(CHEMBL2022698)Show InChI InChI=1S/C20H23FN2O2/c21-16-11-9-15(10-12-16)14-23-13-5-8-18(20(23)25)19(24)22-17-6-3-1-2-4-7-17/h5,8-13,17H,1-4,6-7,14H2,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495631
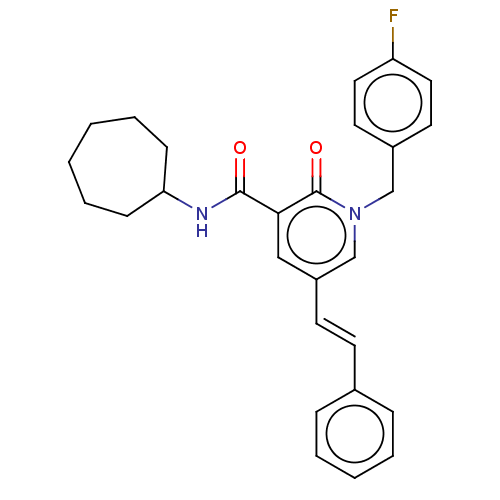
(CHEMBL3114343)Show SMILES Fc1ccc(Cn2cc(\C=C\c3ccccc3)cc(C(=O)NC3CCCCCC3)c2=O)cc1 Show InChI InChI=1S/C28H29FN2O2/c29-24-16-14-22(15-17-24)19-31-20-23(13-12-21-8-4-3-5-9-21)18-26(28(31)33)27(32)30-25-10-6-1-2-7-11-25/h3-5,8-9,12-18,20,25H,1-2,6-7,10-11,19H2,(H,30,32)/b13-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
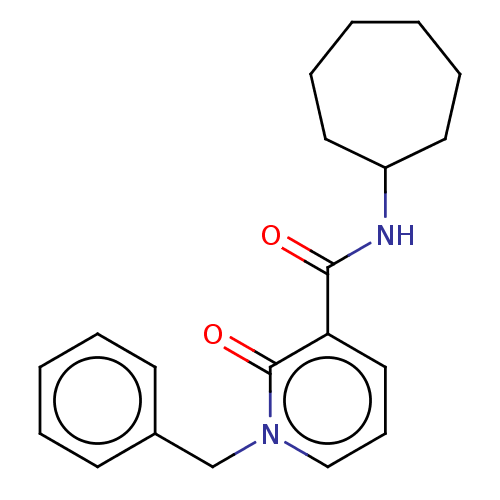
(Homo sapiens (Human)) | BDBM50495625
(CHEMBL3114349)Show InChI InChI=1S/C20H24N2O2/c23-19(21-17-11-6-1-2-7-12-17)18-13-8-14-22(20(18)24)15-16-9-4-3-5-10-16/h3-5,8-10,13-14,17H,1-2,6-7,11-12,15H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
| Assay Description
Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... |
J Med Chem 40: 2525-32 (1997)
Article DOI: 10.1021/jm960871c
BindingDB Entry DOI: 10.7270/Q2MW2FC7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50495628

(CHEMBL3114178)Show SMILES Fc1ccc(Cn2cc(Br)cc(C(=O)NC3CCCCCC3)c2=O)cc1 Show InChI InChI=1S/C20H22BrFN2O2/c21-15-11-18(19(25)23-17-5-3-1-2-4-6-17)20(26)24(13-15)12-14-7-9-16(22)10-8-14/h7-11,13,17H,1-6,12H2,(H,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB1 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077662
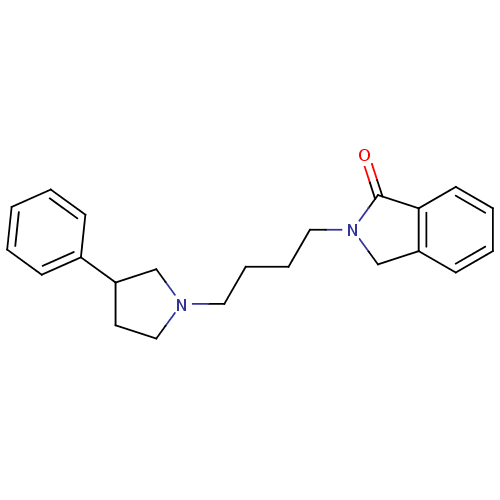
(2-[4-(3-Phenyl-pyrrolidin-1-yl)-butyl]-2,3-dihydro...)Show InChI InChI=1S/C22H26N2O/c25-22-21-11-5-4-10-20(21)17-24(22)14-7-6-13-23-15-12-19(16-23)18-8-2-1-3-9-18/h1-5,8-11,19H,6-7,12-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082771
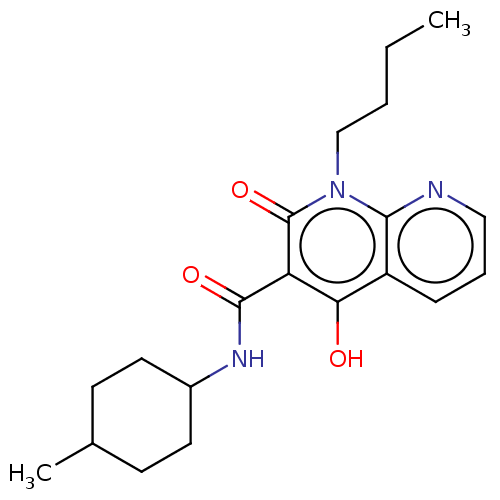
(CHEMBL3422792)Show SMILES CCCCn1c2ncccc2c(O)c(C(=O)NC2CCC(C)CC2)c1=O |(3.74,-6,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.33,2.77,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C20H27N3O3/c1-3-4-12-23-18-15(6-5-11-21-18)17(24)16(20(23)26)19(25)22-14-9-7-13(2)8-10-14/h5-6,11,13-14,24H,3-4,7-10,12H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50062506
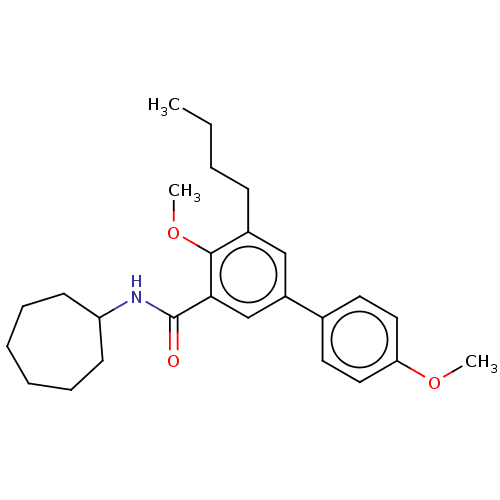
(CHEMBL3397520)Show SMILES CCCCc1cc(cc(C(=O)NC2CCCCCC2)c1OC)-c1ccc(OC)cc1 Show InChI InChI=1S/C26H35NO3/c1-4-5-10-20-17-21(19-13-15-23(29-2)16-14-19)18-24(25(20)30-3)26(28)27-22-11-8-6-7-9-12-22/h13-18,22H,4-12H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay |
Eur J Med Chem 90: 526-36 (2015)
Article DOI: 10.1016/j.ejmech.2014.11.066
BindingDB Entry DOI: 10.7270/Q270833D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50077662

(2-[4-(3-Phenyl-pyrrolidin-1-yl)-butyl]-2,3-dihydro...)Show InChI InChI=1S/C22H26N2O/c25-22-21-11-5-4-10-20(21)17-24(22)14-7-6-13-23-15-12-19(16-23)18-8-2-1-3-9-18/h1-5,8-11,19H,6-7,12-17H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077660
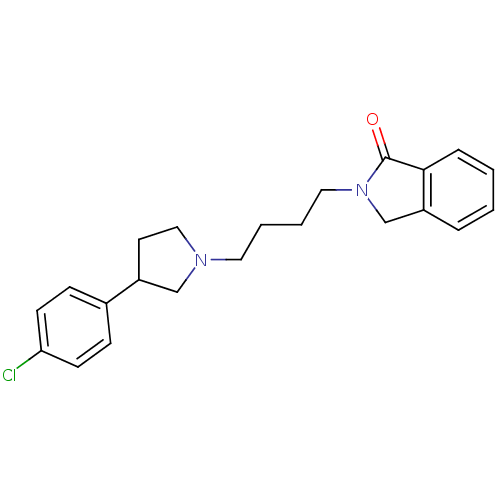
(2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...)Show InChI InChI=1S/C22H25ClN2O/c23-20-9-7-17(8-10-20)18-11-14-24(15-18)12-3-4-13-25-16-19-5-1-2-6-21(19)22(25)26/h1-2,5-10,18H,3-4,11-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50495630
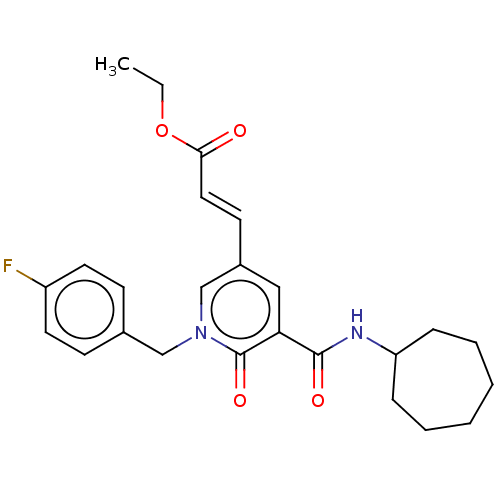
(CHEMBL3114344)Show SMILES CCOC(=O)\C=C\c1cc(C(=O)NC2CCCCCC2)c(=O)n(Cc2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN2O4/c1-2-32-23(29)14-11-19-15-22(24(30)27-21-7-5-3-4-6-8-21)25(31)28(17-19)16-18-9-12-20(26)13-10-18/h9-15,17,21H,2-8,16H2,1H3,(H,27,30)/b14-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50077663

(CHEMBL280534 | {3-[3-(4-Chloro-phenyl)-pyrrolidin-...)Show SMILES Clc1ccc(cc1)C1CCN(Cc2cccc(c2)C(=O)N2CCCCC2)C1 Show InChI InChI=1S/C23H27ClN2O/c24-22-9-7-19(8-10-22)21-11-14-25(17-21)16-18-5-4-6-20(15-18)23(27)26-12-2-1-3-13-26/h4-10,15,21H,1-3,11-14,16-17H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077658

(1,1-Dioxo-2-[4-(3-phenyl-pyrrolidin-1-yl)-butyl]-1...)Show SMILES O=C1N(CCCCN2CCC(C2)c2ccccc2)S(=O)(=O)c2ccccc12 Show InChI InChI=1S/C21H24N2O3S/c24-21-19-10-4-5-11-20(19)27(25,26)23(21)14-7-6-13-22-15-12-18(16-22)17-8-2-1-3-9-17/h1-5,8-11,18H,6-7,12-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082777

(CHEMBL3422784)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(Cc2ccc(F)cc2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.68,-5.39,;4.01,-6.15,;5.34,-5.38,;6.41,-5.99,;5.34,-3.84,;4,-3.07,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C24H25FN2O2/c1-16-6-12-20(13-7-16)26-23(28)21-14-18-4-2-3-5-22(18)27(24(21)29)15-17-8-10-19(25)11-9-17/h2-5,8-11,14,16,20H,6-7,12-13,15H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082774
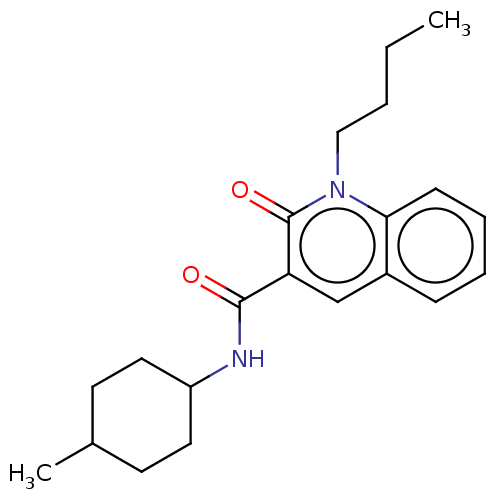
(CHEMBL3422787)Show SMILES CCCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(3.74,-6,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C21H28N2O2/c1-3-4-13-23-19-8-6-5-7-16(19)14-18(21(23)25)20(24)22-17-11-9-15(2)10-12-17/h5-8,14-15,17H,3-4,9-13H2,1-2H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
| Assay Description
Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... |
J Med Chem 40: 2525-32 (1997)
Article DOI: 10.1021/jm960871c
BindingDB Entry DOI: 10.7270/Q2MW2FC7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50062508
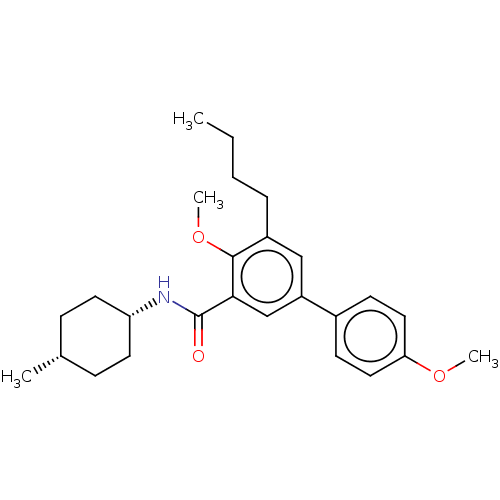
(CHEMBL3397522)Show SMILES CCCCc1cc(cc(C(=O)N[C@@H]2CC[C@H](C)CC2)c1OC)-c1ccc(OC)cc1 |r,wD:12.11,15.15,(-1.6,8.32,;-2.67,7.7,;-2.67,6.16,;-4,5.39,;-4.01,3.85,;-2.67,3.08,;-2.67,1.54,;-4,.77,;-5.34,1.54,;-6.67,.76,;-7.74,1.37,;-6.66,-.78,;-7.99,-1.56,;-7.99,-3.1,;-9.32,-3.87,;-10.66,-3.1,;-11.72,-3.72,;-10.66,-1.56,;-9.33,-.79,;-5.34,3.08,;-6.68,3.84,;-7.74,3.22,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;2.67,-1.54,;2.66,-2.78,;,-1.54,;-1.33,-.77,)| Show InChI InChI=1S/C26H35NO3/c1-5-6-7-20-16-21(19-10-14-23(29-3)15-11-19)17-24(25(20)30-4)26(28)27-22-12-8-18(2)9-13-22/h10-11,14-18,22H,5-9,12-13H2,1-4H3,(H,27,28)/t18-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB2 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 90: 526-36 (2015)
Article DOI: 10.1016/j.ejmech.2014.11.066
BindingDB Entry DOI: 10.7270/Q270833D |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM13122
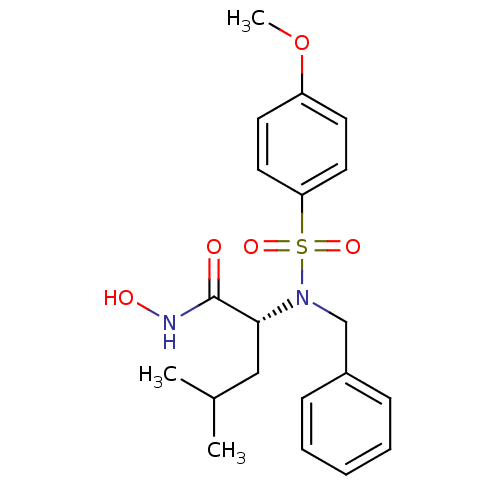
((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](CC(C)C)C(=O)NO |r| Show InChI InChI=1S/C20H26N2O5S/c1-15(2)13-19(20(23)21-24)22(14-16-7-5-4-6-8-16)28(25,26)18-11-9-17(27-3)10-12-18/h4-12,15,19,24H,13-14H2,1-3H3,(H,21,23)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
| Assay Description
Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... |
J Med Chem 40: 2525-32 (1997)
Article DOI: 10.1021/jm960871c
BindingDB Entry DOI: 10.7270/Q2MW2FC7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50495634

(CHEMBL3114180)Show InChI InChI=1S/C17H25BrN2O2/c1-2-3-10-20-12-13(18)11-15(17(20)22)16(21)19-14-8-6-4-5-7-9-14/h11-12,14H,2-10H2,1H3,(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB1 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50495632

(CHEMBL3114341)Show SMILES Fc1ccc(Cn2cc(cc(C(=O)NC3CCCCCC3)c2=O)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C26H26F2N2O2/c27-21-11-7-18(8-12-21)16-30-17-20(19-9-13-22(28)14-10-19)15-24(26(30)32)25(31)29-23-5-3-1-2-4-6-23/h7-15,17,23H,1-6,16H2,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB1 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50495631

(CHEMBL3114343)Show SMILES Fc1ccc(Cn2cc(\C=C\c3ccccc3)cc(C(=O)NC3CCCCCC3)c2=O)cc1 Show InChI InChI=1S/C28H29FN2O2/c29-24-16-14-22(15-17-24)19-31-20-23(13-12-21-8-4-3-5-9-21)18-26(28(31)33)27(32)30-25-10-6-1-2-7-11-25/h3-5,8-9,12-18,20,25H,1-2,6-7,10-11,19H2,(H,30,32)/b13-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB1 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077659
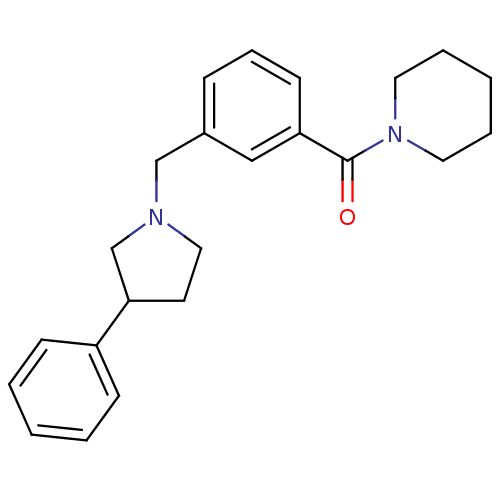
(CHEMBL281214 | [3-(3-Phenyl-pyrrolidin-1-ylmethyl)...)Show InChI InChI=1S/C23H28N2O/c26-23(25-13-5-2-6-14-25)21-11-7-8-19(16-21)17-24-15-12-22(18-24)20-9-3-1-4-10-20/h1,3-4,7-11,16,22H,2,5-6,12-15,17-18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 |
Bioorg Med Chem Lett 9: 1379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2862FMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082775
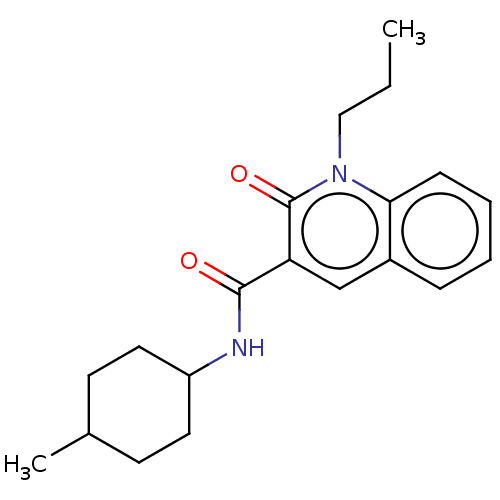
(CHEMBL3422786)Show SMILES CCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(2.67,-5.08,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C20H26N2O2/c1-3-12-22-18-7-5-4-6-15(18)13-17(20(22)24)19(23)21-16-10-8-14(2)9-11-16/h4-7,13-14,16H,3,8-12H2,1-2H3,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50495630

(CHEMBL3114344)Show SMILES CCOC(=O)\C=C\c1cc(C(=O)NC2CCCCCC2)c(=O)n(Cc2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN2O4/c1-2-32-23(29)14-11-19-15-22(24(30)27-21-7-5-3-4-6-8-21)25(31)28(17-19)16-18-9-12-20(26)13-10-18/h9-15,17,21H,2-8,16H2,1H3,(H,27,30)/b14-11+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human recombinant CB1 receptor expressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 74: 524-32 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.070
BindingDB Entry DOI: 10.7270/Q21V5HXR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM13133
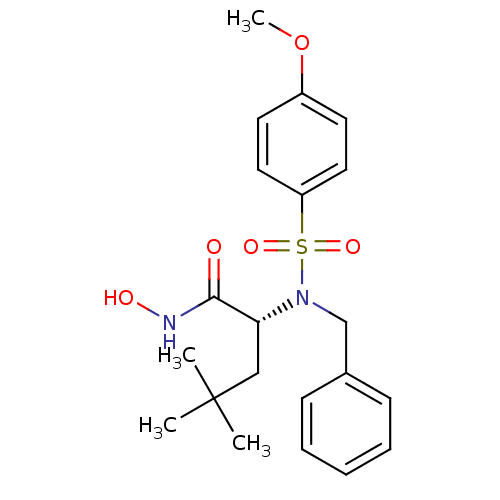
((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](CC(C)(C)C)C(=O)NO |r| Show InChI InChI=1S/C21H28N2O5S/c1-21(2,3)14-19(20(24)22-25)23(15-16-8-6-5-7-9-16)29(26,27)18-12-10-17(28-4)11-13-18/h5-13,19,25H,14-15H2,1-4H3,(H,22,24)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
| Assay Description
Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... |
J Med Chem 40: 2525-32 (1997)
Article DOI: 10.1021/jm960871c
BindingDB Entry DOI: 10.7270/Q2MW2FC7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data