 Found 1973 hits with Last Name = 'park' and Initial = 'wk'
Found 1973 hits with Last Name = 'park' and Initial = 'wk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50298360

((R)-1-(7-ethyl-1H-furo[2,3-g]indazol-1-yl)propan-2...)Show InChI InChI=1S/C14H17N3O/c1-3-11-6-12-13(18-11)5-4-10-7-16-17(14(10)12)8-9(2)15/h4-7,9H,3,8,15H2,1-2H3/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C receptor |
Bioorg Med Chem 17: 4559-68 (2009)
Article DOI: 10.1016/j.bmc.2009.05.003
BindingDB Entry DOI: 10.7270/Q20V8CV1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50257963

(2-(3-chlorobenzyloxy)-6-(piperazin-1-yl)pyrazine |...)Show InChI InChI=1S/C15H17ClN4O/c16-13-3-1-2-12(8-13)11-21-15-10-18-9-14(19-15)20-6-4-17-5-7-20/h1-3,8-10,17H,4-7,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C receptor |
Bioorg Med Chem 17: 4559-68 (2009)
Article DOI: 10.1016/j.bmc.2009.05.003
BindingDB Entry DOI: 10.7270/Q20V8CV1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50228649
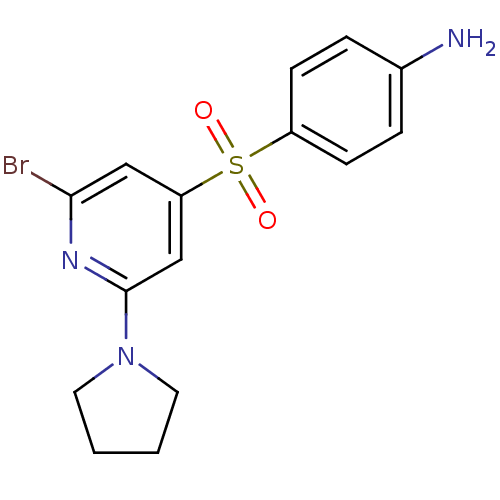
(4-(2-Bromo-6-pyrrolidin-1-yl-pyridine-4-sulfonyl)-...)Show InChI InChI=1S/C15H16BrN3O2S/c16-14-9-13(10-15(18-14)19-7-1-2-8-19)22(20,21)12-5-3-11(17)4-6-12/h3-6,9-10H,1-2,7-8,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50298361

((R)-1-(6-chloro-5-fluoro-1H-indol-1-yl)propan-2-am...)Show InChI InChI=1S/C11H12ClFN2/c1-7(14)6-15-3-2-8-4-10(13)9(12)5-11(8)15/h2-5,7H,6,14H2,1H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C receptor |
Bioorg Med Chem 17: 4559-68 (2009)
Article DOI: 10.1016/j.bmc.2009.05.003
BindingDB Entry DOI: 10.7270/Q20V8CV1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
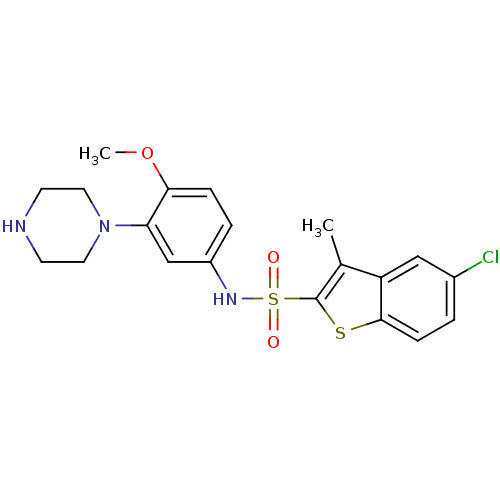
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130264
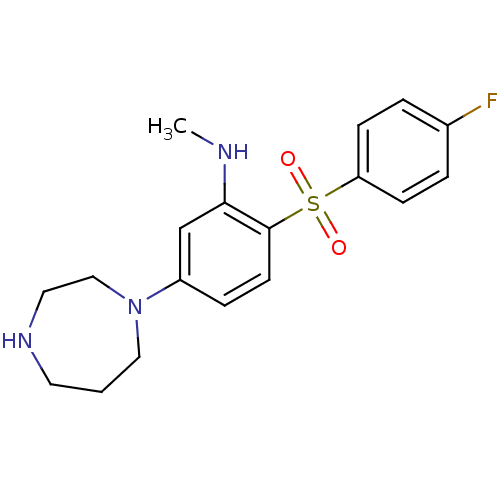
(5-(1,4-diazepan-1-yl)-2-(4-fluorophenylsulfonyl)-N...)Show InChI InChI=1S/C18H22FN3O2S/c1-20-17-13-15(22-11-2-9-21-10-12-22)5-8-18(17)25(23,24)16-6-3-14(19)4-7-16/h3-8,13,20-21H,2,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM34141
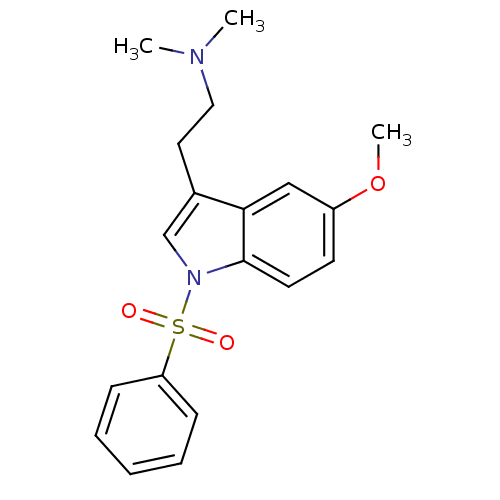
(CHEMBL76237 | MS-245)Show SMILES COc1ccc2n(cc(CCN(C)C)c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H22N2O3S/c1-20(2)12-11-15-14-21(19-10-9-16(24-3)13-18(15)19)25(22,23)17-7-5-4-6-8-17/h4-10,13-14H,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50298362
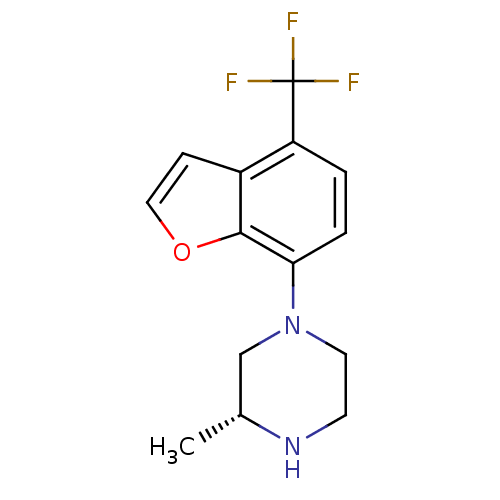
((R)-3-methyl-1-(4-(trifluoromethyl)-2,3-dihydroben...)Show SMILES C[C@@H]1CN(CCN1)c1ccc(c2ccoc12)C(F)(F)F |r| Show InChI InChI=1S/C14H15F3N2O/c1-9-8-19(6-5-18-9)12-3-2-11(14(15,16)17)10-4-7-20-13(10)12/h2-4,7,9,18H,5-6,8H2,1H3/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C receptor |
Bioorg Med Chem 17: 4559-68 (2009)
Article DOI: 10.1016/j.bmc.2009.05.003
BindingDB Entry DOI: 10.7270/Q20V8CV1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130268
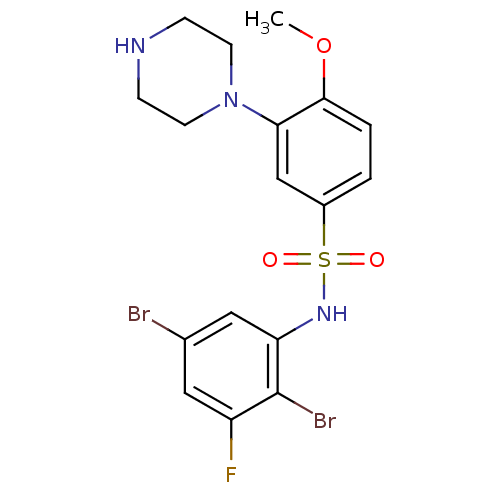
(CHEMBL329383 | N-(2,5-Dibromo-3-fluoro-phenyl)-4-m...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)Nc1cc(Br)cc(F)c1Br Show InChI InChI=1S/C17H18Br2FN3O3S/c1-26-16-3-2-12(10-15(16)23-6-4-21-5-7-23)27(24,25)22-14-9-11(18)8-13(20)17(14)19/h2-3,8-10,21-22H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50091831

((R)-8,9-Dichloro-2,3,4,4a-tetrahydro-1H,6H-pyrazin...)Show InChI InChI=1S/C11H11Cl2N3O/c12-6-3-8-9(4-7(6)13)16-2-1-14-5-10(16)11(17)15-8/h3-4,10,14H,1-2,5H2,(H,15,17)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C receptor |
Bioorg Med Chem 17: 4559-68 (2009)
Article DOI: 10.1016/j.bmc.2009.05.003
BindingDB Entry DOI: 10.7270/Q20V8CV1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50179073
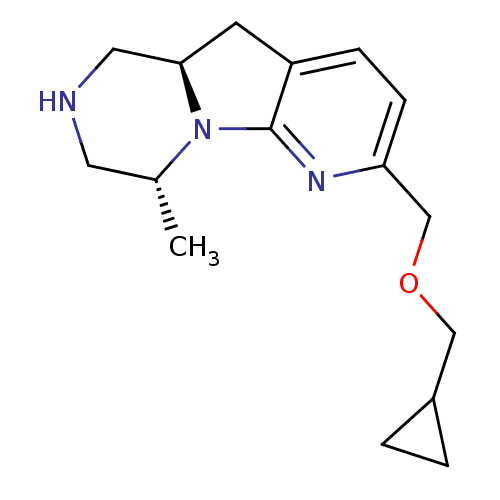
((4R,9aR)-6-Cyclopropylmethoxymethyl-4-methyl-1,2,3...)Show InChI InChI=1S/C16H23N3O/c1-11-7-17-8-15-6-13-4-5-14(18-16(13)19(11)15)10-20-9-12-2-3-12/h4-5,11-12,15,17H,2-3,6-10H2,1H3/t11-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C receptor |
Bioorg Med Chem 17: 4559-68 (2009)
Article DOI: 10.1016/j.bmc.2009.05.003
BindingDB Entry DOI: 10.7270/Q20V8CV1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50298359

((8aR,12aS)-2-(2,6-difluorophenyl)-6,7,8a,9,10,11,1...)Show SMILES Fc1cccc(F)c1-c1cc2[C@H]3CNCC[C@H]3N3CCCSc(c1)c23 |r| Show InChI InChI=1S/C20H20F2N2S/c21-15-3-1-4-16(22)19(15)12-9-13-14-11-23-6-5-17(14)24-7-2-8-25-18(10-12)20(13)24/h1,3-4,9-10,14,17,23H,2,5-8,11H2/t14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C receptor |
Bioorg Med Chem 17: 4559-68 (2009)
Article DOI: 10.1016/j.bmc.2009.05.003
BindingDB Entry DOI: 10.7270/Q20V8CV1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50069447
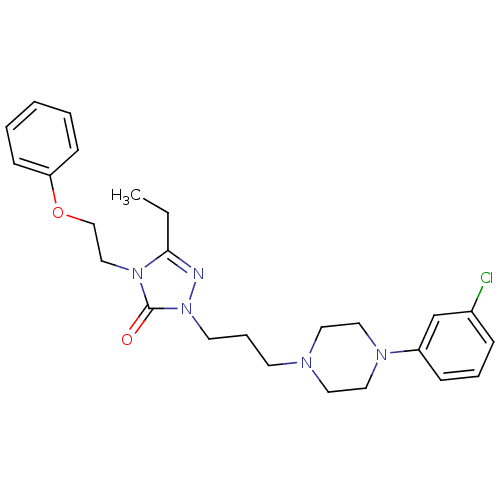
(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1B (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50228656

(5,7-dichloro-3-(4-(diethylamino)phenyl)-3-methylqu...)Show SMILES CCN(CC)c1ccc(cc1)C1(C)C(=O)Nc2cc(Cl)cc(Cl)c2C1=O |w:11.12| Show InChI InChI=1S/C20H20Cl2N2O2/c1-4-24(5-2)14-8-6-12(7-9-14)20(3)18(25)17-15(22)10-13(21)11-16(17)23-19(20)26/h6-11H,4-5H2,1-3H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1A (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50228646

(5,7-dichloro-3-(4-hydroxyphenyl)-3-methylquinoline...)Show SMILES CC1(C(=O)Nc2cc(Cl)cc(Cl)c2C1=O)c1ccc(O)cc1 |w:1.0| Show InChI InChI=1S/C16H11Cl2NO3/c1-16(8-2-4-10(20)5-3-8)14(21)13-11(18)6-9(17)7-12(13)19-15(16)22/h2-7,20H,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1A (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058162
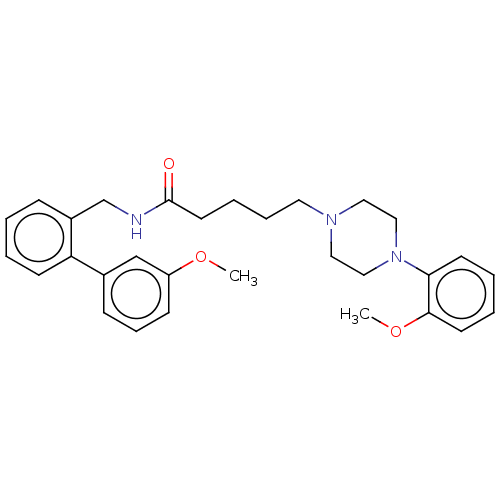
(CHEMBL3326986)Show SMILES COc1cccc(c1)-c1ccccc1CNC(=O)CCCCN1CCN(CC1)c1ccccc1OC Show InChI InChI=1S/C30H37N3O3/c1-35-26-12-9-11-24(22-26)27-13-4-3-10-25(27)23-31-30(34)16-7-8-17-32-18-20-33(21-19-32)28-14-5-6-15-29(28)36-2/h3-6,9-15,22H,7-8,16-21,23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058156
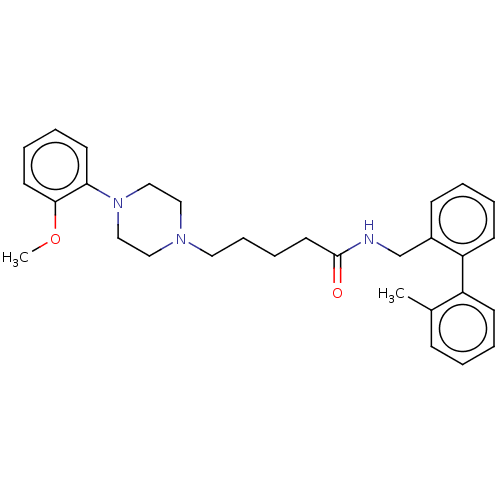
(CHEMBL3326989)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2C)CC1 Show InChI InChI=1S/C30H37N3O2/c1-24-11-3-5-13-26(24)27-14-6-4-12-25(27)23-31-30(34)17-9-10-18-32-19-21-33(22-20-32)28-15-7-8-16-29(28)35-2/h3-8,11-16H,9-10,17-23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058211

(CHEMBL3326984)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-11-5-4-10-27(28)33-20-18-32(19-21-33)17-7-6-12-29(34)31-22-24-8-2-3-9-26(24)23-13-15-25(30)16-14-23/h2-5,8-11,13-16H,6-7,12,17-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50228671
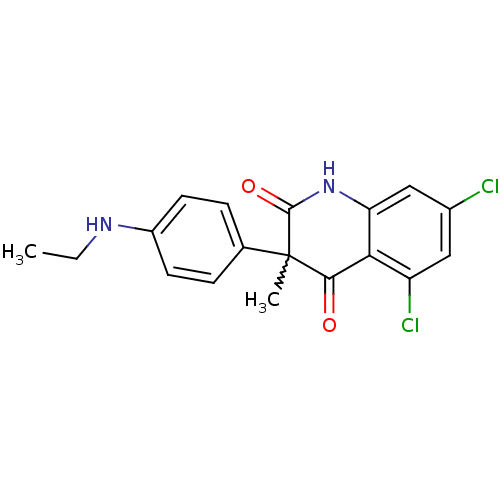
(5,7-dichloro-3-(4-(ethylamino)phenyl)-3-methylquin...)Show SMILES CCNc1ccc(cc1)C1(C)C(=O)Nc2cc(Cl)cc(Cl)c2C1=O |w:9.10| Show InChI InChI=1S/C18H16Cl2N2O2/c1-3-21-12-6-4-10(5-7-12)18(2)16(23)15-13(20)8-11(19)9-14(15)22-17(18)24/h4-9,21H,3H2,1-2H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50228648

(5,7-dichloro-3-(3-hydroxyphenyl)-3-methylquinoline...)Show SMILES CC1(C(=O)Nc2cc(Cl)cc(Cl)c2C1=O)c1cccc(O)c1 |w:1.0| Show InChI InChI=1S/C16H11Cl2NO3/c1-16(8-3-2-4-10(20)5-8)14(21)13-11(18)6-9(17)7-12(13)19-15(16)22/h2-7,20H,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058209

(CHEMBL3326985)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2OC)CC1 Show InChI InChI=1S/C30H37N3O3/c1-35-28-15-7-5-13-26(28)25-12-4-3-11-24(25)23-31-30(34)17-9-10-18-32-19-21-33(22-20-32)27-14-6-8-16-29(27)36-2/h3-8,11-16H,9-10,17-23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058157
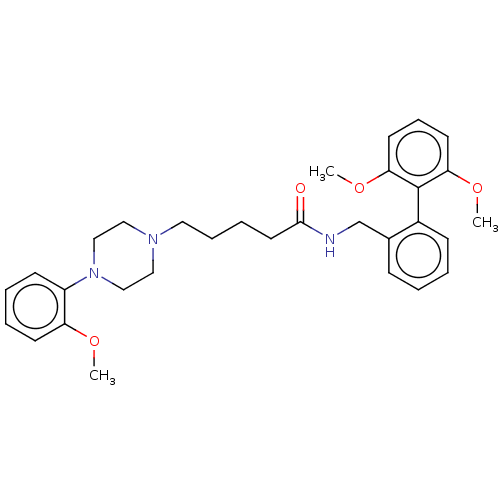
(CHEMBL3326988)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2c(OC)cccc2OC)CC1 |(26.8,-10.21,;25.47,-10.98,;25.48,-12.52,;26.82,-13.29,;26.82,-14.83,;25.49,-15.6,;24.15,-14.83,;24.16,-13.3,;22.83,-12.53,;21.49,-13.3,;20.16,-12.53,;20.16,-10.98,;18.82,-10.22,;17.49,-11,;16.16,-10.23,;14.83,-11.01,;13.49,-10.25,;13.48,-8.71,;12.16,-11.02,;10.82,-10.25,;9.49,-11.03,;9.5,-12.58,;8.16,-13.35,;6.82,-12.58,;6.83,-11.04,;8.16,-10.27,;8.15,-8.73,;6.81,-7.97,;5.47,-8.76,;5.49,-10.31,;6.8,-6.43,;8.14,-5.65,;9.48,-6.42,;9.48,-7.96,;10.81,-8.73,;12.16,-7.95,;21.49,-10.21,;22.82,-10.99,)| Show InChI InChI=1S/C31H39N3O4/c1-36-27-14-7-6-13-26(27)34-21-19-33(20-22-34)18-9-8-17-30(35)32-23-24-11-4-5-12-25(24)31-28(37-2)15-10-16-29(31)38-3/h4-7,10-16H,8-9,17-23H2,1-3H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058216

(CHEMBL3326980)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2cccc(F)c2)CC1 Show InChI InChI=1S/C29H34FN3O2/c1-35-28-14-5-4-13-27(28)33-19-17-32(18-20-33)16-7-6-15-29(34)31-22-24-9-2-3-12-26(24)23-10-8-11-25(30)21-23/h2-5,8-14,21H,6-7,15-20,22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058215

(CHEMBL3326981)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccc(F)cc2)CC1 Show InChI InChI=1S/C29H34FN3O2/c1-35-28-11-5-4-10-27(28)33-20-18-32(19-21-33)17-7-6-12-29(34)31-22-24-8-2-3-9-26(24)23-13-15-25(30)16-14-23/h2-5,8-11,13-16H,6-7,12,17-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058217

(CHEMBL3326979)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2F)CC1 Show InChI InChI=1S/C29H34FN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058219
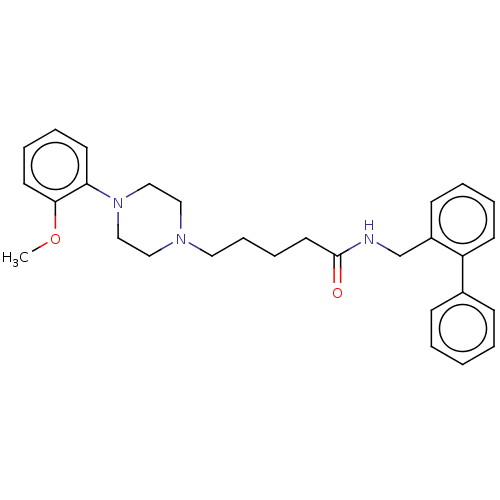
(CHEMBL3325464)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2)CC1 Show InChI InChI=1S/C29H35N3O2/c1-34-28-16-8-7-15-27(28)32-21-19-31(20-22-32)18-10-9-17-29(33)30-23-25-13-5-6-14-26(25)24-11-3-2-4-12-24/h2-8,11-16H,9-10,17-23H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058218

(CHEMBL3326978)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2)CC1 Show InChI InChI=1S/C30H37N3O2/c1-35-29-17-10-9-16-28(29)33-22-20-32(21-23-33)19-11-3-6-18-30(34)31-24-26-14-7-8-15-27(26)25-12-4-2-5-13-25/h2,4-5,7-10,12-17H,3,6,11,18-24H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058159

(CHEMBL3326987)Show SMILES COc1ccc(cc1)-c1ccccc1CNC(=O)CCCCN1CCN(CC1)c1ccccc1OC Show InChI InChI=1S/C30H37N3O3/c1-35-26-16-14-24(15-17-26)27-10-4-3-9-25(27)23-31-30(34)13-7-8-18-32-19-21-33(22-20-32)28-11-5-6-12-29(28)36-2/h3-6,9-12,14-17H,7-8,13,18-23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058070
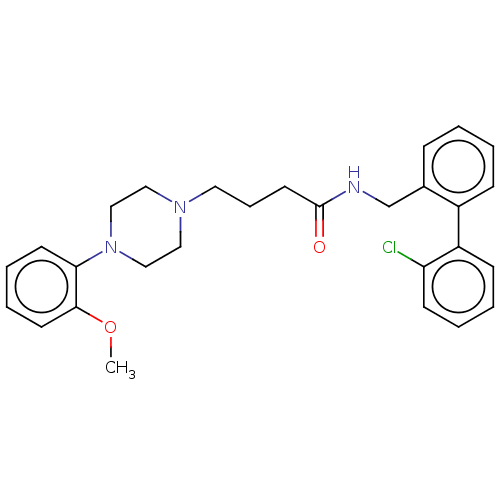
(CHEMBL3326992)Show SMILES COc1ccccc1N1CCN(CCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C28H32ClN3O2/c1-34-27-14-7-6-13-26(27)32-19-17-31(18-20-32)16-8-15-28(33)30-21-22-9-2-3-10-23(22)24-11-4-5-12-25(24)29/h2-7,9-14H,8,15-21H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058151

(CHEMBL3326990)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2cccc(C)c2)CC1 Show InChI InChI=1S/C30H37N3O2/c1-24-10-9-12-25(22-24)27-13-4-3-11-26(27)23-31-30(34)16-7-8-17-32-18-20-33(21-19-32)28-14-5-6-15-29(28)35-2/h3-6,9-15,22H,7-8,16-21,23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50228645
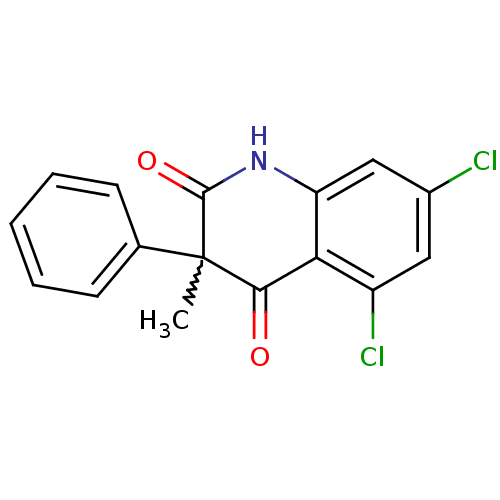
((+/-)-5,7-dichloro-3-phenyl-3-methylquinoline-2,4-...)Show SMILES CC1(C(=O)Nc2cc(Cl)cc(Cl)c2C1=O)c1ccccc1 |w:1.0| Show InChI InChI=1S/C16H11Cl2NO2/c1-16(9-5-3-2-4-6-9)14(20)13-11(18)7-10(17)8-12(13)19-15(16)21/h2-8H,1H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50090528
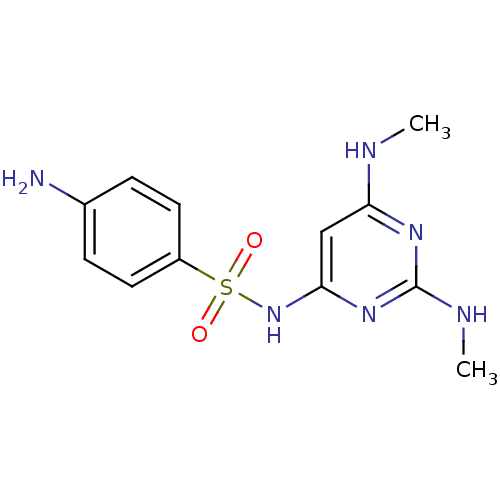
(4-Amino-N-(2,6-bis-dimethylamino-pyrimidin-4-yl)-b...)Show InChI InChI=1S/C12H16N6O2S/c1-14-10-7-11(17-12(15-2)16-10)18-21(19,20)9-5-3-8(13)4-6-9/h3-7H,13H2,1-2H3,(H3,14,15,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058282
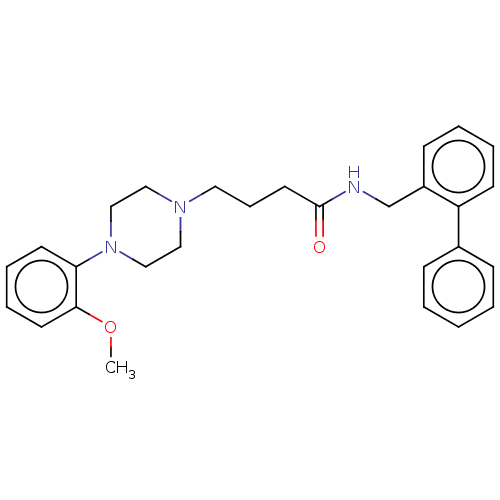
(CHEMBL3326977)Show SMILES COc1ccccc1N1CCN(CCCC(=O)NCc2ccccc2-c2ccccc2)CC1 Show InChI InChI=1S/C28H33N3O2/c1-33-27-15-8-7-14-26(27)31-20-18-30(19-21-31)17-9-16-28(32)29-22-24-12-5-6-13-25(24)23-10-3-2-4-11-23/h2-8,10-15H,9,16-22H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058071

(CHEMBL3326991)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccc(C)cc2)CC1 Show InChI InChI=1S/C30H37N3O2/c1-24-14-16-25(17-15-24)27-10-4-3-9-26(27)23-31-30(34)13-7-8-18-32-19-21-33(22-20-32)28-11-5-6-12-29(28)35-2/h3-6,9-12,14-17H,7-8,13,18-23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50228666
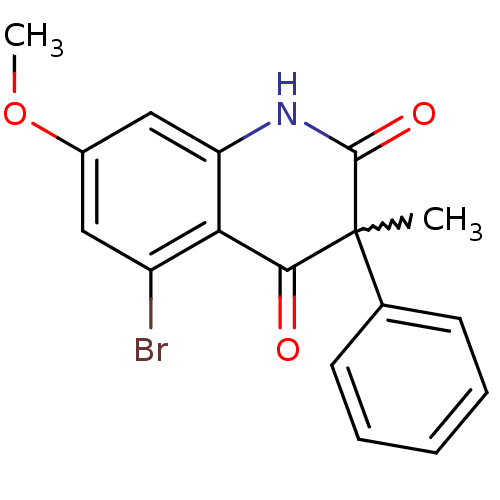
(5-bromo-7-methoxy-3-methyl-3-phenylquinoline-2,4(1...)Show SMILES COc1cc(Br)c2C(=O)C(C)(C(=O)Nc2c1)c1ccccc1 |w:9.9| Show InChI InChI=1S/C17H14BrNO3/c1-17(10-6-4-3-5-7-10)15(20)14-12(18)8-11(22-2)9-13(14)19-16(17)21/h3-9H,1-2H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 73.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human cloned 5HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 738-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.045
BindingDB Entry DOI: 10.7270/Q2XK8F99 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data