 Found 57 hits with Last Name = 'parmar' and Initial = 'b'
Found 57 hits with Last Name = 'parmar' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
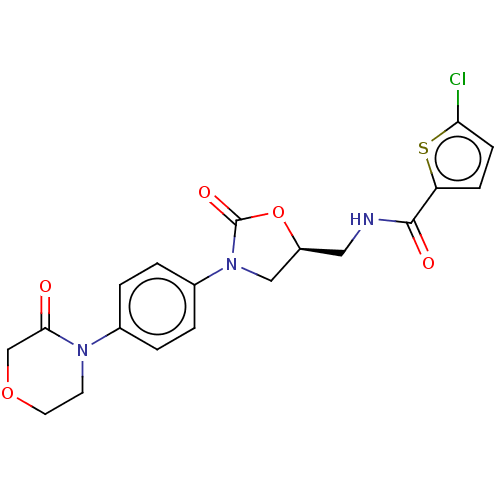
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392595
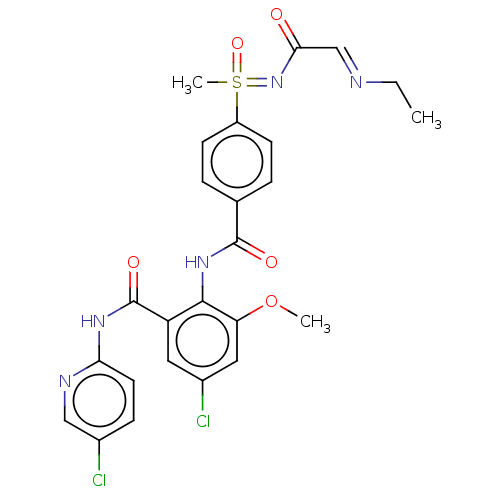
(CHEMBL2153392)Show SMILES CC\N=C\C(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1c(OC)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H25Cl2N5O5S/c1-4-28-14-22(33)32-38(3,36)18-8-5-15(6-9-18)24(34)31-23-19(11-17(27)12-20(23)37-2)25(35)30-21-10-7-16(26)13-29-21/h5-14,38H,4H2,1-3H3,(H,31,34)(H,29,30,35)(H,32,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392593
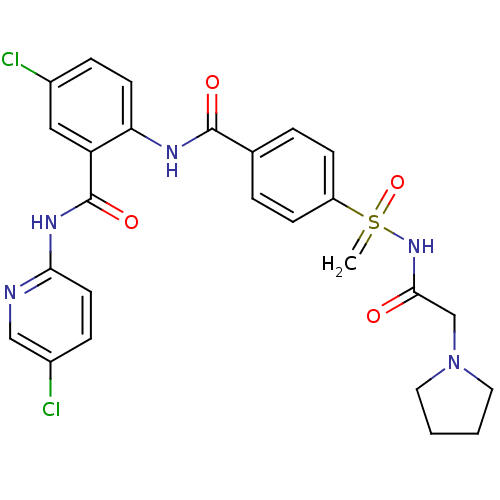
(CHEMBL2153383)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)S(=C)(=O)NC(=O)CN2CCCC2)nc1 Show InChI InChI=1S/C26H25Cl2N5O4S/c1-38(37,32-24(34)16-33-12-2-3-13-33)20-8-4-17(5-9-20)25(35)30-22-10-6-18(27)14-21(22)26(36)31-23-11-7-19(28)15-29-23/h4-11,14-15H,1-3,12-13,16H2,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392596
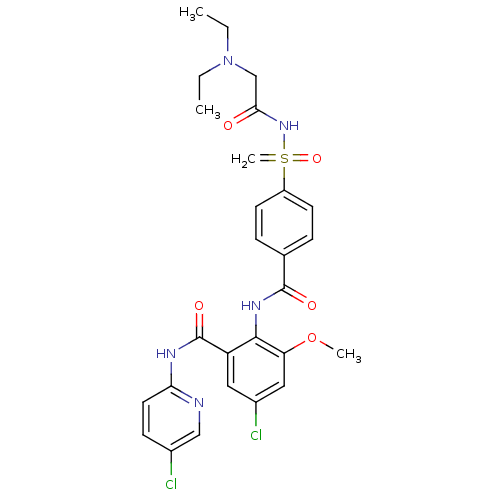
(CHEMBL2153393)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1c(OC)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C27H29Cl2N5O5S/c1-5-34(6-2)16-24(35)33-40(4,38)20-10-7-17(8-11-20)26(36)32-25-21(13-19(29)14-22(25)39-3)27(37)31-23-12-9-18(28)15-30-23/h7-15H,4-6,16H2,1-3H3,(H,32,36)(H,30,31,37)(H,33,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392590
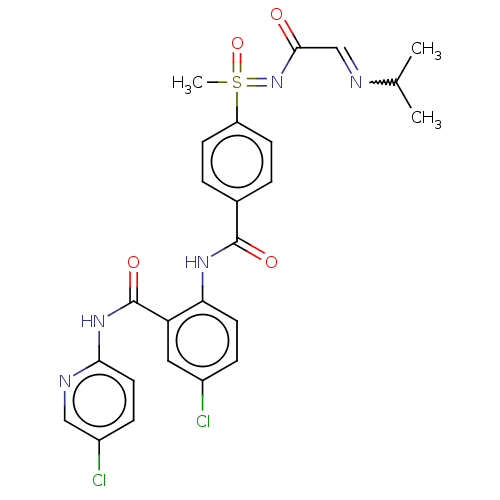
(CHEMBL2153378)Show SMILES CC(C)N=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:4.4| Show InChI InChI=1S/C25H25Cl2N5O4S/c1-15(2)28-14-23(33)32-37(3,36)19-8-4-16(5-9-19)24(34)30-21-10-6-17(26)12-20(21)25(35)31-22-11-7-18(27)13-29-22/h4-15,37H,1-3H3,(H,30,34)(H,29,31,35)(H,32,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392588
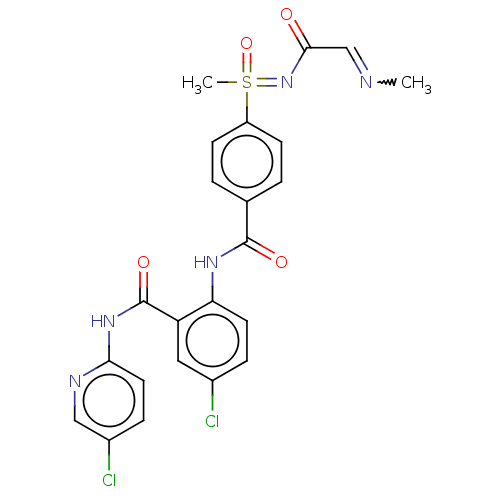
(CHEMBL2153376)Show SMILES CN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:1.0| Show InChI InChI=1S/C23H21Cl2N5O4S/c1-26-13-21(31)30-35(2,34)17-7-3-14(4-8-17)22(32)28-19-9-5-15(24)11-18(19)23(33)29-20-10-6-16(25)12-27-20/h3-13,35H,1-2H3,(H,28,32)(H,27,29,33)(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392594
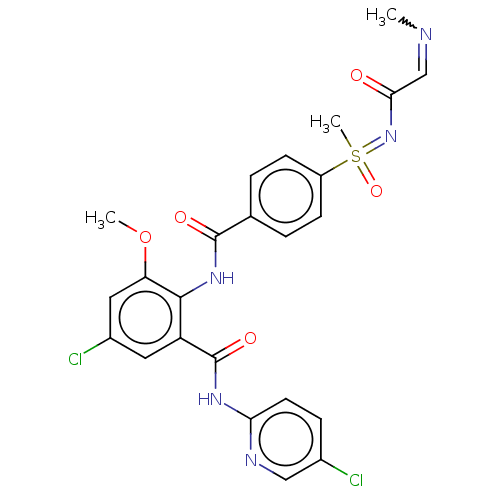
(CHEMBL2153391)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)S(C)(=O)=NC(=O)C=NC |w:35.38| Show InChI InChI=1S/C24H23Cl2N5O5S/c1-27-13-21(32)31-37(3,35)17-7-4-14(5-8-17)23(33)30-22-18(10-16(26)11-19(22)36-2)24(34)29-20-9-6-15(25)12-28-20/h4-13,37H,1-3H3,(H,30,33)(H,28,29,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392597
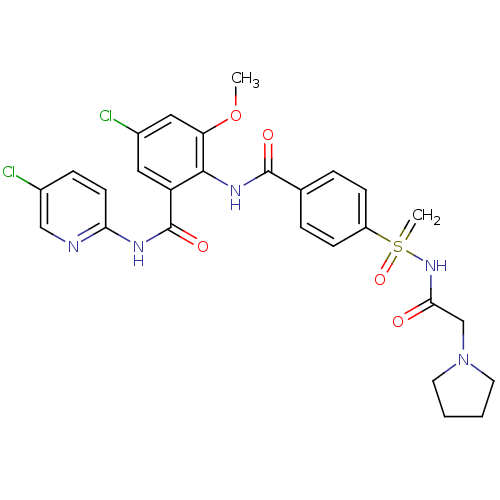
(CHEMBL2153394)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)S(=C)(=O)NC(=O)CN1CCCC1 Show InChI InChI=1S/C27H27Cl2N5O5S/c1-39-22-14-19(29)13-21(27(37)31-23-10-7-18(28)15-30-23)25(22)32-26(36)17-5-8-20(9-6-17)40(2,38)33-24(35)16-34-11-3-4-12-34/h5-10,13-15H,2-4,11-12,16H2,1H3,(H,32,36)(H,30,31,37)(H,33,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392591

(CHEMBL2153380)Show SMILES CN(C)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C24H23Cl2N5O4S/c1-31(2)14-22(32)30-36(3,35)18-8-4-15(5-9-18)23(33)28-20-10-6-16(25)12-19(20)24(34)29-21-11-7-17(26)13-27-21/h4-13H,3,14H2,1-2H3,(H,28,33)(H,27,29,34)(H,30,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392598
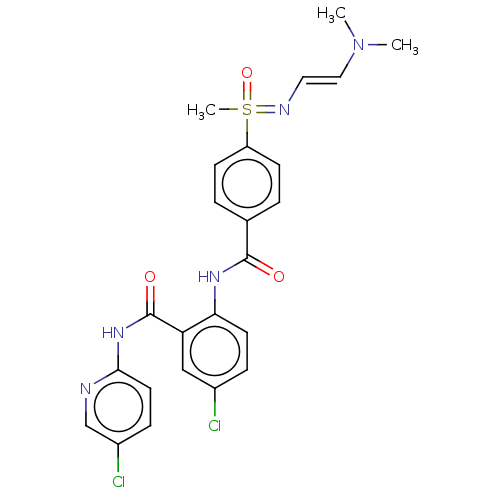
(CHEMBL2153395)Show SMILES CN(C)\C=C\N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C24H25Cl2N5O3S/c1-31(2)13-12-28-35(3,34)19-8-4-16(5-9-19)23(32)29-21-10-6-17(25)14-20(21)24(33)30-22-11-7-18(26)15-27-22/h4-15,35H,1-3H3,(H,28,34)(H,29,32)(H,27,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2765 as substrate after 45 mins |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50139534
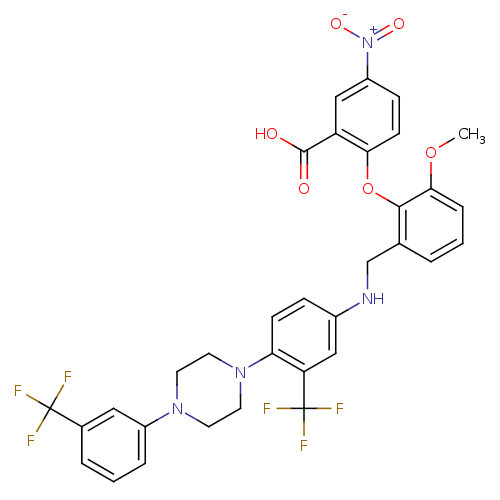
(2-[2-Methoxy-6-({3-trifluoromethyl-4-[4-(3-trifluo...)Show SMILES COc1cccc(CNc2ccc(N3CCN(CC3)c3cccc(c3)C(F)(F)F)c(c2)C(F)(F)F)c1Oc1ccc(cc1C(O)=O)[N+]([O-])=O Show InChI InChI=1S/C33H28F6N4O6/c1-48-29-7-2-4-20(30(29)49-28-11-9-24(43(46)47)18-25(28)31(44)45)19-40-22-8-10-27(26(17-22)33(37,38)39)42-14-12-41(13-15-42)23-6-3-5-21(16-23)32(34,35)36/h2-11,16-18,40H,12-15,19H2,1H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50354841

(CHEMBL1834496)Show SMILES COc1cccc(Cn2ccc3cc(ccc23)N2CCN(CC2)c2cccc(c2)C(F)(F)F)c1Oc1ccc(cc1C(O)=O)[N+]([O-])=O Show InChI InChI=1S/C34H29F3N4O6/c1-46-31-7-2-4-23(32(31)47-30-11-9-27(41(44)45)20-28(30)33(42)43)21-40-13-12-22-18-26(8-10-29(22)40)39-16-14-38(15-17-39)25-6-3-5-24(19-25)34(35,36)37/h2-13,18-20H,14-17,21H2,1H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50354839

(CHEMBL1834494)Show SMILES OC(=O)c1cc(ccc1Oc1ccccc1Cn1ccc2cc(ccc12)N1CCN(CC1)c1cccc(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C33H27F3N4O5/c34-33(35,36)24-5-3-6-25(19-24)37-14-16-38(17-15-37)26-8-10-29-22(18-26)12-13-39(29)21-23-4-1-2-7-30(23)45-31-11-9-27(40(43)44)20-28(31)32(41)42/h1-13,18-20H,14-17,21H2,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50354834

(CHEMBL1834489)Show SMILES COc1cccc(Cn2ccc3cc(ccc23)-c2ccc(OC(F)(F)F)cc2)c1Oc1ccc(cc1C(O)=O)[N+]([O-])=O Show InChI InChI=1S/C30H21F3N2O7/c1-40-27-4-2-3-21(28(27)41-26-12-8-22(35(38)39)16-24(26)29(36)37)17-34-14-13-20-15-19(7-11-25(20)34)18-5-9-23(10-6-18)42-30(31,32)33/h2-16H,17H2,1H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242808

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(3-(trifluor...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(NS(=O)(=O)c2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C24H15F9N2O5S/c25-22(26,27)14-2-1-3-19(11-14)41(39,40)34-17-4-6-18(7-5-17)35(20(36)21(37)38)12-13-8-15(23(28,29)30)10-16(9-13)24(31,32)33/h1-11,34H,12H2,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50354835

(CHEMBL1834490)Show SMILES OC(=O)c1cc(ccc1Oc1ccccc1Cn1ccc2cc(ccc12)-c1ccc(OC(F)(F)F)cc1)[N+]([O-])=O Show InChI InChI=1S/C29H19F3N2O6/c30-29(31,32)40-23-9-5-18(6-10-23)19-7-11-25-20(15-19)13-14-33(25)17-21-3-1-2-4-26(21)39-27-12-8-22(34(37)38)16-24(27)28(35)36/h1-16H,17H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242811

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-methyl-3,...)Show SMILES CN(c1ccc(cc1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)C(O)=O)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H16F12N2O5S/c1-39(46(44,45)20-10-16(25(33,34)35)9-17(11-20)26(36,37)38)18-2-4-19(5-3-18)40(21(41)22(42)43)12-13-6-14(23(27,28)29)8-15(7-13)24(30,31)32/h2-11H,12H2,1H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242810
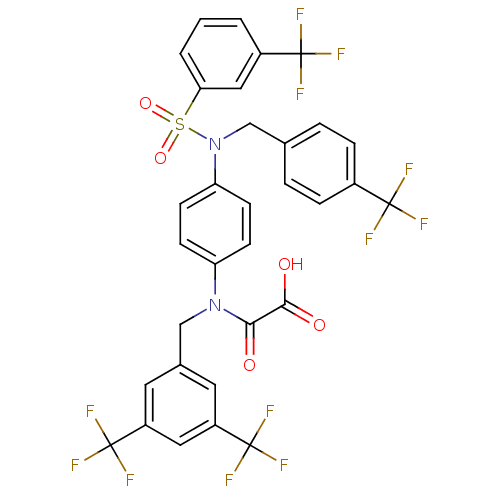
(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-(4-(trifl...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C32H20F12N2O5S/c33-29(34,35)20-6-4-18(5-7-20)17-46(52(50,51)26-3-1-2-21(15-26)30(36,37)38)25-10-8-24(9-11-25)45(27(47)28(48)49)16-19-12-22(31(39,40)41)14-23(13-19)32(42,43)44/h1-15H,16-17H2,(H,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242812

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-propyl-3,...)Show SMILES CCCN(c1ccc(cc1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)C(O)=O)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H20F12N2O5S/c1-2-7-42(48(46,47)22-12-18(27(35,36)37)11-19(13-22)28(38,39)40)21-5-3-20(4-6-21)41(23(43)24(44)45)14-15-8-16(25(29,30)31)10-17(9-15)26(32,33)34/h3-6,8-13H,2,7,14H2,1H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242805
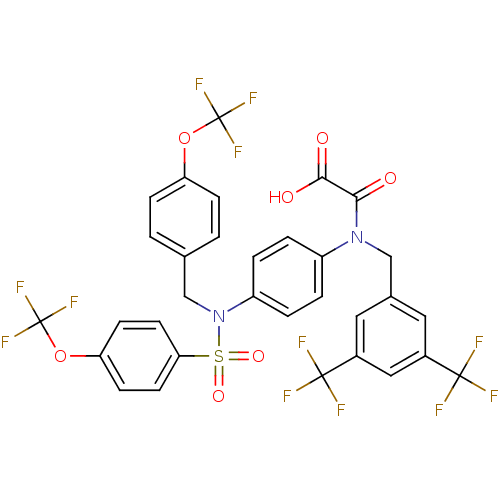
(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-(4-(trifl...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(cc1)N(Cc1ccc(OC(F)(F)F)cc1)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C32H20F12N2O7S/c33-29(34,35)20-13-19(14-21(15-20)30(36,37)38)16-45(27(47)28(48)49)22-3-5-23(6-4-22)46(17-18-1-7-24(8-2-18)52-31(39,40)41)54(50,51)26-11-9-25(10-12-26)53-32(42,43)44/h1-15H,16-17H2,(H,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242790

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(4-(trifluor...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(NS(=O)(=O)c2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C24H15F9N2O6S/c25-22(26,27)14-9-13(10-15(11-14)23(28,29)30)12-35(20(36)21(37)38)17-3-1-16(2-4-17)34-42(39,40)19-7-5-18(6-8-19)41-24(31,32)33/h1-11,34H,12H2,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50149275

(2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...)Show SMILES OC(=O)C(=O)c1cn(Cc2ccccc2)c2ccc(cc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H16F3NO4/c25-24(26,27)32-18-9-6-16(7-10-18)17-8-11-21-19(12-17)20(22(29)23(30)31)14-28(21)13-15-4-2-1-3-5-15/h1-12,14H,13H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242809
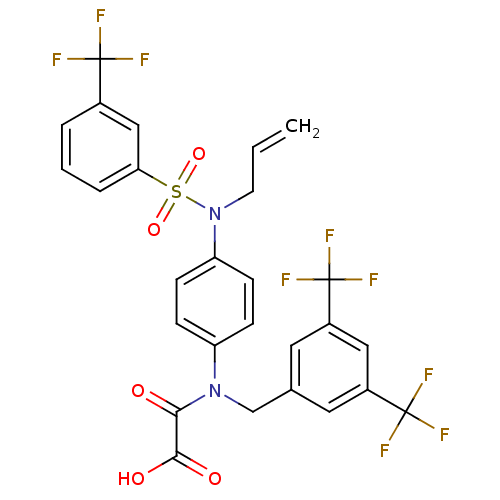
(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-allyl-3-(...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(cc1)N(CC=C)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H19F9N2O5S/c1-2-10-38(44(42,43)22-5-3-4-17(14-22)25(28,29)30)21-8-6-20(7-9-21)37(23(39)24(40)41)15-16-11-18(26(31,32)33)13-19(12-16)27(34,35)36/h2-9,11-14H,1,10,15H2,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242813

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-allyl-3,5...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(cc1)N(CC=C)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H18F12N2O5S/c1-2-7-42(48(46,47)22-12-18(27(35,36)37)11-19(13-22)28(38,39)40)21-5-3-20(4-6-21)41(23(43)24(44)45)14-15-8-16(25(29,30)31)10-17(9-15)26(32,33)34/h2-6,8-13H,1,7,14H2,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242803
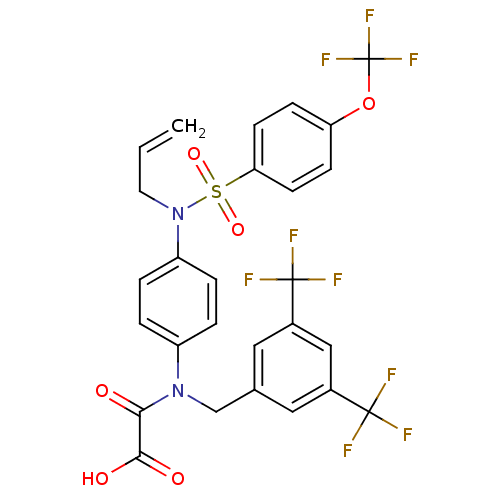
(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-allyl-4-(...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(cc1)N(CC=C)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H19F9N2O6S/c1-2-11-38(45(42,43)22-9-7-21(8-10-22)44-27(34,35)36)20-5-3-19(4-6-20)37(23(39)24(40)41)15-16-12-17(25(28,29)30)14-18(13-16)26(31,32)33/h2-10,12-14H,1,11,15H2,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242774

(CHEMBL460054 | N-(3,5-Bis-trifluoromethyl-benzyl)-...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(cc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H14F9NO4/c25-22(26,27)16-9-13(10-17(11-16)23(28,29)30)12-34(20(35)21(36)37)18-5-1-14(2-6-18)15-3-7-19(8-4-15)38-24(31,32)33/h1-11H,12H2,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50354840
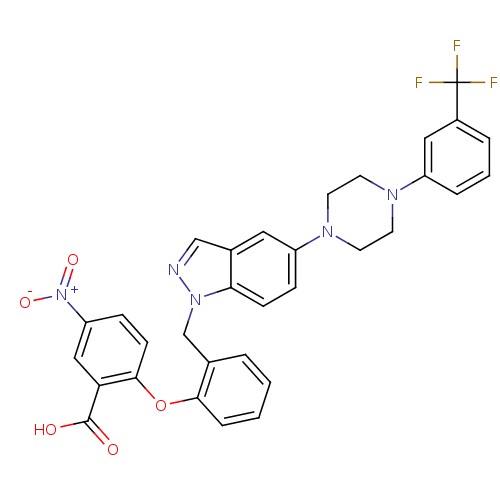
(CHEMBL1834495)Show SMILES OC(=O)c1cc(ccc1Oc1ccccc1Cn1ncc2cc(ccc12)N1CCN(CC1)c1cccc(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C32H26F3N5O5/c33-32(34,35)23-5-3-6-24(17-23)37-12-14-38(15-13-37)25-8-10-28-22(16-25)19-36-39(28)20-21-4-1-2-7-29(21)45-30-11-9-26(40(43)44)18-27(30)31(41)42/h1-11,16-19H,12-15,20H2,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50149275

(2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...)Show SMILES OC(=O)C(=O)c1cn(Cc2ccccc2)c2ccc(cc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H16F3NO4/c25-24(26,27)32-18-9-6-16(7-10-18)17-8-11-21-19(12-17)20(22(29)23(30)31)14-28(21)13-15-4-2-1-3-5-15/h1-12,14H,13H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242802
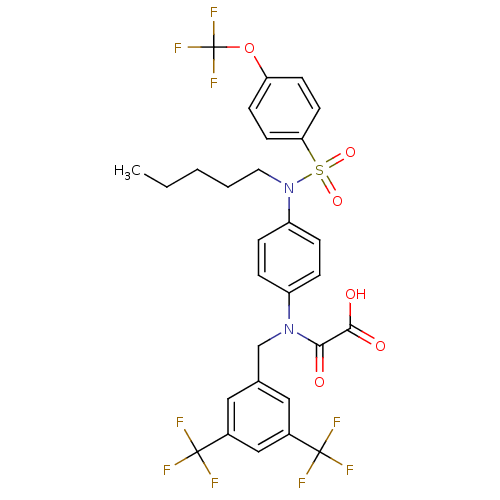
(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-pentyl-4-...)Show SMILES CCCCCN(c1ccc(cc1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)C(O)=O)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H25F9N2O6S/c1-2-3-4-13-40(47(44,45)24-11-9-23(10-12-24)46-29(36,37)38)22-7-5-21(6-8-22)39(25(41)26(42)43)17-18-14-19(27(30,31)32)16-20(15-18)28(33,34)35/h5-12,14-16H,2-4,13,17H2,1H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242791

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(3-(trifluor...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(NS(=O)(=O)c2cccc(OC(F)(F)F)c2)cc1 Show InChI InChI=1S/C24H15F9N2O6S/c25-22(26,27)14-8-13(9-15(10-14)23(28,29)30)12-35(20(36)21(37)38)17-6-4-16(5-7-17)34-42(39,40)19-3-1-2-18(11-19)41-24(31,32)33/h1-11,34H,12H2,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242801

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-propyl-4-...)Show SMILES CCCN(c1ccc(cc1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)C(O)=O)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H21F9N2O6S/c1-2-11-38(45(42,43)22-9-7-21(8-10-22)44-27(34,35)36)20-5-3-19(4-6-20)37(23(39)24(40)41)15-16-12-17(25(28,29)30)14-18(13-16)26(31,32)33/h3-10,12-14H,2,11,15H2,1H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50354842

(CHEMBL1834497)Show SMILES COc1cccc(Cn2ccc3cc(ccc23)N2CCN(CC2)c2ccc(cc2)C(F)(F)F)c1Oc1ccc(cc1C(O)=O)[N+]([O-])=O Show InChI InChI=1S/C34H29F3N4O6/c1-46-31-4-2-3-23(32(31)47-30-12-10-27(41(44)45)20-28(30)33(42)43)21-40-14-13-22-19-26(9-11-29(22)40)39-17-15-38(16-18-39)25-7-5-24(6-8-25)34(35,36)37/h2-14,19-20H,15-18,21H2,1H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242773
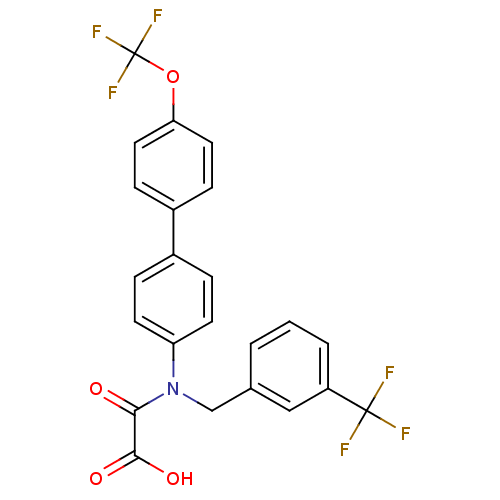
(CHEMBL460053 | N-(4'-Trifluoromethoxy-biphenyl-4-y...)Show SMILES OC(=O)C(=O)N(Cc1cccc(c1)C(F)(F)F)c1ccc(cc1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H15F6NO4/c24-22(25,26)17-3-1-2-14(12-17)13-30(20(31)21(32)33)18-8-4-15(5-9-18)16-6-10-19(11-7-16)34-23(27,28)29/h1-12H,13H2,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242800

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-methyl-4-...)Show SMILES CN(c1ccc(cc1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)C(O)=O)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C25H17F9N2O6S/c1-35(43(40,41)20-8-6-19(7-9-20)42-25(32,33)34)17-2-4-18(5-3-17)36(21(37)22(38)39)13-14-10-15(23(26,27)28)12-16(11-14)24(29,30)31/h2-12H,13H2,1H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50354837

(CHEMBL1834492)Show SMILES OC(=O)c1cc(ccc1Oc1ccccc1CN1CCc2cc(ccc12)N1CCN(CC1)c1cccc(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C33H29F3N4O5/c34-33(35,36)24-5-3-6-25(19-24)37-14-16-38(17-15-37)26-8-10-29-22(18-26)12-13-39(29)21-23-4-1-2-7-30(23)45-31-11-9-27(40(43)44)20-28(31)32(41)42/h1-11,18-20H,12-17,21H2,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human Plasminogen activator inhibitor 1 using HRP substrate after 30 mins by chromogenic assay |
Bioorg Med Chem Lett 21: 5701-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.031
BindingDB Entry DOI: 10.7270/Q2B858JV |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242789
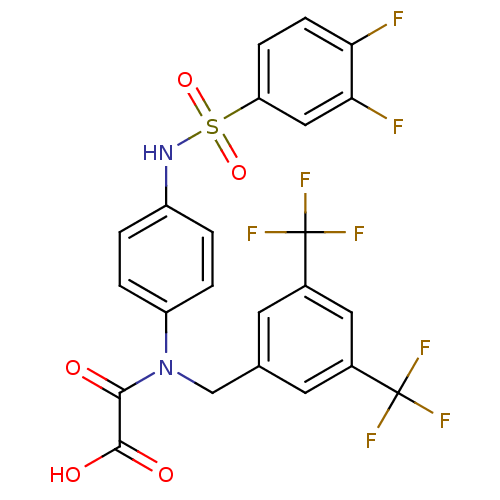
(2-((3,5-bis(trifluoromethyl)benzyl)(4-(3,4-difluor...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(NS(=O)(=O)c2ccc(F)c(F)c2)cc1 Show InChI InChI=1S/C23H14F8N2O5S/c24-18-6-5-17(10-19(18)25)39(37,38)32-15-1-3-16(4-2-15)33(20(34)21(35)36)11-12-7-13(22(26,27)28)9-14(8-12)23(29,30)31/h1-10,32H,11H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242804

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-benzyl-4-...)Show SMILES OC(=O)C(=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ccc(cc1)N(Cc1ccccc1)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C31H21F9N2O6S/c32-29(33,34)21-14-20(15-22(16-21)30(35,36)37)17-41(27(43)28(44)45)23-6-8-24(9-7-23)42(18-19-4-2-1-3-5-19)49(46,47)26-12-10-25(11-13-26)48-31(38,39)40/h1-16H,17-18H2,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50242807

(2-((3,5-bis(trifluoromethyl)benzyl)(4-(N-methyl-4-...)Show SMILES CN(c1ccc(cc1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)C(O)=O)S(=O)(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C25H17F9N2O5S/c1-35(42(40,41)20-8-2-15(3-9-20)23(26,27)28)18-4-6-19(7-5-18)36(21(37)22(38)39)13-14-10-16(24(29,30)31)12-17(11-14)25(32,33)34/h2-12H,13H2,1H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PAI1 by chromogenic assay |
Eur J Med Chem 43: 880-4 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.011
BindingDB Entry DOI: 10.7270/Q25Q4VWX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data