 Found 239 hits with Last Name = 'parthasarathy' and Initial = 's'
Found 239 hits with Last Name = 'parthasarathy' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017233
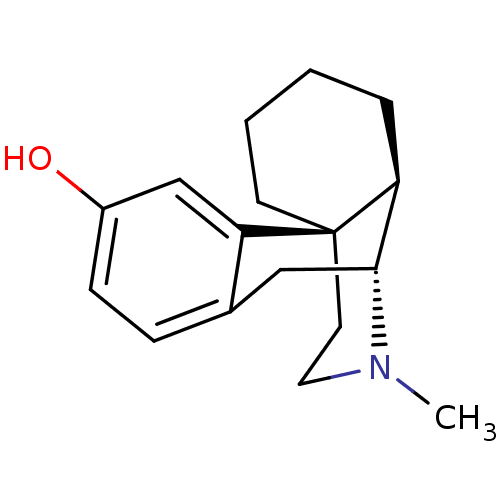
(CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL)Show SMILES CN1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(O)cc31 |r,TLB:0:1:12.18.11:9| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595702
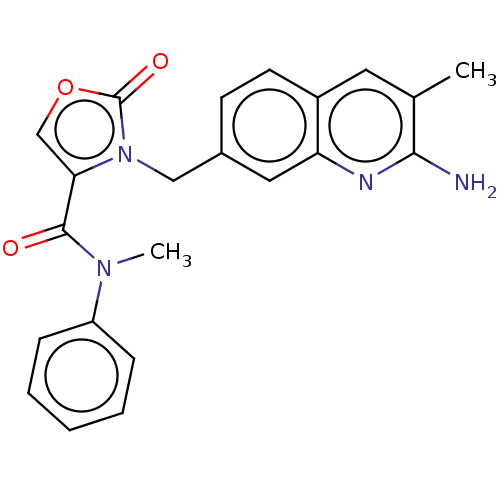
(CHEMBL5172637)Show SMILES CN(C(=O)c1coc(=O)n1Cc1ccc2cc(C)c(N)nc2c1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595695

(CHEMBL5172447)Show SMILES CN(C(=O)c1cn(C)c(=O)n1Cc1ccc2cc(C)c(N)nc2c1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595698

(CHEMBL5174634)Show SMILES CN(C(=O)c1nn(C)c(=O)n1Cc1ccc2cc(C)c(N)nc2c1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50271974
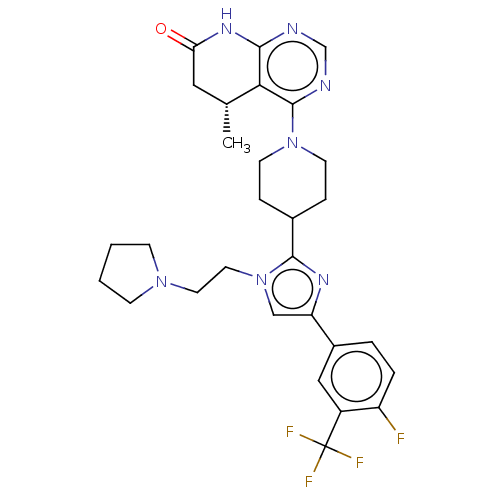
(CHEMBL4128457)Show SMILES C[C@@H]1CC(=O)Nc2ncnc(N3CCC(CC3)c3nc(cn3CCN3CCCC3)-c3ccc(F)c(c3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C29H33F4N7O/c1-18-14-24(41)37-26-25(18)28(35-17-34-26)39-10-6-19(7-11-39)27-36-23(16-40(27)13-12-38-8-2-3-9-38)20-4-5-22(30)21(15-20)29(31,32)33/h4-5,15-19H,2-3,6-14H2,1H3,(H,34,35,37,41)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKT1 expressed in insect cells incubated for 60 mins measured at apparent ATP Km level by kinase ADP-FP assay |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50271976

(LY 2584702 | LY-2584702 | LY2584702)Show SMILES Cn1cc(nc1C1CCN(CC1)c1ncnc2[nH]ncc12)-c1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C21H19F4N7/c1-31-10-17(13-2-3-16(22)15(8-13)21(23,24)25)29-19(31)12-4-6-32(7-5-12)20-14-9-28-30-18(14)26-11-27-20/h2-3,8-12H,4-7H2,1H3,(H,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human p70S6K measured at apparent ATP Km level |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50271973

(CHEMBL4126067)Show SMILES C[C@@H]1CC(=O)Nc2ncnc(N3CCC(CC3)c3nc(cn3CCN3CCCC3)C3CCOCC3)c12 |r| Show InChI InChI=1S/C27H39N7O2/c1-19-16-23(35)31-25-24(19)27(29-18-28-25)33-10-4-21(5-11-33)26-30-22(20-6-14-36-15-7-20)17-34(26)13-12-32-8-2-3-9-32/h17-21H,2-16H2,1H3,(H,28,29,31,35)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKT1 expressed in insect cells incubated for 60 mins measured at apparent ATP Km level by kinase ADP-FP assay |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50017233

(CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL)Show SMILES CN1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(O)cc31 |r,TLB:0:1:12.18.11:9| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
In vitro binding affinity against cloned human Opioid receptor kappa 1 expressed in HEK 293S cells |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50271964
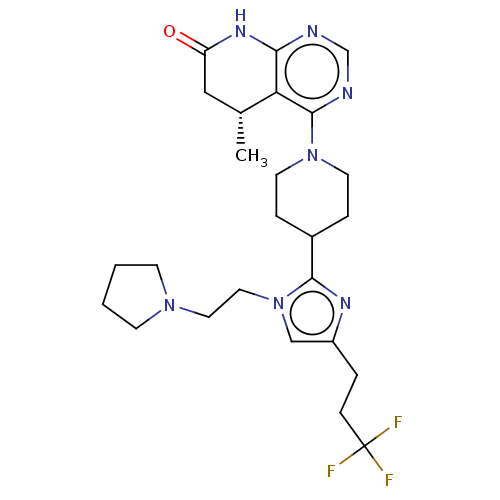
(CHEMBL4129379)Show SMILES C[C@@H]1CC(=O)Nc2ncnc(N3CCC(CC3)c3nc(CCC(F)(F)F)cn3CCN3CCCC3)c12 |r| Show InChI InChI=1S/C25H34F3N7O/c1-17-14-20(36)32-22-21(17)24(30-16-29-22)34-10-5-18(6-11-34)23-31-19(4-7-25(26,27)28)15-35(23)13-12-33-8-2-3-9-33/h15-18H,2-14H2,1H3,(H,29,30,32,36)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKT1 expressed in insect cells incubated for 60 mins measured at apparent ATP Km level by kinase ADP-FP assay |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50017233

(CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL)Show SMILES CN1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(O)cc31 |r,TLB:0:1:12.18.11:9| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
In vitro binding affinity against cloned human Opioid receptor delta 1 expressed in HEK 293S cells |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50122542
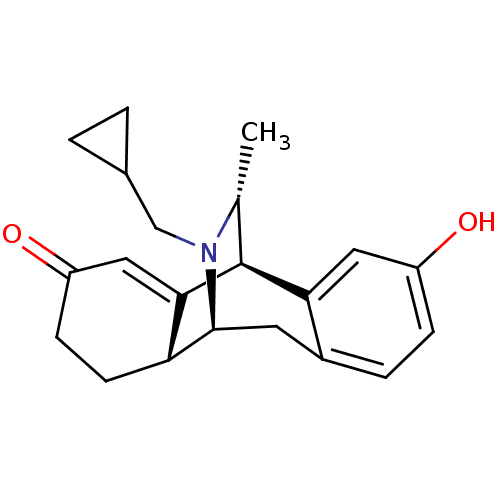
(17-cyclopropylmethyl-16-methyl-17-azatetracyclo[7....)Show SMILES C[C@@H]1[C@@H]2C3=CC(=O)CC[C@H]3[C@H](Cc3ccc(O)cc23)N1CC1CC1 |wU:2.2,10.21,9.8,1.0,t:3,TLB:0:1:3.9:18.12.11,17:18:19.1:3.9,THB:13:12:19.1:3.9,20:19:3.9:18.12.11,8:9:19.1:18.12.11,(9.83,-1.73,;8.73,-2.83,;4.91,-1.57,;4.91,-.02,;4.91,1.53,;6.24,2.3,;6.24,3.84,;7.57,1.53,;7.57,-.02,;6.24,-.79,;6.45,-4.65,;4.91,-4.65,;3.58,-3.88,;2.25,-4.65,;.91,-3.88,;.91,-2.34,;-.44,-1.55,;2.25,-1.57,;3.58,-2.34,;7.89,-4.11,;8.85,-5.3,;8.29,-6.75,;7.09,-7.7,;8.52,-8.27,)| Show InChI InChI=1S/C21H25NO2/c1-12-21-18-9-15(23)5-4-14(18)8-20(22(12)11-13-2-3-13)17-7-6-16(24)10-19(17)21/h4-5,9-10,12-13,17,20-21,23H,2-3,6-8,11H2,1H3/t12-,17-,20+,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50122549
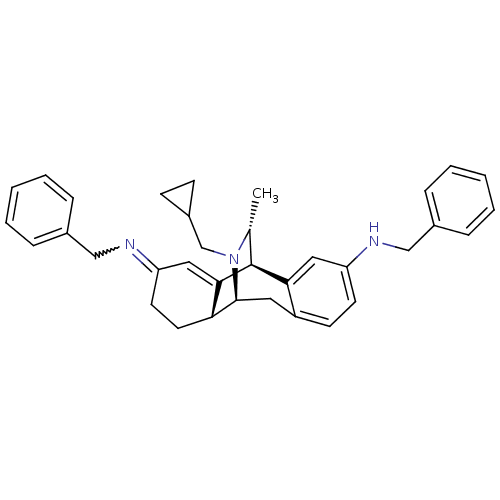
(4N,13N-dibenzyl-17-cyclopropylmethyl-16-methyl-17-...)Show SMILES C[C@@H]1[C@@H]2C3=CC(CC[C@H]3[C@H](Cc3ccc(NCc4ccccc4)cc23)N1CC1CC1)=NCc1ccccc1 |w:30.36,wU:2.2,9.28,8.7,1.0,t:3,TLB:0:1:3.8:24.11.10,23:24:25.1:3.8,THB:12:11:25.1:3.8,26:25:3.8:24.11.10,7:8:25.1:24.11.10,(11.14,-1.71,;10.05,-2.81,;6.2,-1.55,;6.2,,;6.2,1.56,;7.54,2.33,;8.88,1.56,;8.88,,;7.54,-.77,;7.76,-4.64,;6.2,-4.64,;4.87,-3.87,;3.54,-4.64,;2.21,-3.87,;2.21,-2.32,;.85,-1.53,;-.49,-2.29,;-.49,-3.83,;-1.84,-4.59,;-1.84,-6.13,;-.51,-6.9,;.83,-6.13,;.83,-4.59,;3.54,-1.55,;4.87,-2.32,;9.2,-4.09,;10.15,-5.29,;9.59,-6.73,;8.4,-7.69,;9.84,-8.25,;7.54,3.87,;6.2,4.64,;4.87,3.87,;3.55,4.64,;2.23,3.9,;2.23,2.35,;3.56,1.59,;4.87,2.36,)| Show InChI InChI=1S/C35H39N3/c1-24-35-32-19-29(36-21-25-8-4-2-5-9-25)15-14-28(32)18-34(38(24)23-27-12-13-27)31-17-16-30(20-33(31)35)37-22-26-10-6-3-7-11-26/h2-11,14-15,19-20,24,27,31,34-36H,12-13,16-18,21-23H2,1H3/t24-,31-,34+,35+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50122523
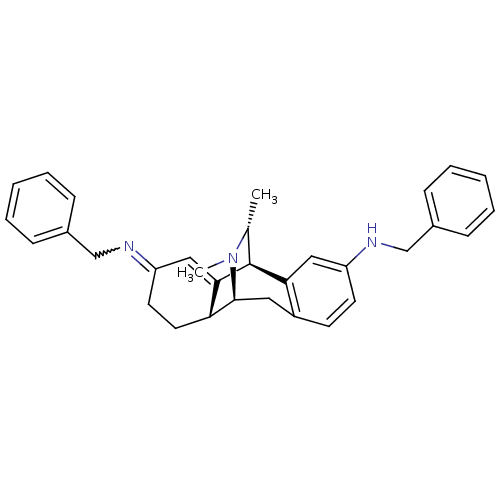
(4N,13N-dibenzyl-16,17-dimethyl-17-azatetracyclo[7....)Show SMILES C[C@@H]1[C@@H]2C3=CC(CC[C@H]3[C@H](Cc3ccc(NCc4ccccc4)cc23)N1C)=NCc1ccccc1 |w:27.32,wU:2.2,9.28,8.8,1.0,t:3,TLB:0:1:3.8:24.11.10,23:24:25.1:3.8,THB:26:25:3.8:24.11.10,7:8:25.1:24.11.10,12:11:25.1:3.8,(11.14,-2.39,;10.05,-3.49,;6.2,-2.23,;6.2,-.68,;6.2,.88,;7.54,1.65,;8.88,.88,;8.88,-.68,;7.54,-1.45,;7.76,-5.32,;6.2,-5.32,;4.87,-4.54,;3.54,-5.32,;2.21,-4.54,;2.21,-3,;.85,-2.21,;-.49,-2.97,;-.49,-4.51,;-1.84,-5.27,;-1.84,-6.8,;-.51,-7.58,;.83,-6.8,;.83,-5.27,;3.54,-2.23,;4.87,-3,;9.2,-4.77,;10.16,-5.97,;7.54,3.2,;6.2,3.97,;4.87,3.2,;4.87,1.68,;3.56,.91,;2.23,1.68,;2.23,3.2,;3.55,3.97,)| Show InChI InChI=1S/C32H35N3/c1-22-32-29-18-26(33-20-23-9-5-3-6-10-23)14-13-25(29)17-31(35(22)2)28-16-15-27(19-30(28)32)34-21-24-11-7-4-8-12-24/h3-14,18-19,22,28,31-33H,15-17,20-21H2,1-2H3/t22-,28-,31+,32+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595696

(CHEMBL5197027)Show SMILES CN(C(=O)c1cn(C)c(=O)n1Cc1ccc2cc(C)c(N)nc2c1)c1ccccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50271975
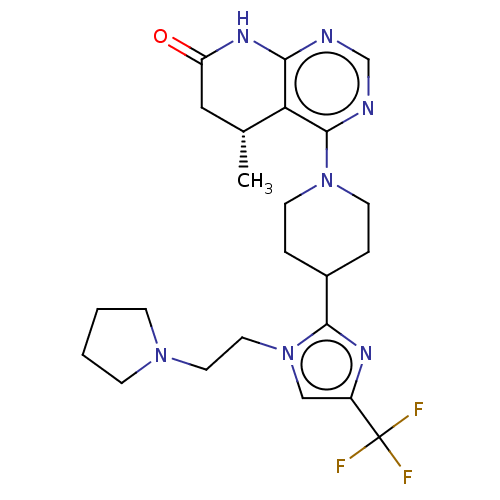
(CHEMBL4129817)Show SMILES C[C@@H]1CC(=O)Nc2ncnc(N3CCC(CC3)c3nc(cn3CCN3CCCC3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C23H30F3N7O/c1-15-12-18(34)30-20-19(15)22(28-14-27-20)32-8-4-16(5-9-32)21-29-17(23(24,25)26)13-33(21)11-10-31-6-2-3-7-31/h13-16H,2-12H2,1H3,(H,27,28,30,34)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKT1 expressed in insect cells incubated for 60 mins measured at apparent ATP Km level by kinase ADP-FP assay |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381390
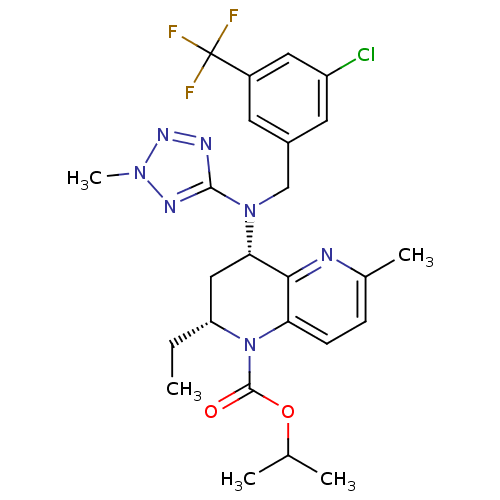
(CHEMBL2017388)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(Cl)cc(c2)C(F)(F)F)c2nnn(C)n2)c2nc(C)ccc2N1C(=O)OC(C)C |r| Show InChI InChI=1S/C25H29ClF3N7O2/c1-6-19-12-21(22-20(8-7-15(4)30-22)36(19)24(37)38-14(2)3)35(23-31-33-34(5)32-23)13-16-9-17(25(27,28)29)11-18(26)10-16/h7-11,14,19,21H,6,12-13H2,1-5H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595705

(CHEMBL5192334)Show SMILES CN(C(=O)c1ccnn1Cc1ccc2cc(C)c(N)nc2c1)c1ncccc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50122548
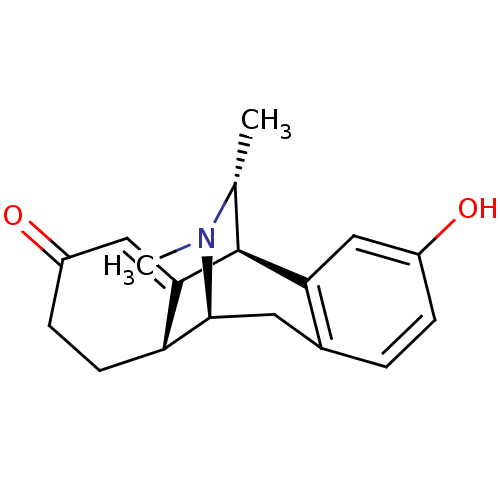
(16,17-dimethyl-17-azatetracyclo[7.6.2.02,7.010,15]...)Show SMILES C[C@@H]1[C@@H]2C3=CC(=O)CC[C@H]3[C@H](Cc3ccc(O)cc23)N1C |wU:2.2,10.21,9.9,1.0,t:3,TLB:0:1:3.9:18.12.11,17:18:19.1:3.9,THB:20:19:3.9:18.12.11,8:9:19.1:18.12.11,13:12:19.1:3.9,(9.83,-3.2,;8.73,-4.3,;4.91,-3.04,;4.91,-1.5,;4.91,.05,;6.24,.82,;6.24,2.36,;7.57,.05,;7.57,-1.5,;6.24,-2.27,;6.45,-6.14,;4.91,-6.14,;3.58,-5.37,;2.25,-6.14,;.91,-5.37,;.91,-3.83,;-.44,-3.03,;2.25,-3.04,;3.58,-3.81,;7.89,-5.58,;8.85,-6.79,)| Show InChI InChI=1S/C18H21NO2/c1-10-18-15-8-12(20)4-3-11(15)7-17(19(10)2)14-6-5-13(21)9-16(14)18/h3-4,8-10,14,17-18,20H,5-7H2,1-2H3/t10-,14-,17+,18+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595700
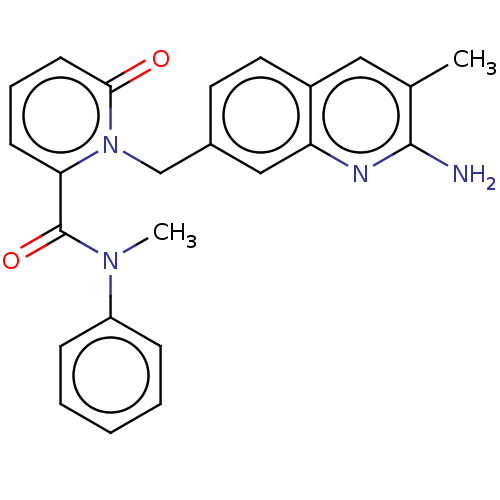
(CHEMBL5175731)Show SMILES CN(C(=O)c1cccc(=O)n1Cc1ccc2cc(C)c(N)nc2c1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381389
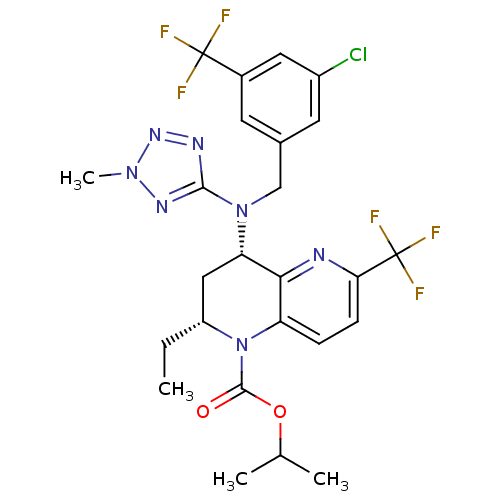
(CHEMBL2017387)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(Cl)cc(c2)C(F)(F)F)c2nnn(C)n2)c2nc(ccc2N1C(=O)OC(C)C)C(F)(F)F |r| Show InChI InChI=1S/C25H26ClF6N7O2/c1-5-17-11-19(21-18(39(17)23(40)41-13(2)3)6-7-20(33-21)25(30,31)32)38(22-34-36-37(4)35-22)12-14-8-15(24(27,28)29)10-16(26)9-14/h6-10,13,17,19H,5,11-12H2,1-4H3/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50271974

(CHEMBL4128457)Show SMILES C[C@@H]1CC(=O)Nc2ncnc(N3CCC(CC3)c3nc(cn3CCN3CCCC3)-c3ccc(F)c(c3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C29H33F4N7O/c1-18-14-24(41)37-26-25(18)28(35-17-34-26)39-10-6-19(7-11-39)27-36-23(16-40(27)13-12-38-8-2-3-9-38)20-4-5-22(30)21(15-20)29(31,32)33/h4-5,15-19H,2-3,6-14H2,1H3,(H,34,35,37,41)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human RSK1 measured at apparent ATP Km level |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381391

(CHEMBL2017389)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C#N)c2nnn(C)n2)c2nc(C)ccc2N1C(=O)OC(C)C |r| Show InChI InChI=1S/C26H29F3N8O2/c1-6-20-12-22(23-21(8-7-16(4)31-23)37(20)25(38)39-15(2)3)36(24-32-34-35(5)33-24)14-18-9-17(13-30)10-19(11-18)26(27,28)29/h7-11,15,20,22H,6,12,14H2,1-5H3/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595699
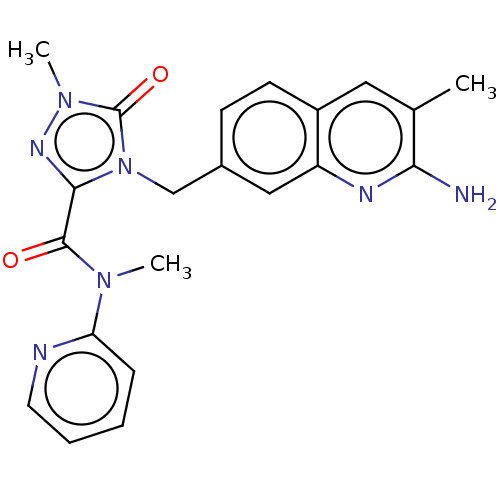
(CHEMBL5185584)Show SMILES CN(C(=O)c1nn(C)c(=O)n1Cc1ccc2cc(C)c(N)nc2c1)c1ccccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381388

(CHEMBL2017386)Show SMILES CCOC(=O)N1[C@H](CC)C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(C)n2)c2nc(cc(C)c12)C(F)(F)F |r| Show InChI InChI=1S/C26H26F9N7O2/c1-5-17-11-18(20-21(42(17)23(43)44-6-2)13(3)7-19(36-20)26(33,34)35)41(22-37-39-40(4)38-22)12-14-8-15(24(27,28)29)10-16(9-14)25(30,31)32/h7-10,17-18H,5-6,11-12H2,1-4H3/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381417
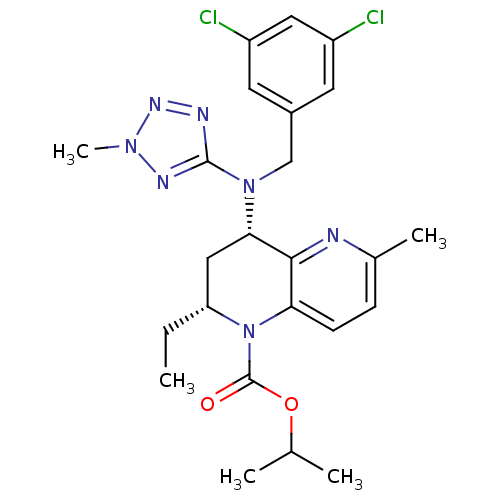
(CHEMBL2017391)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(Cl)cc(Cl)c2)c2nnn(C)n2)c2nc(C)ccc2N1C(=O)OC(C)C |r| Show InChI InChI=1S/C24H29Cl2N7O2/c1-6-19-12-21(22-20(8-7-15(4)27-22)33(19)24(34)35-14(2)3)32(23-28-30-31(5)29-23)13-16-9-17(25)11-18(26)10-16/h7-11,14,19,21H,6,12-13H2,1-5H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381415
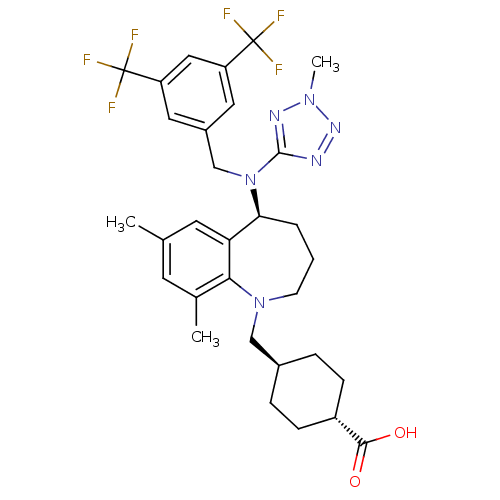
(EVACETRAPIB | LY2484595)Show SMILES Cc1cc(C)c2N(C[C@H]3CC[C@@H](CC3)C(O)=O)CCC[C@H](N(Cc3cc(cc(c3)C(F)(F)F)C(F)(F)F)c3nnn(C)n3)c2c1 |r,wU:20.21,11.14,wD:8.7,(-5.45,-18.22,;-4.12,-17.45,;-2.78,-18.22,;-1.45,-17.44,;-.11,-18.2,;-1.45,-15.91,;-.01,-15.34,;1.12,-16.38,;2.59,-15.92,;3.91,-16.71,;5.25,-15.96,;5.27,-14.42,;3.95,-13.63,;2.6,-14.38,;6.62,-13.68,;7.94,-14.47,;6.65,-12.14,;.44,-13.86,;-.45,-12.58,;-1.99,-12.47,;-3.03,-13.61,;-4.36,-12.84,;-4.37,-11.31,;-5.7,-10.54,;-5.7,-8.99,;-7.04,-8.22,;-8.37,-8.99,;-8.37,-10.54,;-7.04,-11.31,;-9.7,-11.31,;-11.04,-10.54,;-9.7,-12.85,;-11.05,-12.07,;-7.04,-6.69,;-5.71,-5.91,;-8.38,-5.92,;-7.06,-5.14,;-5.69,-13.62,;-7.1,-12.98,;-8.13,-14.13,;-7.35,-15.46,;-7.98,-16.87,;-5.85,-15.14,;-2.79,-15.14,;-4.12,-15.91,)| Show InChI InChI=1S/C31H36F6N6O2/c1-18-11-19(2)27-25(12-18)26(5-4-10-42(27)16-20-6-8-22(9-7-20)28(44)45)43(29-38-40-41(3)39-29)17-21-13-23(30(32,33)34)15-24(14-21)31(35,36)37/h11-15,20,22,26H,4-10,16-17H2,1-3H3,(H,44,45)/t20-,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381395

(CHEMBL2017394)Show SMILES CCOC(=O)N1[C@H](CC)C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(C)n2)c2nc(ccc12)C(F)(F)F |r| Show InChI InChI=1S/C25H24F9N7O2/c1-4-16-11-18(20-17(41(16)22(42)43-5-2)6-7-19(35-20)25(32,33)34)40(21-36-38-39(3)37-21)12-13-8-14(23(26,27)28)10-15(9-13)24(29,30)31/h6-10,16,18H,4-5,11-12H2,1-3H3/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595697
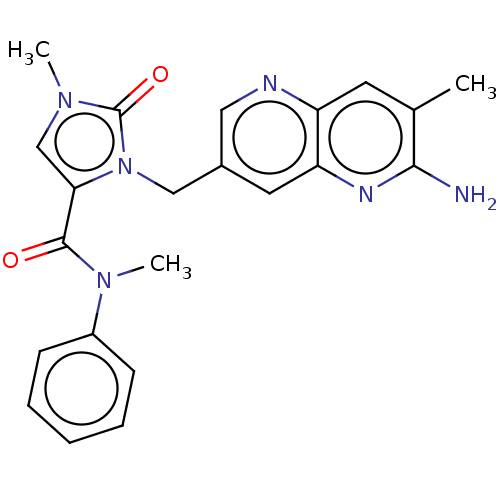
(CHEMBL5171279)Show SMILES CN(C(=O)c1cn(C)c(=O)n1Cc1cnc2cc(C)c(N)nc2c1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381392
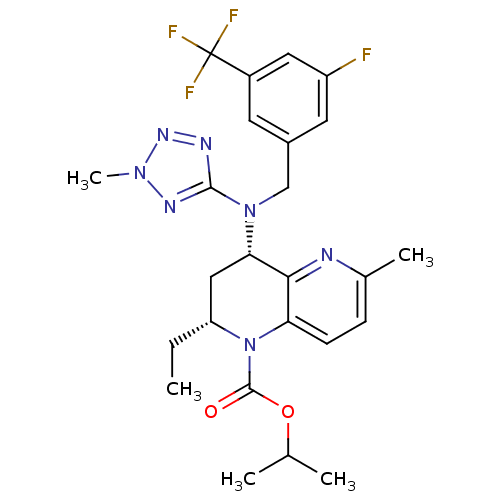
(CHEMBL2017390)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(F)cc(c2)C(F)(F)F)c2nnn(C)n2)c2nc(C)ccc2N1C(=O)OC(C)C |r| Show InChI InChI=1S/C25H29F4N7O2/c1-6-19-12-21(22-20(8-7-15(4)30-22)36(19)24(37)38-14(2)3)35(23-31-33-34(5)32-23)13-16-9-17(25(27,28)29)11-18(26)10-16/h7-11,14,19,21H,6,12-13H2,1-5H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50122549

(4N,13N-dibenzyl-17-cyclopropylmethyl-16-methyl-17-...)Show SMILES C[C@@H]1[C@@H]2C3=CC(CC[C@H]3[C@H](Cc3ccc(NCc4ccccc4)cc23)N1CC1CC1)=NCc1ccccc1 |w:30.36,wU:2.2,9.28,8.7,1.0,t:3,TLB:0:1:3.8:24.11.10,23:24:25.1:3.8,THB:12:11:25.1:3.8,26:25:3.8:24.11.10,7:8:25.1:24.11.10,(11.14,-1.71,;10.05,-2.81,;6.2,-1.55,;6.2,,;6.2,1.56,;7.54,2.33,;8.88,1.56,;8.88,,;7.54,-.77,;7.76,-4.64,;6.2,-4.64,;4.87,-3.87,;3.54,-4.64,;2.21,-3.87,;2.21,-2.32,;.85,-1.53,;-.49,-2.29,;-.49,-3.83,;-1.84,-4.59,;-1.84,-6.13,;-.51,-6.9,;.83,-6.13,;.83,-4.59,;3.54,-1.55,;4.87,-2.32,;9.2,-4.09,;10.15,-5.29,;9.59,-6.73,;8.4,-7.69,;9.84,-8.25,;7.54,3.87,;6.2,4.64,;4.87,3.87,;3.55,4.64,;2.23,3.9,;2.23,2.35,;3.56,1.59,;4.87,2.36,)| Show InChI InChI=1S/C35H39N3/c1-24-35-32-19-29(36-21-25-8-4-2-5-9-25)15-14-28(32)18-34(38(24)23-27-12-13-27)31-17-16-30(20-33(31)35)37-22-26-10-6-3-7-11-26/h2-11,14-15,19-20,24,27,31,34-36H,12-13,16-18,21-23H2,1H3/t24-,31-,34+,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
In vitro binding affinity against cloned human Opioid receptor kappa 1 expressed in HEK 293S cells |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381429

(CHEMBL2017203)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(C)n2)c2nc(ccc2N1C(=O)OC(C)C)N(C)C |r| Show InChI InChI=1S/C27H32F6N8O2/c1-7-19-13-21(23-20(8-9-22(34-23)38(4)5)41(19)25(42)43-15(2)3)40(24-35-37-39(6)36-24)14-16-10-17(26(28,29)30)12-18(11-16)27(31,32)33/h8-12,15,19,21H,7,13-14H2,1-6H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50271974

(CHEMBL4128457)Show SMILES C[C@@H]1CC(=O)Nc2ncnc(N3CCC(CC3)c3nc(cn3CCN3CCCC3)-c3ccc(F)c(c3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C29H33F4N7O/c1-18-14-24(41)37-26-25(18)28(35-17-34-26)39-10-6-19(7-11-39)27-36-23(16-40(27)13-12-38-8-2-3-9-38)20-4-5-22(30)21(15-20)29(31,32)33/h4-5,15-19H,2-3,6-14H2,1H3,(H,34,35,37,41)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human p70S6K measured at apparent ATP Km level |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50271974

(CHEMBL4128457)Show SMILES C[C@@H]1CC(=O)Nc2ncnc(N3CCC(CC3)c3nc(cn3CCN3CCCC3)-c3ccc(F)c(c3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C29H33F4N7O/c1-18-14-24(41)37-26-25(18)28(35-17-34-26)39-10-6-19(7-11-39)27-36-23(16-40(27)13-12-38-8-2-3-9-38)20-4-5-22(30)21(15-20)29(31,32)33/h4-5,15-19H,2-3,6-14H2,1H3,(H,34,35,37,41)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human PKCbeta2 measured at apparent ATP Km level |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381437

(CHEMBL2017384)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(C)n2)c2nc(OC)c(C)cc2N1C(=O)OC(C)C |r| Show InChI InChI=1S/C27H31F6N7O3/c1-7-19-12-20(22-21(8-15(4)23(34-22)42-6)40(19)25(41)43-14(2)3)39(24-35-37-38(5)36-24)13-16-9-17(26(28,29)30)11-18(10-16)27(31,32)33/h8-11,14,19-20H,7,12-13H2,1-6H3/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595701

(CHEMBL5203872)Show SMILES CN(C(=O)c1nccc(=O)n1Cc1ccc2cc(C)c(N)nc2c1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50271974

(CHEMBL4128457)Show SMILES C[C@@H]1CC(=O)Nc2ncnc(N3CCC(CC3)c3nc(cn3CCN3CCCC3)-c3ccc(F)c(c3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C29H33F4N7O/c1-18-14-24(41)37-26-25(18)28(35-17-34-26)39-10-6-19(7-11-39)27-36-23(16-40(27)13-12-38-8-2-3-9-38)20-4-5-22(30)21(15-20)29(31,32)33/h4-5,15-19H,2-3,6-14H2,1H3,(H,34,35,37,41)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 in human U87MG cells assessed as reduction in GSK3beta Ser9 phosphorylation incubated for 1 hr by alpha screen Surefire assay |
Bioorg Med Chem Lett 28: 1887-1891 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.092
BindingDB Entry DOI: 10.7270/Q2GF0X0M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50312718
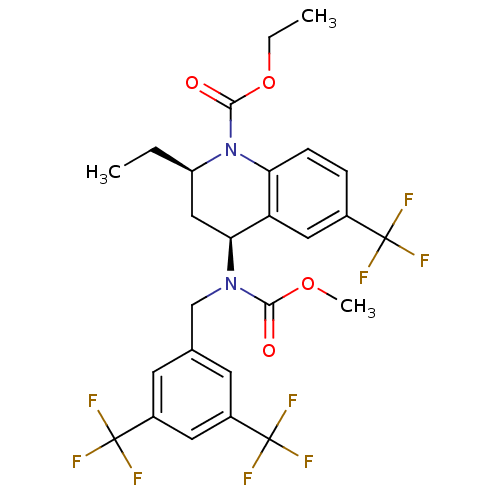
(CHEMBL479527 | torcetrapib)Show SMILES CCOC(=O)N1[C@H](CC)C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)C(=O)OC)c2cc(ccc12)C(F)(F)F |r| Show InChI InChI=1S/C26H25F9N2O4/c1-4-18-12-21(19-11-15(24(27,28)29)6-7-20(19)37(18)23(39)41-5-2)36(22(38)40-3)13-14-8-16(25(30,31)32)10-17(9-14)26(33,34)35/h6-11,18,21H,4-5,12-13H2,1-3H3/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50122526
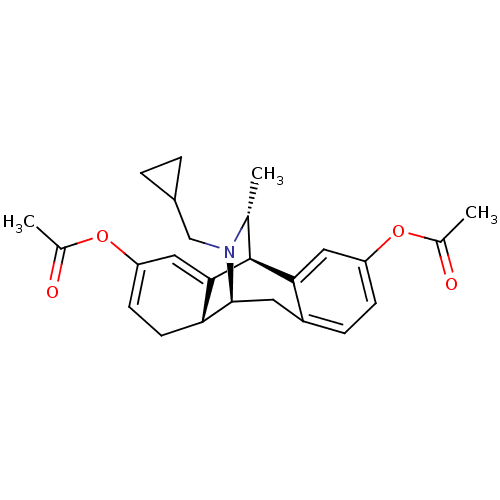
(17-cyclopropylmethyl-16-methyl-13-methylcarbonylox...)Show SMILES C[C@@H]1[C@@H]2C3=CC(OC(C)=O)=CC[C@H]3[C@H](Cc3ccc(OC(C)=O)cc23)N1CC1CC1 |wU:2.2,13.27,12.11,1.0,c:9,t:3,TLB:0:1:3.12:24.15.14,23:24:25.1:3.12,THB:16:15:25.1:3.12,26:25:3.12:24.15.14,11:12:25.1:24.15.14,(10.46,-2.45,;9.36,-3.55,;5.54,-2.29,;5.54,-.73,;5.54,.81,;6.87,1.58,;6.87,3.12,;8.2,3.9,;8.2,5.44,;9.53,3.13,;8.2,.81,;8.2,-.73,;6.87,-1.5,;7.08,-5.37,;5.54,-5.37,;4.21,-4.6,;2.88,-5.37,;1.54,-4.6,;1.54,-3.06,;.19,-2.27,;.19,-.73,;1.54,.04,;-1.14,.06,;2.88,-2.29,;4.21,-3.06,;8.52,-4.81,;9.48,-6.03,;8.92,-7.47,;7.72,-8.41,;9.15,-8.98,)| Show InChI InChI=1S/C25H29NO4/c1-14-25-22-11-19(29-15(2)27)7-6-18(22)10-24(26(14)13-17-4-5-17)21-9-8-20(12-23(21)25)30-16(3)28/h6-8,11-12,14,17,21,24-25H,4-5,9-10,13H2,1-3H3/t14-,21-,24+,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381428
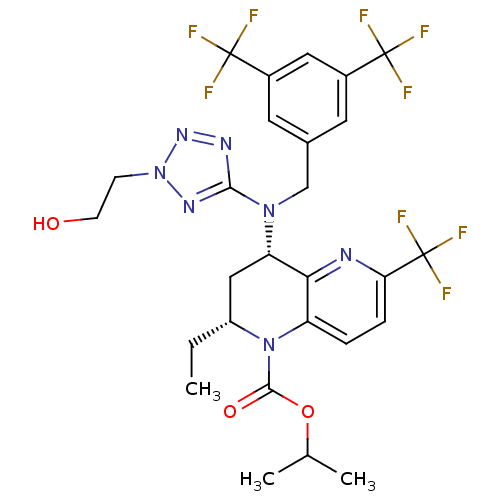
(CHEMBL2017200)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(CCO)n2)c2nc(ccc2N1C(=O)OC(C)C)C(F)(F)F |r| Show InChI InChI=1S/C27H28F9N7O3/c1-4-18-12-20(22-19(43(18)24(45)46-14(2)3)5-6-21(37-22)27(34,35)36)41(23-38-40-42(39-23)7-8-44)13-15-9-16(25(28,29)30)11-17(10-15)26(31,32)33/h5-6,9-11,14,18,20,44H,4,7-8,12-13H2,1-3H3/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50348228
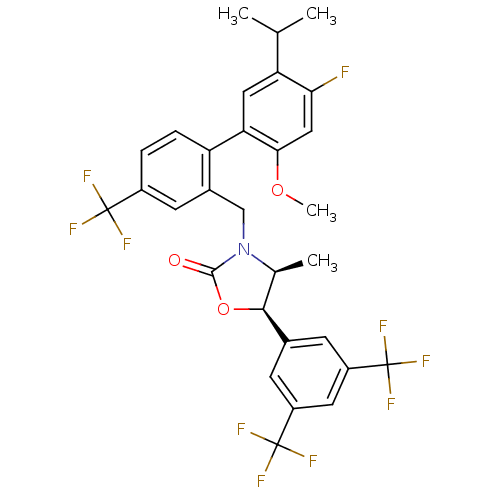
(CHEMBL1800807)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO3/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-18(28(32,33)34)9-17(21)13-41-15(3)26(44-27(41)42)16-7-19(29(35,36)37)10-20(8-16)30(38,39)40/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381425
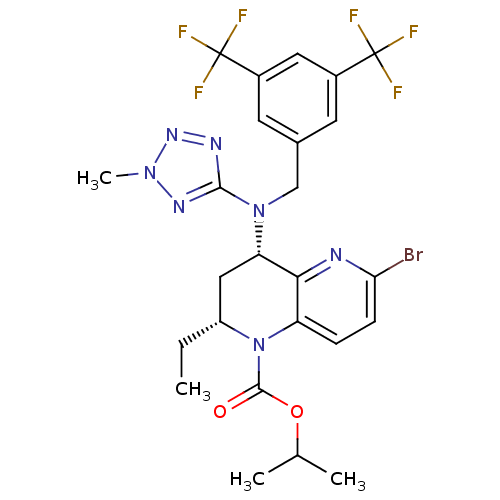
(CHEMBL2017197)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(C)n2)c2nc(Br)ccc2N1C(=O)OC(C)C |r| Show InChI InChI=1S/C25H26BrF6N7O2/c1-5-17-11-19(21-18(6-7-20(26)33-21)39(17)23(40)41-13(2)3)38(22-34-36-37(4)35-22)12-14-8-15(24(27,28)29)10-16(9-14)25(30,31)32/h6-10,13,17,19H,5,11-12H2,1-4H3/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381424

(CHEMBL2017195)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(C)n2)c2nc(ccc2N1C(=O)OC(C)C)C(F)(F)F |r| Show InChI InChI=1S/C26H26F9N7O2/c1-5-17-11-19(21-18(42(17)23(43)44-13(2)3)6-7-20(36-21)26(33,34)35)41(22-37-39-40(4)38-22)12-14-8-15(24(27,28)29)10-16(9-14)25(30,31)32/h6-10,13,17,19H,5,11-12H2,1-4H3/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50122549

(4N,13N-dibenzyl-17-cyclopropylmethyl-16-methyl-17-...)Show SMILES C[C@@H]1[C@@H]2C3=CC(CC[C@H]3[C@H](Cc3ccc(NCc4ccccc4)cc23)N1CC1CC1)=NCc1ccccc1 |w:30.36,wU:2.2,9.28,8.7,1.0,t:3,TLB:0:1:3.8:24.11.10,23:24:25.1:3.8,THB:12:11:25.1:3.8,26:25:3.8:24.11.10,7:8:25.1:24.11.10,(11.14,-1.71,;10.05,-2.81,;6.2,-1.55,;6.2,,;6.2,1.56,;7.54,2.33,;8.88,1.56,;8.88,,;7.54,-.77,;7.76,-4.64,;6.2,-4.64,;4.87,-3.87,;3.54,-4.64,;2.21,-3.87,;2.21,-2.32,;.85,-1.53,;-.49,-2.29,;-.49,-3.83,;-1.84,-4.59,;-1.84,-6.13,;-.51,-6.9,;.83,-6.13,;.83,-4.59,;3.54,-1.55,;4.87,-2.32,;9.2,-4.09,;10.15,-5.29,;9.59,-6.73,;8.4,-7.69,;9.84,-8.25,;7.54,3.87,;6.2,4.64,;4.87,3.87,;3.55,4.64,;2.23,3.9,;2.23,2.35,;3.56,1.59,;4.87,2.36,)| Show InChI InChI=1S/C35H39N3/c1-24-35-32-19-29(36-21-25-8-4-2-5-9-25)15-14-28(32)18-34(38(24)23-27-12-13-27)31-17-16-30(20-33(31)35)37-22-26-10-6-3-7-11-26/h2-11,14-15,19-20,24,27,31,34-36H,12-13,16-18,21-23H2,1H3/t24-,31-,34+,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
In vitro binding affinity against cloned human Opioid receptor delta 1 expressed in HEK 293S cells |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595703

(CHEMBL5181402)Show SMILES CN(C(=O)c1noc(=O)n1Cc1ccc2cc(C)c(N)nc2c1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381424

(CHEMBL2017195)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(C)n2)c2nc(ccc2N1C(=O)OC(C)C)C(F)(F)F |r| Show InChI InChI=1S/C26H26F9N7O2/c1-5-17-11-19(21-18(42(17)23(43)44-13(2)3)6-7-20(36-21)26(33,34)35)41(22-37-39-40(4)38-22)12-14-8-15(24(27,28)29)10-16(9-14)25(30,31)32/h6-10,13,17,19H,5,11-12H2,1-4H3/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50595704
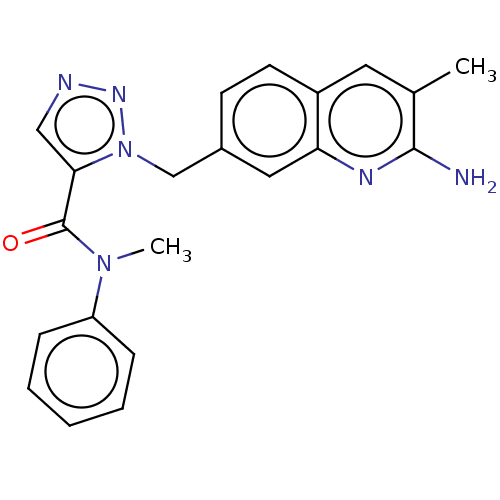
(CHEMBL5190045)Show SMILES CN(C(=O)c1cnnn1Cc1ccc2cc(C)c(N)nc2c1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MW2N51 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50122540
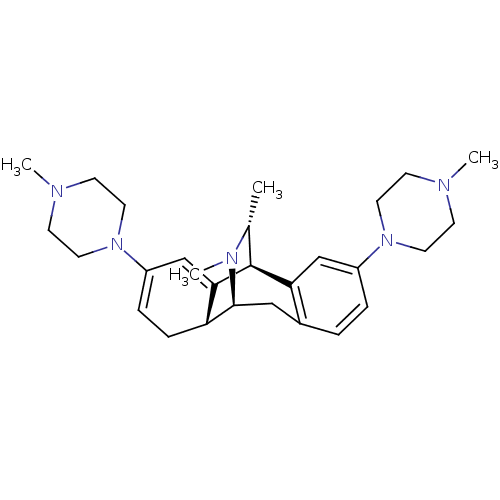
(16,17-dimethyl-4,13-di(4-methylhexahydro-1-pyrazin...)Show SMILES C[C@@H]1[C@@H]2C3=CC(=CC[C@H]3[C@H](Cc3ccc(cc23)N2CCN(C)CC2)N1C)N1CCN(C)CC1 |wU:2.2,9.27,8.8,1.0,c:5,t:3,TLB:15:16:24.1:3.8,0:1:3.8:16.11.10,THB:7:8:24.1:16.11.10,12:11:24.1:3.8,25:24:3.8:16.11.10,(13.17,-7.29,;12.07,-8.38,;8.24,-7.12,;8.24,-5.56,;8.24,-4.02,;9.57,-3.25,;10.9,-4.02,;10.9,-5.56,;9.57,-6.33,;9.78,-10.2,;8.24,-10.2,;6.91,-9.43,;5.58,-10.2,;4.25,-9.43,;4.25,-7.89,;5.58,-7.12,;6.91,-7.89,;2.9,-7.1,;2.9,-5.54,;1.57,-4.76,;.21,-5.51,;-1.11,-4.74,;.19,-7.08,;1.55,-7.87,;11.23,-9.64,;12.19,-10.86,;9.57,-1.71,;10.9,-.94,;10.9,.58,;9.57,1.37,;9.59,2.91,;8.24,.6,;8.24,-.94,)| Show InChI InChI=1S/C28H41N5/c1-20-28-25-18-22(32-13-9-29(2)10-14-32)6-5-21(25)17-27(31(20)4)24-8-7-23(19-26(24)28)33-15-11-30(3)12-16-33/h5-7,18-20,24,27-28H,8-17H2,1-4H3/t20-,24-,27+,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381404

(CHEMBL2017403)Show SMILES CC[C@@H]1C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)c2nnn(C)n2)c2nc(ccc2N1C(=O)C(C)C)C(F)(F)F |r| Show InChI InChI=1S/C26H26F9N7O/c1-5-17-11-19(21-18(42(17)22(43)13(2)3)6-7-20(36-21)26(33,34)35)41(23-37-39-40(4)38-23)12-14-8-15(24(27,28)29)10-16(9-14)25(30,31)32/h6-10,13,17,19H,5,11-12H2,1-4H3/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma assessed as reduction in fluorescent intensity by fluorescence analysis |
Bioorg Med Chem Lett 22: 3056-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.075
BindingDB Entry DOI: 10.7270/Q28G8MQT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50122541

(4N,13N-diphenyl-16,17-dimethyl-(16R)-17-azatetracy...)Show SMILES C[C@@H]1[C@@H]2C3=CC(CC[C@H]3[C@H](Cc3ccc(Nc4ccccc4)cc23)N1C)=Nc1ccccc1 |w:26.31,wU:2.2,9.27,8.8,1.0,t:3,TLB:0:1:3.8:23.11.10,22:23:24.1:3.8,THB:12:11:24.1:3.8,25:24:3.8:23.11.10,7:8:24.1:23.11.10,(11.76,-4.31,;10.67,-5.4,;6.84,-4.14,;6.84,-2.59,;6.84,-1.06,;8.17,-.29,;9.51,-1.06,;9.51,-2.59,;8.17,-3.36,;8.39,-7.22,;6.84,-7.22,;5.52,-6.46,;4.19,-7.22,;2.85,-6.46,;2.85,-4.91,;1.51,-4.13,;.17,-4.87,;-1.16,-4.1,;-2.48,-4.85,;-2.49,-6.4,;-1.16,-7.18,;.17,-6.41,;4.19,-4.14,;5.52,-4.91,;9.82,-6.67,;10.78,-7.87,;8.17,1.25,;6.83,2.02,;5.52,1.26,;4.19,2.02,;4.17,3.56,;5.52,4.35,;6.84,3.58,)| Show InChI InChI=1S/C30H31N3/c1-20-30-27-18-24(31-22-9-5-3-6-10-22)14-13-21(27)17-29(33(20)2)26-16-15-25(19-28(26)30)32-23-11-7-4-8-12-23/h3-14,18-20,26,29-31H,15-17H2,1-2H3/t20-,26-,29+,30+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Tested for effective concentration against cloned human Opioid receptor mu 1 |
J Med Chem 46: 34-48 (2002)
Article DOI: 10.1021/jm020164l
BindingDB Entry DOI: 10.7270/Q2TM7BTT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data