 Found 72 hits with Last Name = 'parmar' and Initial = 'r'
Found 72 hits with Last Name = 'parmar' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85357
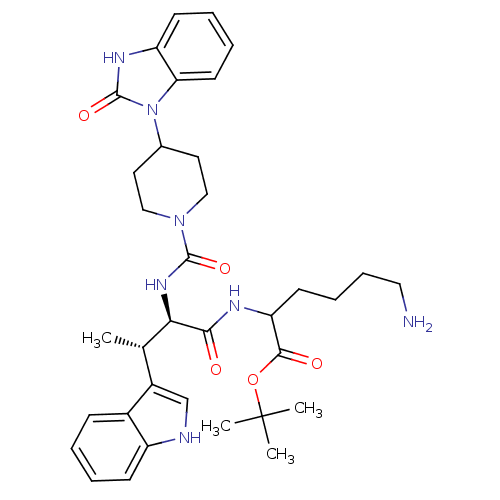
(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81766
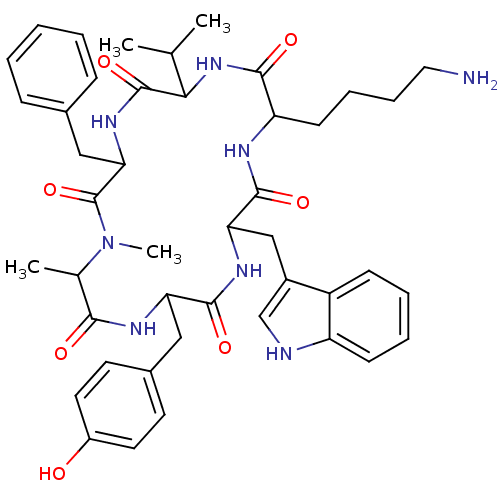
(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50059090
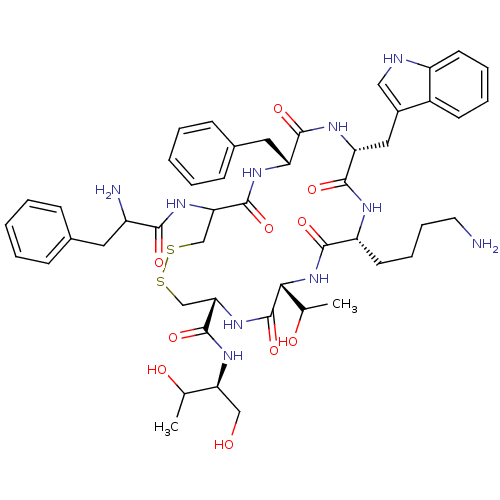
(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064778
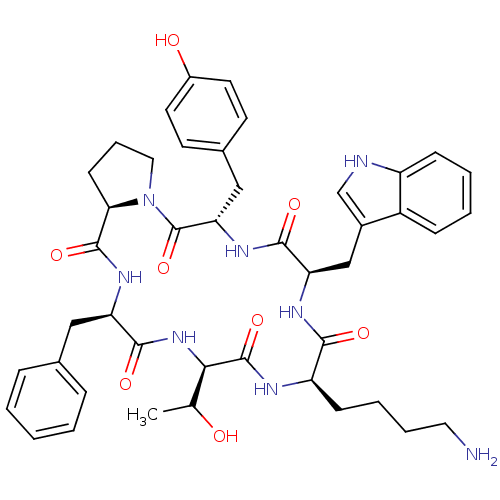
((5S,8R,11R,14R,17R,19aR)-11-(4-Amino-butyl)-17-ben...)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCCN)NC1=O Show InChI InChI=1S/C44H54N8O8/c1-26(53)38-43(59)47-33(14-7-8-20-45)39(55)48-35(24-29-25-46-32-13-6-5-12-31(29)32)40(56)50-36(23-28-16-18-30(54)19-17-28)44(60)52-21-9-15-37(52)42(58)49-34(41(57)51-38)22-27-10-3-2-4-11-27/h2-6,10-13,16-19,25-26,33-38,46,53-54H,7-9,14-15,20-24,45H2,1H3,(H,47,59)(H,48,55)(H,49,58)(H,50,56)(H,51,57)/t26?,33-,34-,35-,36+,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM85357

(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50059090

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM85357

(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50059090

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM85357

(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50059090

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM81766

(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81766

(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064778

((5S,8R,11R,14R,17R,19aR)-11-(4-Amino-butyl)-17-ben...)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCCN)NC1=O Show InChI InChI=1S/C44H54N8O8/c1-26(53)38-43(59)47-33(14-7-8-20-45)39(55)48-35(24-29-25-46-32-13-6-5-12-31(29)32)40(56)50-36(23-28-16-18-30(54)19-17-28)44(60)52-21-9-15-37(52)42(58)49-34(41(57)51-38)22-27-10-3-2-4-11-27/h2-6,10-13,16-19,25-26,33-38,46,53-54H,7-9,14-15,20-24,45H2,1H3,(H,47,59)(H,48,55)(H,49,58)(H,50,56)(H,51,57)/t26?,33-,34-,35-,36+,37-,38-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50059090

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064778

((5S,8R,11R,14R,17R,19aR)-11-(4-Amino-butyl)-17-ben...)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCCN)NC1=O Show InChI InChI=1S/C44H54N8O8/c1-26(53)38-43(59)47-33(14-7-8-20-45)39(55)48-35(24-29-25-46-32-13-6-5-12-31(29)32)40(56)50-36(23-28-16-18-30(54)19-17-28)44(60)52-21-9-15-37(52)42(58)49-34(41(57)51-38)22-27-10-3-2-4-11-27/h2-6,10-13,16-19,25-26,33-38,46,53-54H,7-9,14-15,20-24,45H2,1H3,(H,47,59)(H,48,55)(H,49,58)(H,50,56)(H,51,57)/t26?,33-,34-,35-,36+,37-,38-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM81766

(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064778

((5S,8R,11R,14R,17R,19aR)-11-(4-Amino-butyl)-17-ben...)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCCN)NC1=O Show InChI InChI=1S/C44H54N8O8/c1-26(53)38-43(59)47-33(14-7-8-20-45)39(55)48-35(24-29-25-46-32-13-6-5-12-31(29)32)40(56)50-36(23-28-16-18-30(54)19-17-28)44(60)52-21-9-15-37(52)42(58)49-34(41(57)51-38)22-27-10-3-2-4-11-27/h2-6,10-13,16-19,25-26,33-38,46,53-54H,7-9,14-15,20-24,45H2,1H3,(H,47,59)(H,48,55)(H,49,58)(H,50,56)(H,51,57)/t26?,33-,34-,35-,36+,37-,38-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81766

(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064778

((5S,8R,11R,14R,17R,19aR)-11-(4-Amino-butyl)-17-ben...)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCCN)NC1=O Show InChI InChI=1S/C44H54N8O8/c1-26(53)38-43(59)47-33(14-7-8-20-45)39(55)48-35(24-29-25-46-32-13-6-5-12-31(29)32)40(56)50-36(23-28-16-18-30(54)19-17-28)44(60)52-21-9-15-37(52)42(58)49-34(41(57)51-38)22-27-10-3-2-4-11-27/h2-6,10-13,16-19,25-26,33-38,46,53-54H,7-9,14-15,20-24,45H2,1H3,(H,47,59)(H,48,55)(H,49,58)(H,50,56)(H,51,57)/t26?,33-,34-,35-,36+,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221982
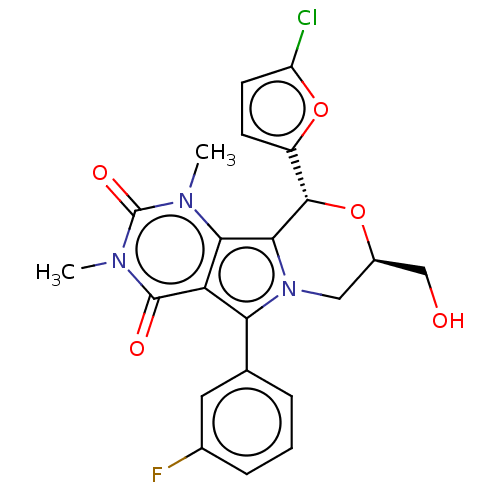
(US9303035, 6)Show SMILES Cn1c2c3[C@@H](O[C@@H](CO)Cn3c(-c3cccc(F)c3)c2c(=O)n(C)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C22H19ClFN3O5/c1-25-18-16(21(29)26(2)22(25)30)17(11-4-3-5-12(24)8-11)27-9-13(10-28)31-20(19(18)27)14-6-7-15(23)32-14/h3-8,13,20,28H,9-10H2,1-2H3/t13-,20+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221986
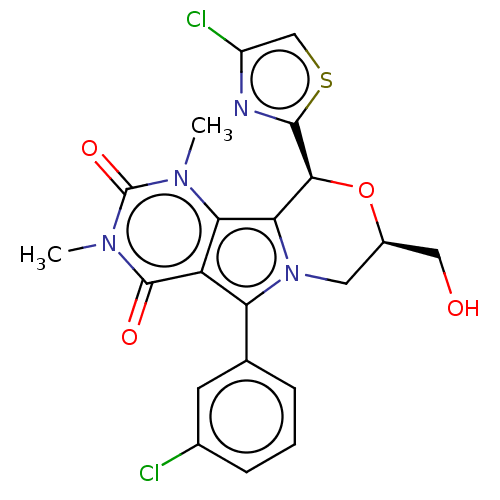
(US9303035, 8.0a)Show SMILES Cn1c2c3[C@H](O[C@@H](CO)Cn3c(-c3cccc(Cl)c3)c2c(=O)n(C)c1=O)c1nc(Cl)cs1 Show InChI InChI=1S/C21H18Cl2N4O4S/c1-25-16-14(20(29)26(2)21(25)30)15(10-4-3-5-11(22)6-10)27-7-12(8-28)31-18(17(16)27)19-24-13(23)9-32-19/h3-6,9,12,18,28H,7-8H2,1-2H3/t12-,18+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221984
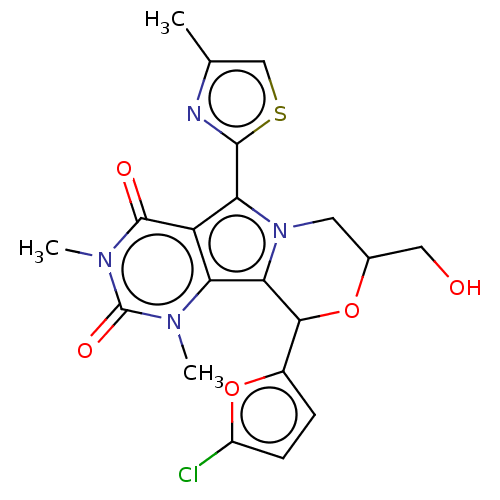
(US9303035, 7.0a)Show SMILES Cc1csc(n1)-c1n2CC(CO)OC(c3ccc(Cl)o3)c2c2n(C)c(=O)n(C)c(=O)c12 Show InChI InChI=1S/C20H19ClN4O5S/c1-9-8-31-18(22-9)15-13-14(23(2)20(28)24(3)19(13)27)16-17(11-4-5-12(21)30-11)29-10(7-26)6-25(15)16/h4-5,8,10,17,26H,6-7H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221987
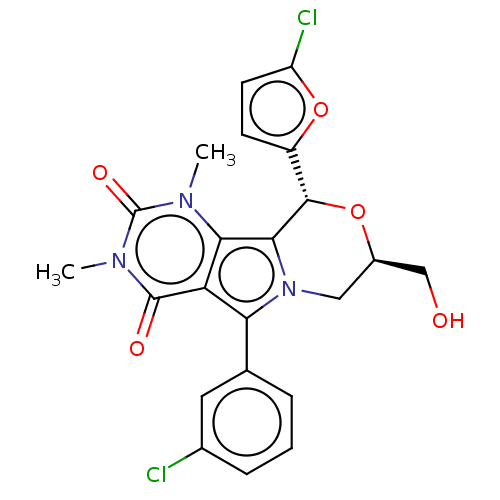
(US9303035, 8.1a)Show SMILES Cn1c2c3[C@@H](O[C@@H](CO)Cn3c(-c3cccc(Cl)c3)c2c(=O)n(C)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C22H19Cl2N3O5/c1-25-18-16(21(29)26(2)22(25)30)17(11-4-3-5-12(23)8-11)27-9-13(10-28)31-20(19(18)27)14-6-7-15(24)32-14/h3-8,13,20,28H,9-10H2,1-2H3/t13-,20+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221983
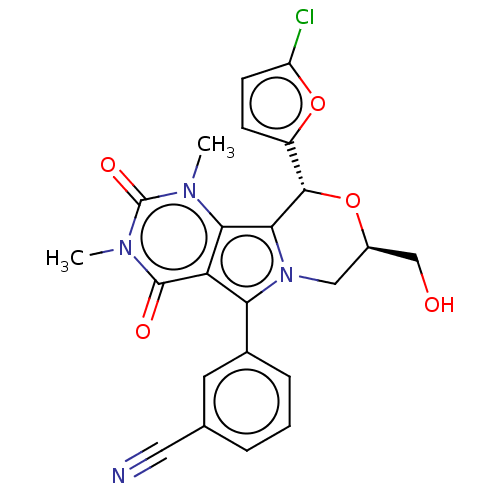
(US9303035, 6.1)Show SMILES Cn1c2c3[C@@H](O[C@@H](CO)Cn3c(-c3cccc(c3)C#N)c2c(=O)n(C)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C23H19ClN4O5/c1-26-19-17(22(30)27(2)23(26)31)18(13-5-3-4-12(8-13)9-25)28-10-14(11-29)32-21(20(19)28)15-6-7-16(24)33-15/h3-8,14,21,29H,10-11H2,1-2H3/t14-,21+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221992
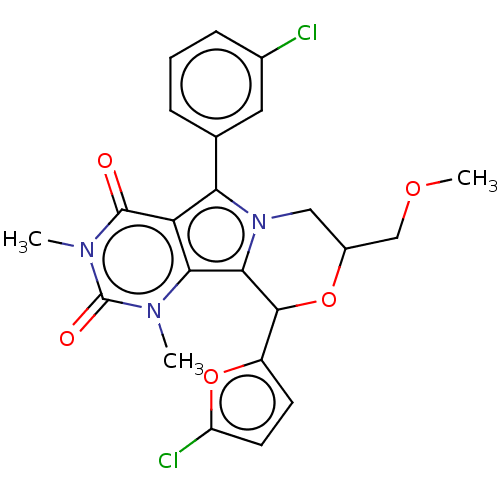
(US9303035, 12.2)Show SMILES COCC1Cn2c(C(O1)c1ccc(Cl)o1)c1n(C)c(=O)n(C)c(=O)c1c2-c1cccc(Cl)c1 Show InChI InChI=1S/C23H21Cl2N3O5/c1-26-19-17(22(29)27(2)23(26)30)18(12-5-4-6-13(24)9-12)28-10-14(11-31-3)32-21(20(19)28)15-7-8-16(25)33-15/h4-9,14,21H,10-11H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221989
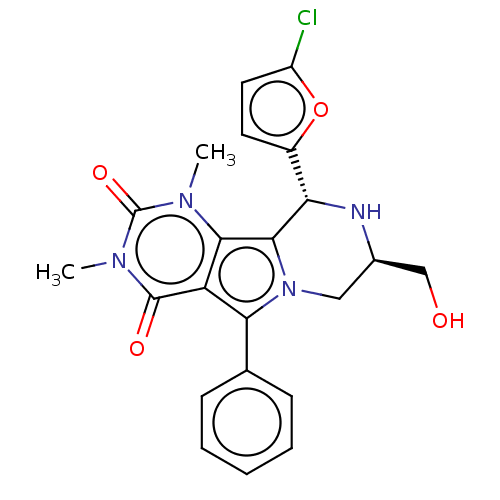
(US9303035, 11a)Show SMILES Cn1c2c3[C@@H](N[C@@H](CO)Cn3c(-c3ccccc3)c2c(=O)n(C)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C22H21ClN4O4/c1-25-19-16(21(29)26(2)22(25)30)18(12-6-4-3-5-7-12)27-10-13(11-28)24-17(20(19)27)14-8-9-15(23)31-14/h3-9,13,17,24,28H,10-11H2,1-2H3/t13-,17+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221993
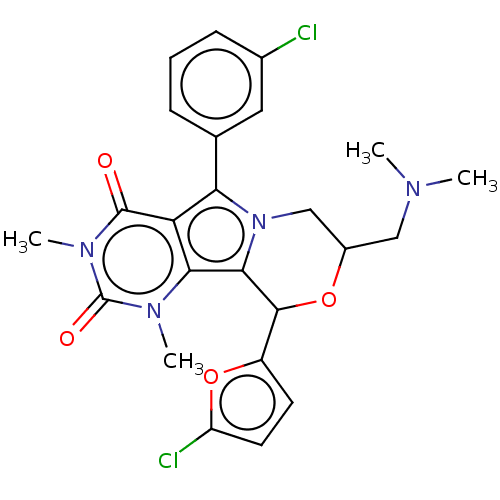
(US9303035, 12.4)Show SMILES CN(C)CC1Cn2c(C(O1)c1ccc(Cl)o1)c1n(C)c(=O)n(C)c(=O)c1c2-c1cccc(Cl)c1 Show InChI InChI=1S/C24H24Cl2N4O4/c1-27(2)11-15-12-30-19(13-6-5-7-14(25)10-13)18-20(28(3)24(32)29(4)23(18)31)21(30)22(33-15)16-8-9-17(26)34-16/h5-10,15,22H,11-12H2,1-4H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
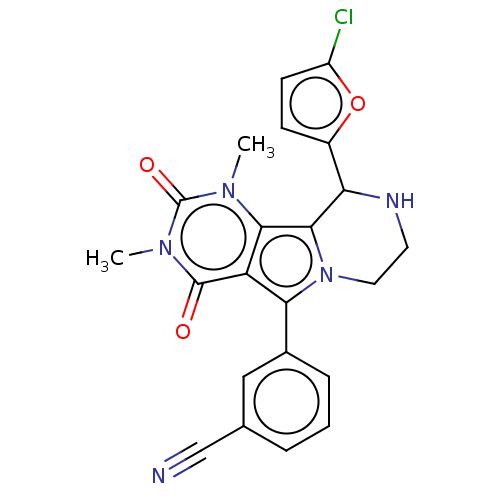
(Cricetulus griseus (Chinese hamster)) | BDBM221967
(US9303035, 2.9)Show SMILES Cn1c2c3C(NCCn3c(-c3cccc(c3)C#N)c2c(=O)n(C)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C22H18ClN5O3/c1-26-19-16(21(29)27(2)22(26)30)18(13-5-3-4-12(10-13)11-24)28-9-8-25-17(20(19)28)14-6-7-15(23)31-14/h3-7,10,17,25H,8-9H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
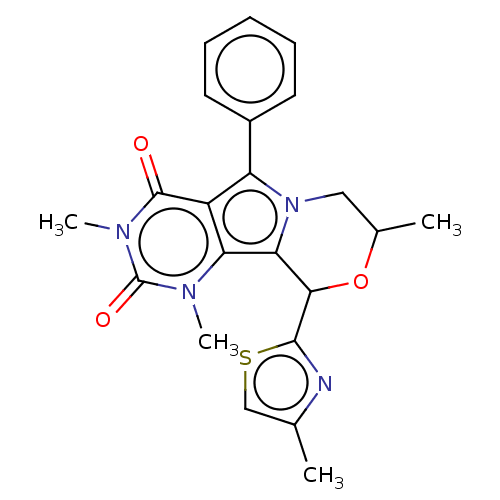
(Cricetulus griseus (Chinese hamster)) | BDBM221990
(US9303035, 12)Show SMILES CC1Cn2c(C(O1)c1nc(C)cs1)c1n(C)c(=O)n(C)c(=O)c1c2-c1ccccc1 Show InChI InChI=1S/C22H22N4O3S/c1-12-11-30-20(23-12)19-18-17-15(21(27)25(4)22(28)24(17)3)16(14-8-6-5-7-9-14)26(18)10-13(2)29-19/h5-9,11,13,19H,10H2,1-4H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
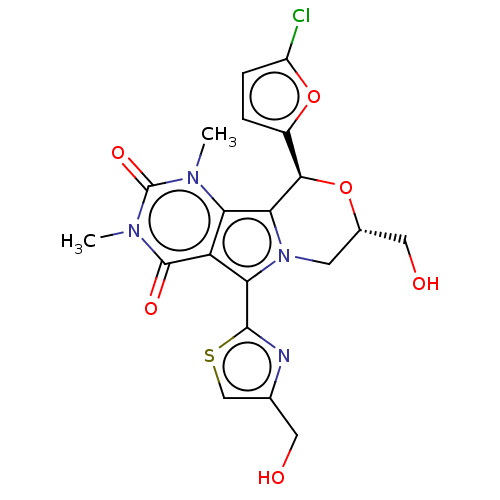
(Cricetulus griseus (Chinese hamster)) | BDBM221985
(US9303035, 7.1a)Show SMILES Cn1c2c3[C@H](O[C@H](CO)Cn3c(-c3nc(CO)cs3)c2c(=O)n(C)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C20H19ClN4O6S/c1-23-14-13(19(28)24(2)20(23)29)15(18-22-9(6-26)8-32-18)25-5-10(7-27)30-17(16(14)25)11-3-4-12(21)31-11/h3-4,8,10,17,26-27H,5-7H2,1-2H3/t10-,17+/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
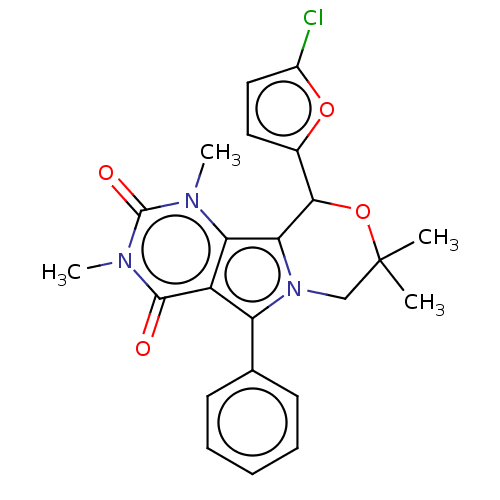
(Cricetulus griseus (Chinese hamster)) | BDBM221994
(US9303035, 12.5)Show SMILES Cn1c2c3C(OC(C)(C)Cn3c(-c3ccccc3)c2c(=O)n(C)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C23H22ClN3O4/c1-23(2)12-27-17(13-8-6-5-7-9-13)16-18(25(3)22(29)26(4)21(16)28)19(27)20(31-23)14-10-11-15(24)30-14/h5-11,20H,12H2,1-4H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221970
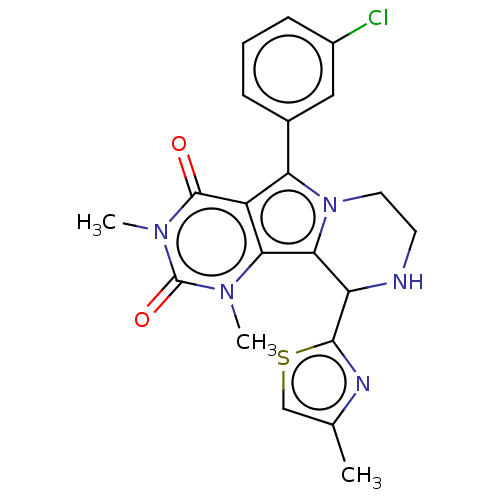
(US9303035, 3.2)Show SMILES Cc1csc(n1)C1NCCn2c1c1n(C)c(=O)n(C)c(=O)c1c2-c1cccc(Cl)c1 Show InChI InChI=1S/C21H20ClN5O2S/c1-11-10-30-19(24-11)15-18-17-14(20(28)26(3)21(29)25(17)2)16(27(18)8-7-23-15)12-5-4-6-13(22)9-12/h4-6,9-10,15,23H,7-8H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
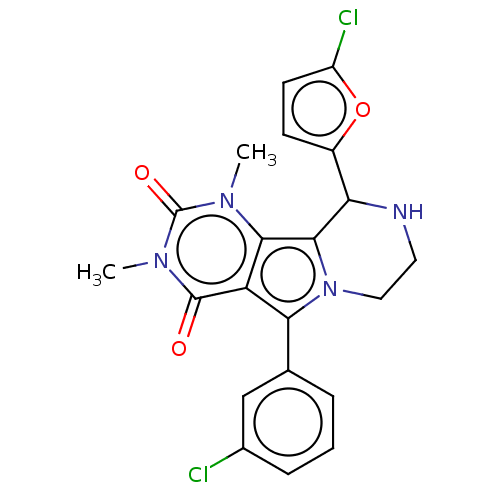
(Cricetulus griseus (Chinese hamster)) | BDBM221969
(US9303035, 3.1)Show SMILES Cn1c2c3C(NCCn3c(-c3cccc(Cl)c3)c2c(=O)n(C)c1=O)c1ccc(Cl)o1 Show InChI InChI=1S/C21H18Cl2N4O3/c1-25-18-15(20(28)26(2)21(25)29)17(11-4-3-5-12(22)10-11)27-9-8-24-16(19(18)27)13-6-7-14(23)30-13/h3-7,10,16,24H,8-9H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
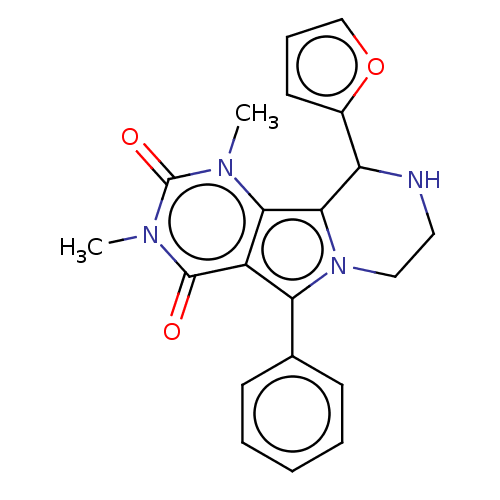
(Cricetulus griseus (Chinese hamster)) | BDBM221952
(US9303035, 1.2)Show SMILES Cn1c2c3C(NCCn3c(-c3ccccc3)c2c(=O)n(C)c1=O)c1ccco1 Show InChI InChI=1S/C21H20N4O3/c1-23-18-15(20(26)24(2)21(23)27)17(13-7-4-3-5-8-13)25-11-10-22-16(19(18)25)14-9-6-12-28-14/h3-9,12,16,22H,10-11H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
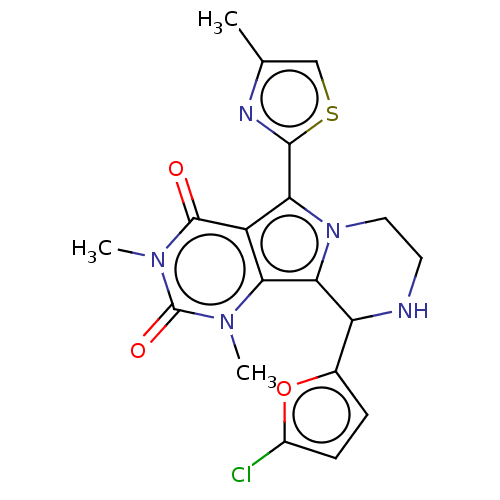
(Cricetulus griseus (Chinese hamster)) | BDBM221958
(US9303035, 1.26)Show SMILES Cc1csc(n1)-c1n2CCNC(c3ccc(Cl)o3)c2c2n(C)c(=O)n(C)c(=O)c12 Show InChI InChI=1S/C19H18ClN5O3S/c1-9-8-29-17(22-9)15-12-14(23(2)19(27)24(3)18(12)26)16-13(21-6-7-25(15)16)10-4-5-11(20)28-10/h4-5,8,13,21H,6-7H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221996
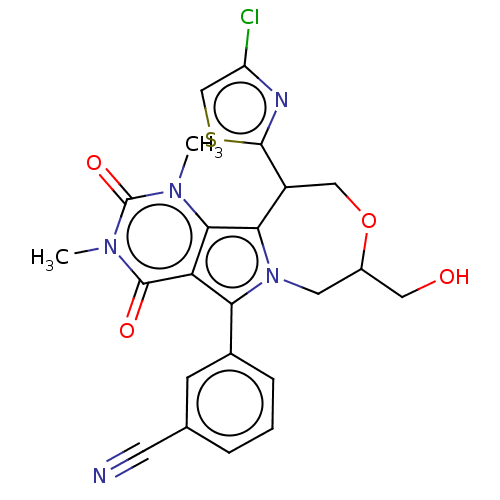
(US9303035, 14)Show SMILES Cn1c2c3C(COC(CO)Cn3c(-c3cccc(c3)C#N)c2c(=O)n(C)c1=O)c1nc(Cl)cs1 Show InChI InChI=1S/C23H20ClN5O4S/c1-27-20-17(22(31)28(2)23(27)32)18(13-5-3-4-12(6-13)7-25)29-8-14(9-30)33-10-15(19(20)29)21-26-16(24)11-34-21/h3-6,11,14-15,30H,8-10H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221978
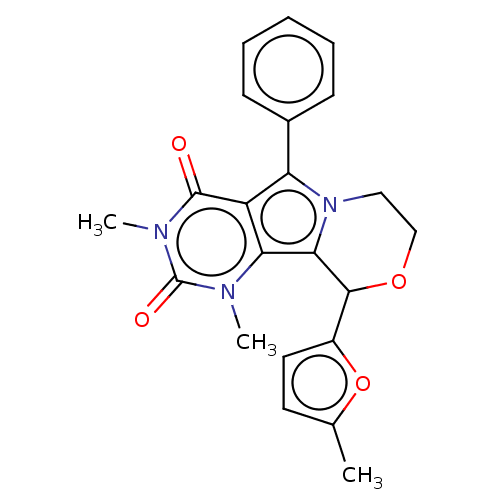
(US9303035, 4.1 | US9303035, 4.3)Show SMILES Cc1ccc(o1)C1OCCn2c1c1n(C)c(=O)n(C)c(=O)c1c2-c1ccccc1 Show InChI InChI=1S/C22H21N3O4/c1-13-9-10-15(29-13)20-19-18-16(21(26)24(3)22(27)23(18)2)17(25(19)11-12-28-20)14-7-5-4-6-8-14/h4-10,20H,11-12H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221981
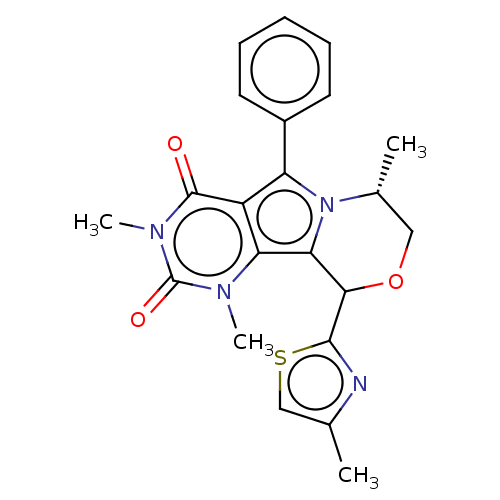
(US9303035, 5.1)Show SMILES C[C@@H]1COC(c2nc(C)cs2)c2n1c(-c1ccccc1)c1c2n(C)c(=O)n(C)c1=O Show InChI InChI=1S/C22H22N4O3S/c1-12-11-30-20(23-12)19-18-17-15(21(27)25(4)22(28)24(17)3)16(14-8-6-5-7-9-14)26(18)13(2)10-29-19/h5-9,11,13,19H,10H2,1-4H3/t13-,19?/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
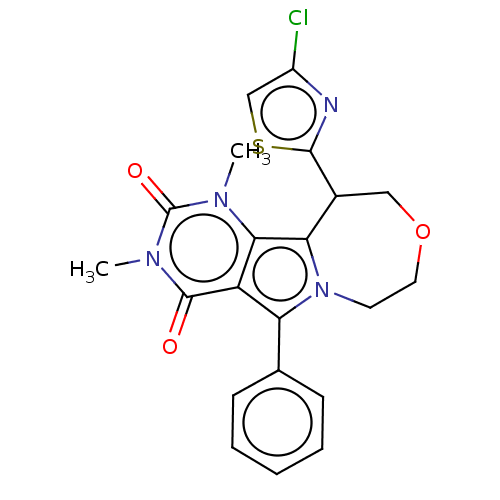
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221995
(US9303035, 13)Show SMILES Cn1c2c3C(COCCn3c(-c3ccccc3)c2c(=O)n(C)c1=O)c1nc(Cl)cs1 Show InChI InChI=1S/C21H19ClN4O3S/c1-24-18-15(20(27)25(2)21(24)28)16(12-6-4-3-5-7-12)26-8-9-29-10-13(17(18)26)19-23-14(22)11-30-19/h3-7,11,13H,8-10H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221978

(US9303035, 4.1 | US9303035, 4.3)Show SMILES Cc1ccc(o1)C1OCCn2c1c1n(C)c(=O)n(C)c(=O)c1c2-c1ccccc1 Show InChI InChI=1S/C22H21N3O4/c1-13-9-10-15(29-13)20-19-18-16(21(26)24(3)22(27)23(18)2)17(25(19)11-12-28-20)14-7-5-4-6-8-14/h4-10,20H,11-12H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
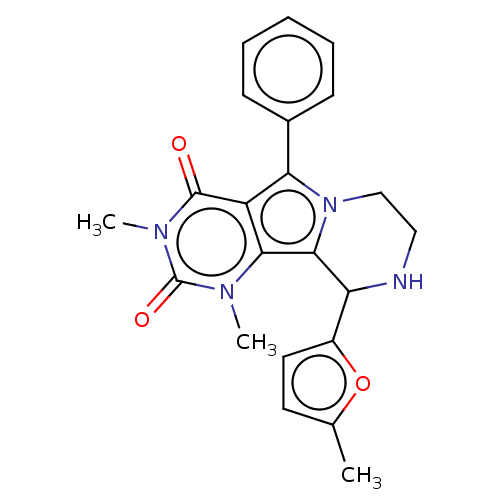
(Cricetulus griseus (Chinese hamster)) | BDBM221934
(US9303035, 1.1)Show SMILES Cc1ccc(o1)C1NCCn2c1c1n(C)c(=O)n(C)c(=O)c1c2-c1ccccc1 Show InChI InChI=1S/C22H22N4O3/c1-13-9-10-15(29-13)17-20-19-16(21(27)25(3)22(28)24(19)2)18(26(20)12-11-23-17)14-7-5-4-6-8-14/h4-10,17,23H,11-12H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
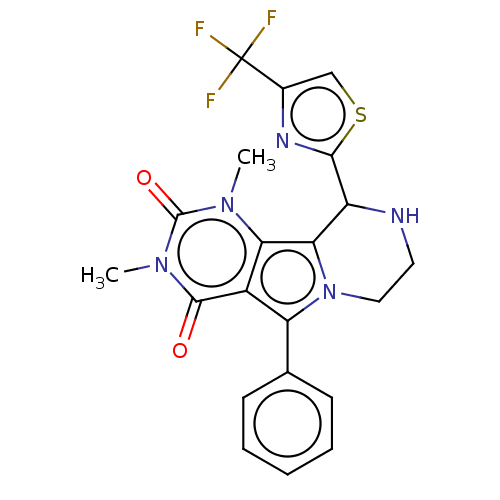
(Cricetulus griseus (Chinese hamster)) | BDBM221941
(US9303035, 1.9 | US9303035, 2.4)Show SMILES Cn1c2c3C(NCCn3c(-c3ccccc3)c2c(=O)n(C)c1=O)c1nc(cs1)C(F)(F)F Show InChI InChI=1S/C21H18F3N5O2S/c1-27-16-13(19(30)28(2)20(27)31)15(11-6-4-3-5-7-11)29-9-8-25-14(17(16)29)18-26-12(10-32-18)21(22,23)24/h3-7,10,14,25H,8-9H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
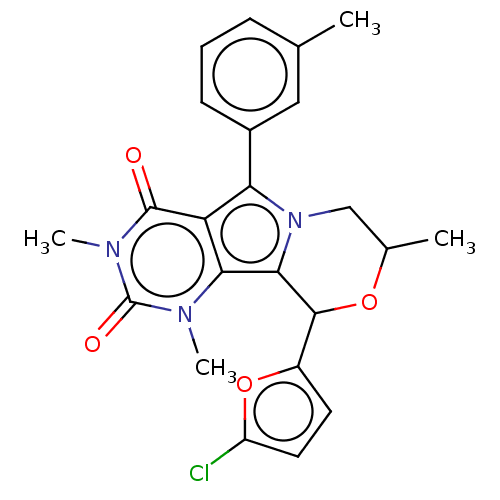
(Cricetulus griseus (Chinese hamster)) | BDBM221991
(US9303035, 12.1)Show SMILES CC1Cn2c(C(O1)c1ccc(Cl)o1)c1n(C)c(=O)n(C)c(=O)c1c2-c1cccc(C)c1 Show InChI InChI=1S/C23H22ClN3O4/c1-12-6-5-7-14(10-12)18-17-19(25(3)23(29)26(4)22(17)28)20-21(15-8-9-16(24)31-15)30-13(2)11-27(18)20/h5-10,13,21H,11H2,1-4H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
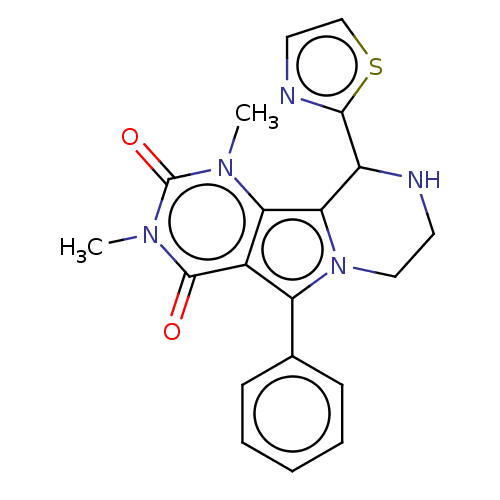
(Cricetulus griseus (Chinese hamster)) | BDBM221939
(US9303035, 1.7)Show SMILES Cn1c2c3C(NCCn3c(-c3ccccc3)c2c(=O)n(C)c1=O)c1nccs1 Show InChI InChI=1S/C20H19N5O2S/c1-23-16-13(19(26)24(2)20(23)27)15(12-6-4-3-5-7-12)25-10-8-21-14(17(16)25)18-22-9-11-28-18/h3-7,9,11,14,21H,8,10H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Cricetulus griseus (Chinese hamster)) | BDBM221941

(US9303035, 1.9 | US9303035, 2.4)Show SMILES Cn1c2c3C(NCCn3c(-c3ccccc3)c2c(=O)n(C)c1=O)c1nc(cs1)C(F)(F)F Show InChI InChI=1S/C21H18F3N5O2S/c1-27-16-13(19(30)28(2)20(27)31)15(11-6-4-3-5-7-11)29-9-8-25-14(17(16)29)18-26-12(10-32-18)21(22,23)24/h3-7,10,14,25H,8-9H2,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
CFTR activity can be quantified by electrophysiology methods, using the whole-cell configuration of the patch clamp technique (Hamill O, Marty A, Neh... |
US Patent US9303035 (2016)
BindingDB Entry DOI: 10.7270/Q2SQ8Z7R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data