 Found 131 hits with Last Name = 'parsons' and Initial = 'rb'
Found 131 hits with Last Name = 'parsons' and Initial = 'rb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM13016

(1,2,3,4-tetrahydroisoquinoline | CHEMBL14346 | THI...)Show InChI InChI=1S/C9H11N/c1-2-4-9-7-10-6-5-8(9)3-1/h1-4,10H,5-7H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.70E+7 | -7.88 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM60920
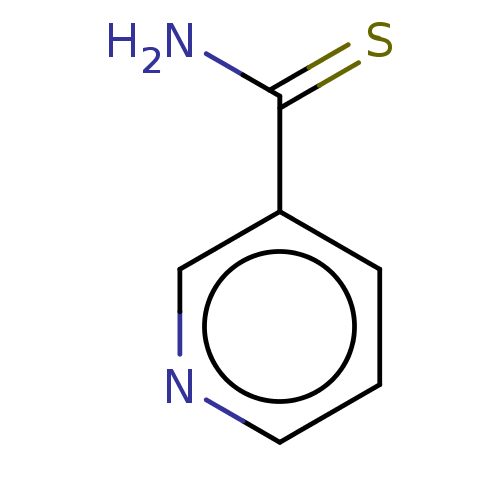
(thionicotinamide)Show InChI InChI=1S/C6H6N2S/c7-6(9)5-2-1-3-8-4-5/h1-4H,(H2,7,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+7 | -7.01 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM60924
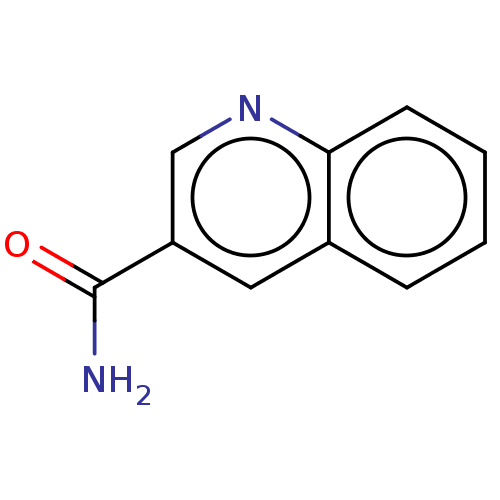
(quinoline 3-carboxamide)Show InChI InChI=1S/C10H8N2O/c11-10(13)8-5-7-3-1-2-4-9(7)12-6-8/h1-6H,(H2,11,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70E+7 | -6.02 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50047015

(CHEMBL14474 | Chinolin | benzo[b]pyridine | quinol...)Show InChI InChI=1S/C9H7N/c1-2-6-9-8(4-1)5-3-7-10-9/h1-7H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.02E+8 | -5.89 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM60921
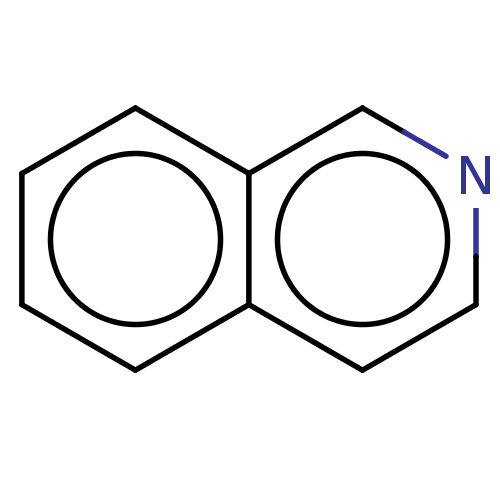
(isoquinoline)Show InChI InChI=1S/C9H7N/c1-2-4-9-7-10-6-5-8(9)3-1/h1-7H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.02E+8 | -4.12 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588067

(CHEMBL5189197)Show SMILES N[C@@H](CCN(C\C=C\c1ccc(cc1)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588085

(CHEMBL5173204)Show SMILES N[C@@H](CCN(CC=Cc1ccc(cc1)[N+]([O-])=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50506000

(CHEMBL4445337)Show SMILES N[C@@H](CCN(CC#Cc1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H28N8O6/c25-15(24(36)37)6-8-31(7-2-4-13-3-1-5-14(9-13)21(27)35)10-16-18(33)19(34)23(38-16)32-12-30-17-20(26)28-11-29-22(17)32/h1,3,5,9,11-12,15-16,18-19,23,33-34H,6-8,10,25H2,(H2,27,35)(H,36,37)(H2,26,28,29)/t15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588063

(CHEMBL5172454)Show SMILES N[C@@H](CCN(C\C=C\c1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588087

(CHEMBL5182376)Show SMILES N[C@@H](CCN(CCCc1ccc(cc1)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588082
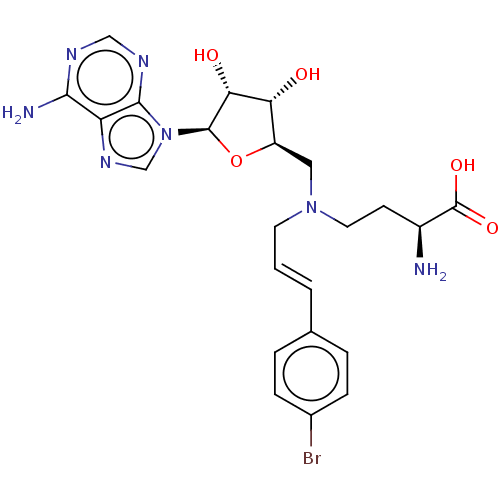
(CHEMBL5196219)Show SMILES N[C@@H](CCN(C\C=C\c1ccc(Br)cc1)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588089

(CHEMBL5175456)Show SMILES N[C@@H](CCN(CC#Cc1ccc(cc1)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50506011

(CHEMBL4436698)Show SMILES N[C@@H](CCN(CCCc1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H32N8O6/c25-15(24(36)37)6-8-31(7-2-4-13-3-1-5-14(9-13)21(27)35)10-16-18(33)19(34)23(38-16)32-12-30-17-20(26)28-11-29-22(17)32/h1,3,5,9,11-12,15-16,18-19,23,33-34H,2,4,6-8,10,25H2,(H2,27,35)(H,36,37)(H2,26,28,29)/t15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588076

(CHEMBL5183289)Show SMILES N[C@@H](CCN(C\C=C\c1ccc(F)cc1)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588079
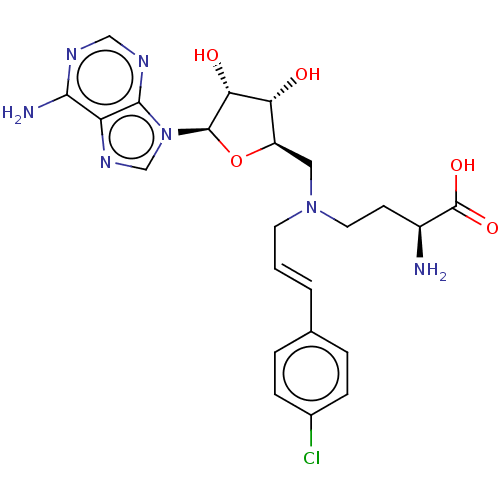
(CHEMBL5198886)Show SMILES N[C@@H](CCN(C\C=C\c1ccc(Cl)cc1)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588095

(CHEMBL5171598)Show SMILES N[C@@H](CCCN(C\C=C\c1ccc(cc1)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588081

(CHEMBL5209274)Show SMILES N[C@@H](CCN(C\C=C\c1cccc(Br)c1)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50445716

(CHEMBL3104317)Show SMILES Cc1ccc(\C=C2/CNCC3=C2N=C2SC=C(N2C3c2ccc(C)cc2)c2ccccc2)cc1 |c:10,16,t:13| Show InChI InChI=1S/C30H27N3S/c1-20-8-12-22(13-9-20)16-25-17-31-18-26-28(25)32-30-33(29(26)24-14-10-21(2)11-15-24)27(19-34-30)23-6-4-3-5-7-23/h3-16,19,29,31H,17-18H2,1-2H3/b25-16+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588049
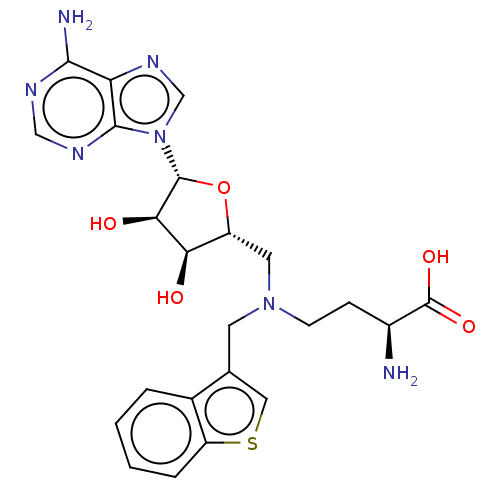
(CHEMBL5196381)Show SMILES N[C@@H](CCN(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)Cc1csc2ccccc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588078

(CHEMBL5205644)Show SMILES N[C@@H](CCN(C\C=C\c1cccc(Cl)c1)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50445693
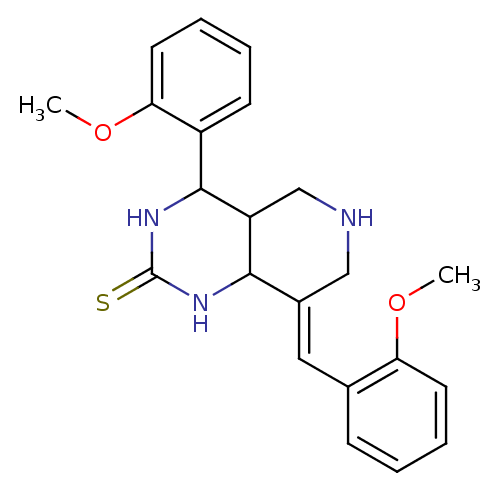
(CHEMBL3104448)Show InChI InChI=1S/C22H25N3O2S/c1-26-18-9-5-3-7-14(18)11-15-12-23-13-17-20(15)24-22(28)25-21(17)16-8-4-6-10-19(16)27-2/h3-11,17,20-21,23H,12-13H2,1-2H3,(H2,24,25,28)/b15-11+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588062

(CHEMBL5203440)Show SMILES N[C@@H](CCN(C\C=C\c1cccc(c1)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588099
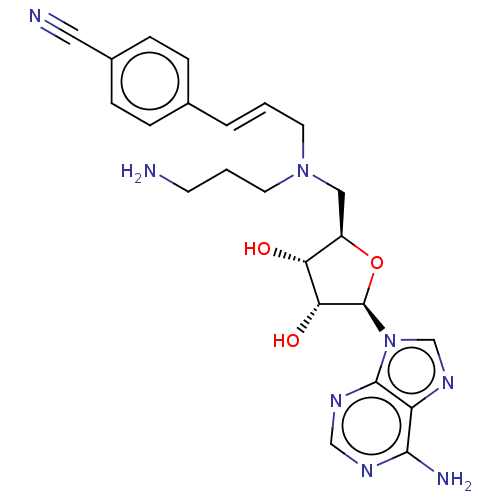
(CHEMBL5191939)Show SMILES NCCCN(C\C=C\c1ccc(cc1)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445693

(CHEMBL3104448)Show InChI InChI=1S/C22H25N3O2S/c1-26-18-9-5-3-7-14(18)11-15-12-23-13-17-20(15)24-22(28)25-21(17)16-8-4-6-10-19(16)27-2/h3-11,17,20-21,23H,12-13H2,1-2H3,(H2,24,25,28)/b15-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588068

(CHEMBL5189085)Show SMILES Cc1ccccc1\C=C\CN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588077
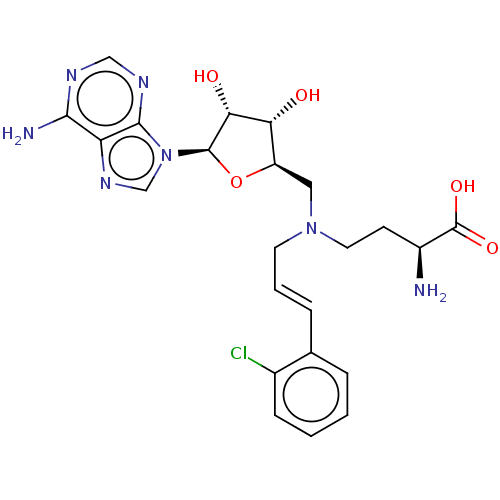
(CHEMBL5184681)Show SMILES N[C@@H](CCN(C\C=C\c1ccccc1Cl)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588088

(CHEMBL5204937)Show SMILES N[C@@H](CCN(CC#Cc1cccc(c1)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588080

(CHEMBL5194159)Show SMILES N[C@@H](CCN(C\C=C\c1ccccc1Br)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50445719
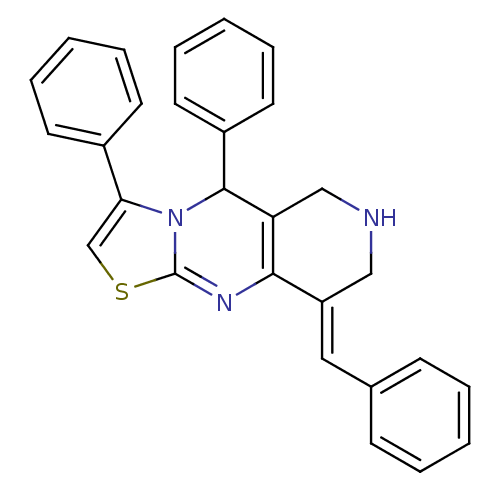
(CHEMBL3104452)Show SMILES C1NCC2=C(N=C3SC=C(N3C2c2ccccc2)c2ccccc2)\C1=C\c1ccccc1 |c:8,t:3,5| Show InChI InChI=1S/C28H23N3S/c1-4-10-20(11-5-1)16-23-17-29-18-24-26(23)30-28-31(27(24)22-14-8-3-9-15-22)25(19-32-28)21-12-6-2-7-13-21/h1-16,19,27,29H,17-18H2/b23-16+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588054

(CHEMBL5184031)Show SMILES N[C@@H](CCN(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)Cc1cccc2ccccc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50445715

(CHEMBL3104318)Show SMILES Clc1ccc(\C=C2/CNCC3=C2N=C2SC=C(N2C3c2ccc(Cl)cc2)c2ccccc2)cc1 |c:10,16,t:13| Show InChI InChI=1S/C28H21Cl2N3S/c29-22-10-6-18(7-11-22)14-21-15-31-16-24-26(21)32-28-33(27(24)20-8-12-23(30)13-9-20)25(17-34-28)19-4-2-1-3-5-19/h1-14,17,27,31H,15-16H2/b21-14+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445716

(CHEMBL3104317)Show SMILES Cc1ccc(\C=C2/CNCC3=C2N=C2SC=C(N2C3c2ccc(C)cc2)c2ccccc2)cc1 |c:10,16,t:13| Show InChI InChI=1S/C30H27N3S/c1-20-8-12-22(13-9-20)16-25-17-31-18-26-28(25)32-30-33(29(26)24-14-10-21(2)11-15-24)27(19-34-30)23-6-4-3-5-7-23/h3-16,19,29,31H,17-18H2,1-2H3/b25-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445719

(CHEMBL3104452)Show SMILES C1NCC2=C(N=C3SC=C(N3C2c2ccccc2)c2ccccc2)\C1=C\c1ccccc1 |c:8,t:3,5| Show InChI InChI=1S/C28H23N3S/c1-4-10-20(11-5-1)16-23-17-29-18-24-26(23)30-28-31(27(24)22-14-8-3-9-15-22)25(19-32-28)21-12-6-2-7-13-21/h1-16,19,27,29H,17-18H2/b23-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588096

(CHEMBL5191670)Show SMILES N[C@@H](CCN(C\C=C\c1ccc(cc1)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50445718
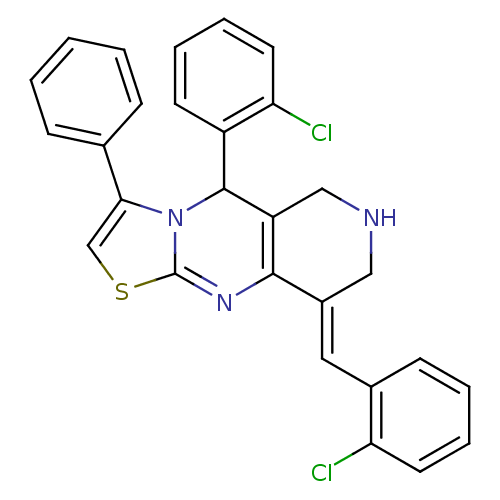
(CHEMBL3104453)Show SMILES Clc1ccccc1\C=C1/CNCC2=C1N=C1SC=C(N1C2c1ccccc1Cl)c1ccccc1 |c:13,19,t:16| Show InChI InChI=1S/C28H21Cl2N3S/c29-23-12-6-4-10-19(23)14-20-15-31-16-22-26(20)32-28-33(27(22)21-11-5-7-13-24(21)30)25(17-34-28)18-8-2-1-3-9-18/h1-14,17,27,31H,15-16H2/b20-14+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445715

(CHEMBL3104318)Show SMILES Clc1ccc(\C=C2/CNCC3=C2N=C2SC=C(N2C3c2ccc(Cl)cc2)c2ccccc2)cc1 |c:10,16,t:13| Show InChI InChI=1S/C28H21Cl2N3S/c29-22-10-6-18(7-11-22)14-21-15-31-16-24-26(21)32-28-33(27(24)20-8-12-23(30)13-9-20)25(17-34-28)19-4-2-1-3-5-19/h1-14,17,27,31H,15-16H2/b21-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50445721

(CHEMBL3104450)Show SMILES Clc1ccc(\C=C2/CNCC3C2NC(=S)NC3c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C20H19Cl2N3S/c21-15-5-1-12(2-6-15)9-14-10-23-11-17-18(24-20(26)25-19(14)17)13-3-7-16(22)8-4-13/h1-9,17-19,23H,10-11H2,(H2,24,25,26)/b14-9+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445718

(CHEMBL3104453)Show SMILES Clc1ccccc1\C=C1/CNCC2=C1N=C1SC=C(N1C2c1ccccc1Cl)c1ccccc1 |c:13,19,t:16| Show InChI InChI=1S/C28H21Cl2N3S/c29-23-12-6-4-10-19(23)14-20-15-31-16-22-26(20)32-28-33(27(22)21-11-5-7-13-24(21)30)25(17-34-28)18-8-2-1-3-9-18/h1-14,17,27,31H,15-16H2/b20-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588086
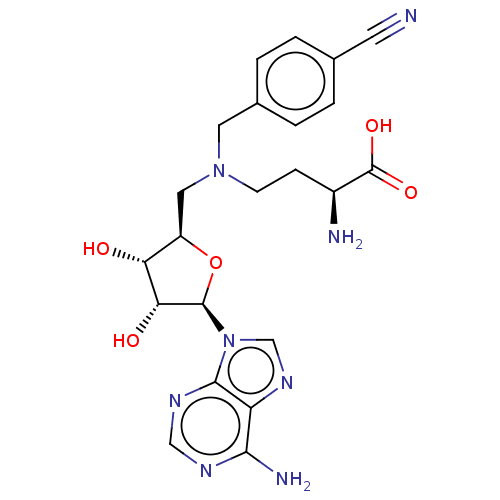
(CHEMBL5190850)Show SMILES N[C@@H](CCN(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)Cc1ccc(cc1)C#N)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588050

(CHEMBL5183652)Show SMILES N[C@@H](CCN(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)Cc1cc2ccccc2s1)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445694
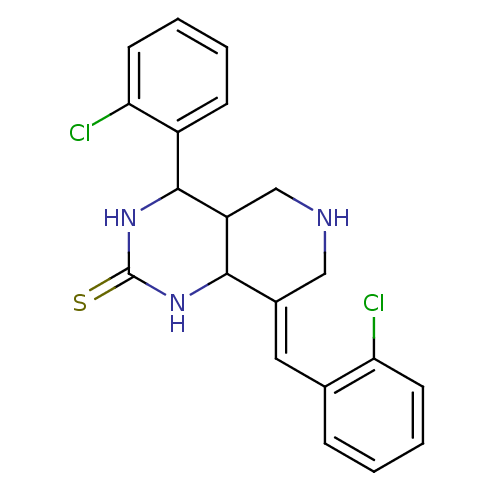
(CHEMBL3104447)Show InChI InChI=1S/C20H19Cl2N3S/c21-16-7-3-1-5-12(16)9-13-10-23-11-15-18(13)24-20(26)25-19(15)14-6-2-4-8-17(14)22/h1-9,15,18-19,23H,10-11H2,(H2,24,25,26)/b13-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588075

(CHEMBL5180479)Show SMILES N[C@@H](CCN(C\C=C\c1cccc(F)c1)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445695
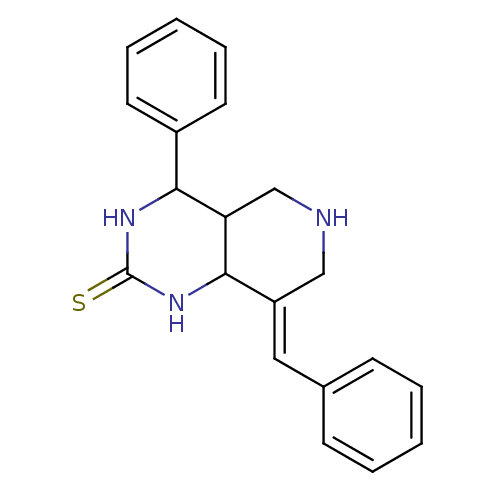
(CHEMBL3104446)Show InChI InChI=1S/C20H21N3S/c24-20-22-18(15-9-5-2-6-10-15)17-13-21-12-16(19(17)23-20)11-14-7-3-1-4-8-14/h1-11,17-19,21H,12-13H2,(H2,22,23,24)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM197173
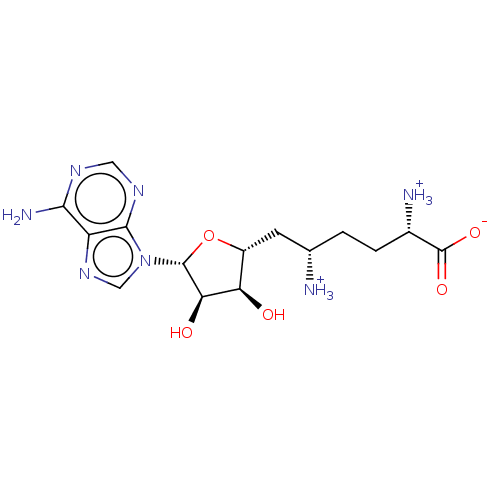
(Sinefungin)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C[C@@H]([NH3+])CC[C@H]([NH3+])C([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/p+1/t6-,7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445714
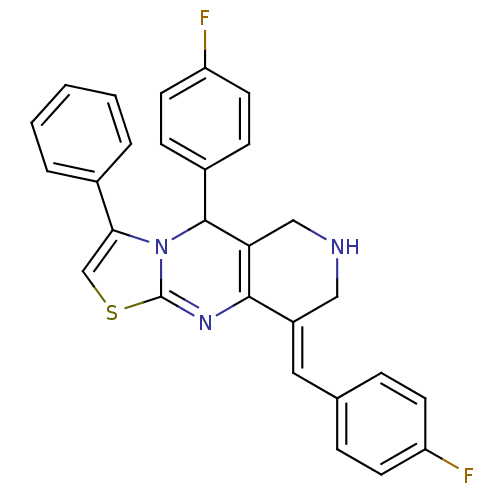
(CHEMBL3104319)Show SMILES Fc1ccc(\C=C2/CNCC3=C2N=C2SC=C(N2C3c2ccc(F)cc2)c2ccccc2)cc1 |c:10,16,t:13| Show InChI InChI=1S/C28H21F2N3S/c29-22-10-6-18(7-11-22)14-21-15-31-16-24-26(21)32-28-33(27(24)20-8-12-23(30)13-9-20)25(17-34-28)19-4-2-1-3-5-19/h1-14,17,27,31H,15-16H2/b21-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50588072

(CHEMBL5180115)Show SMILES COc1cccc(\C=C\CN(CC[C@H](N)C(O)=O)C[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01094
BindingDB Entry DOI: 10.7270/Q22V2M2Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50445714

(CHEMBL3104319)Show SMILES Fc1ccc(\C=C2/CNCC3=C2N=C2SC=C(N2C3c2ccc(F)cc2)c2ccccc2)cc1 |c:10,16,t:13| Show InChI InChI=1S/C28H21F2N3S/c29-22-10-6-18(7-11-22)14-21-15-31-16-24-26(21)32-28-33(27(24)20-8-12-23(30)13-9-20)25(17-34-28)19-4-2-1-3-5-19/h1-14,17,27,31H,15-16H2/b21-14+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50445717

(CHEMBL3104454)Show SMILES COc1ccccc1\C=C1/CNCC2=C1N=C1SC=C(N1C2c1ccccc1OC)c1ccccc1 |c:14,20,t:17| Show InChI InChI=1S/C30H27N3O2S/c1-34-26-14-8-6-12-21(26)16-22-17-31-18-24-28(22)32-30-33(25(19-36-30)20-10-4-3-5-11-20)29(24)23-13-7-9-15-27(23)35-2/h3-16,19,29,31H,17-18H2,1-2H3/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50445717

(CHEMBL3104454)Show SMILES COc1ccccc1\C=C1/CNCC2=C1N=C1SC=C(N1C2c1ccccc1OC)c1ccccc1 |c:14,20,t:17| Show InChI InChI=1S/C30H27N3O2S/c1-34-26-14-8-6-12-21(26)16-22-17-31-18-24-28(22)32-30-33(25(19-36-30)20-10-4-3-5-11-20)29(24)23-13-7-9-15-27(23)35-2/h3-16,19,29,31H,17-18H2,1-2H3/b22-16+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... |
Bioorg Med Chem 22: 906-16 (2014)
Article DOI: 10.1016/j.bmc.2013.11.020
BindingDB Entry DOI: 10.7270/Q23R0VBZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data