

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410041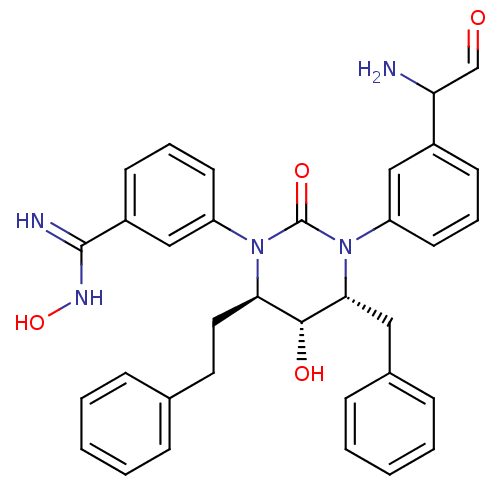 (CHEMBL178951) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410036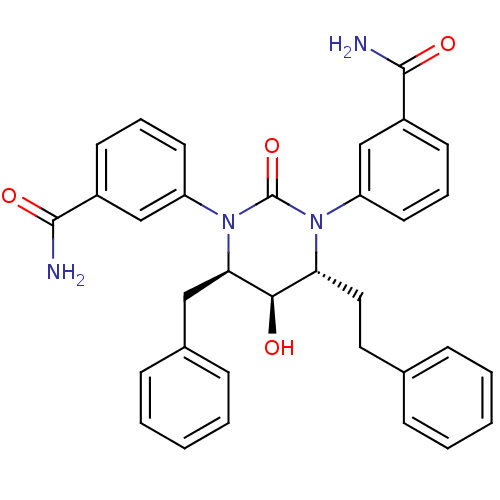 (CHEMBL179761) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410038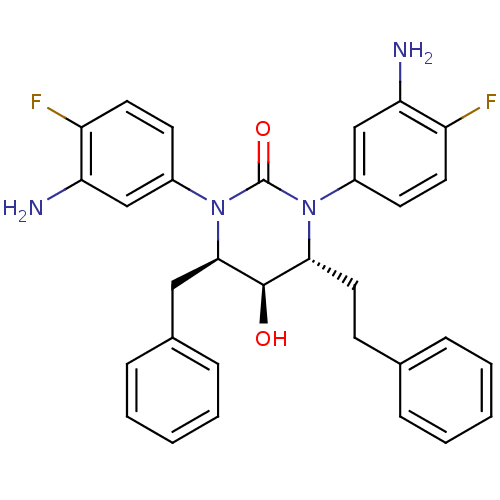 (CHEMBL179103) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410028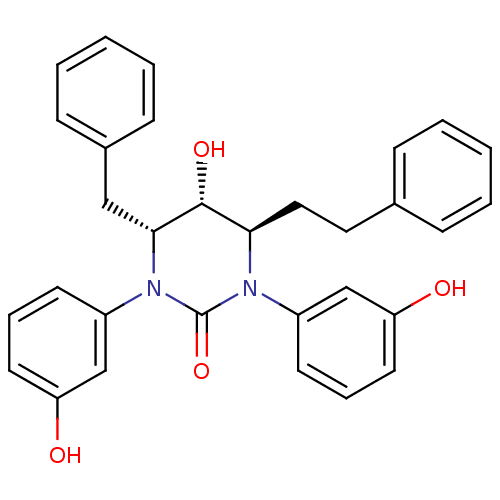 (CHEMBL359753) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410031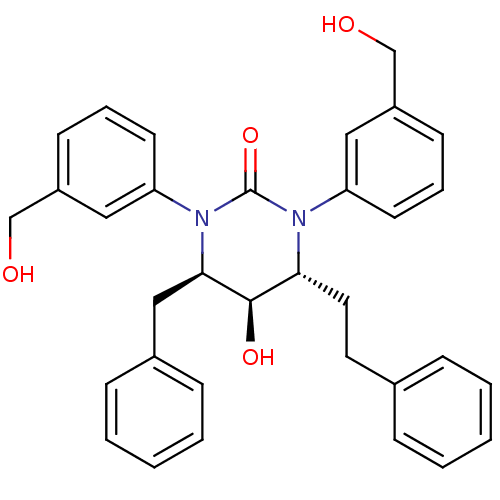 (CHEMBL362069) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263671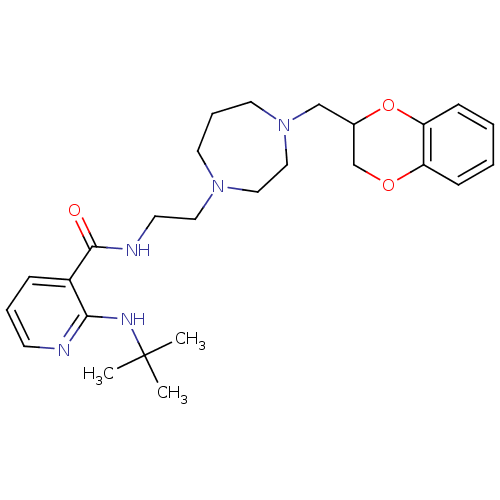 (2-(tert-butylamino)-N-(2-(4-((2,3-dihydrobenzo[b][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263673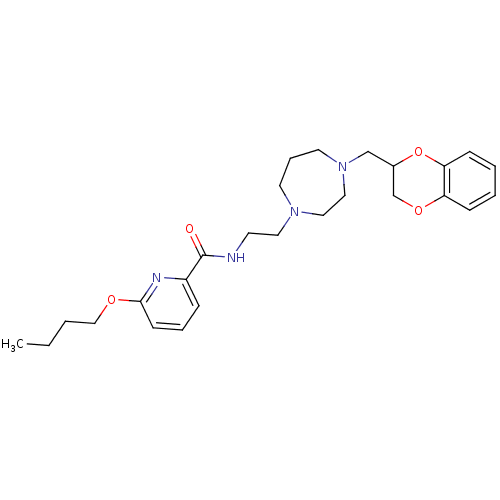 (6-butoxy-N-(2-(4-((2,3-dihydrobenzo[b][1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50326659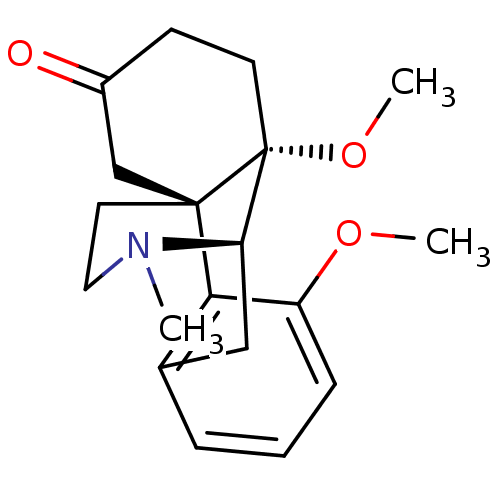 (3,10-dimethoxy-17-methyl-(10S)-17-azatetracyclo[7....) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]DAMGO from mu receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263621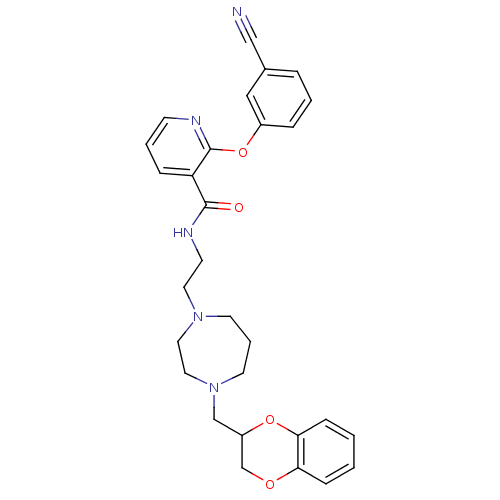 (2-(3-cyanophenoxy)-N-(2-(4-((2,3-dihydrobenzo[b][1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263672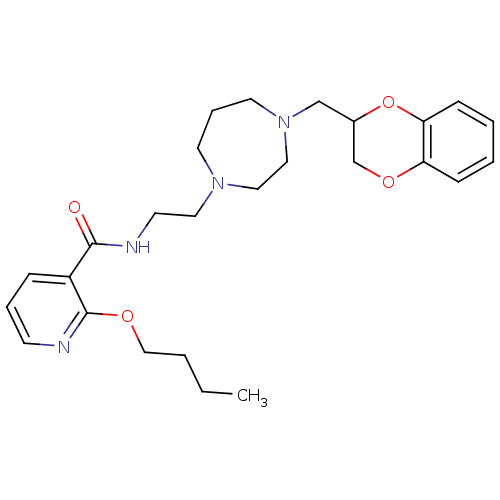 (2-butoxy-N-(2-(4-((2,3-dihydrobenzo[b][1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50285003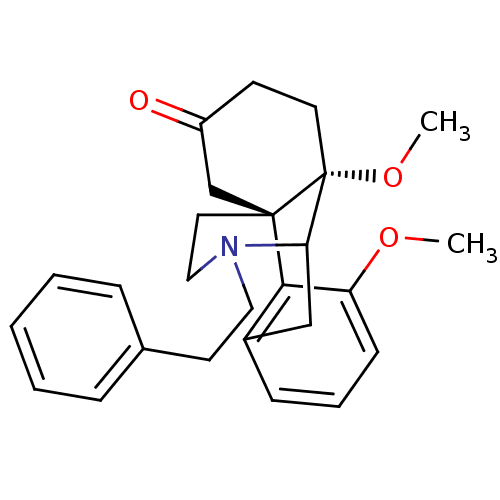 (3,10-dimethoxy-17-phenethyl-17-azatetracyclo[7.5.3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]DAMGO from mu receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50084621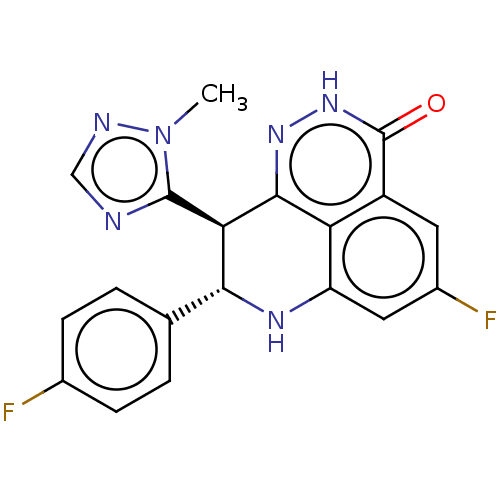 (BMN 673 | Talazoparib) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University Curated by ChEMBL | Assay Description Inhibition of PARP2 (unknown origin) after 1 min in presence of NAD by top count analysis | Eur J Med Chem 165: 198-215 (2019) Article DOI: 10.1016/j.ejmech.2019.01.024 BindingDB Entry DOI: 10.7270/Q2Z03CPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263774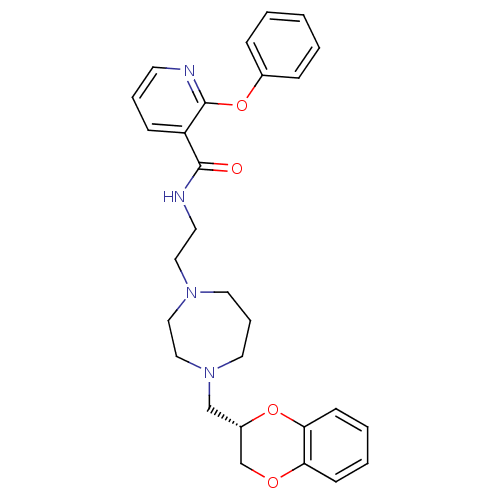 ((S)-N-{2-[4-(2,3-dihydro-benzo[1,4]dioxin-2-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002926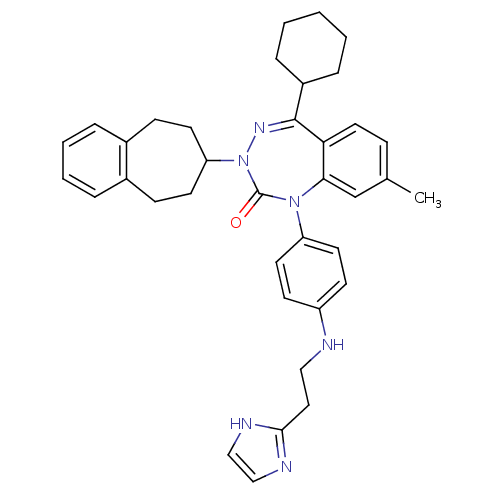 (CHEMBL244325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50084621 (BMN 673 | Talazoparib) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University Curated by ChEMBL | Assay Description Inhibition of PARP1 (unknown origin) after 1 min in presence of NAD by top count analysis | Eur J Med Chem 165: 198-215 (2019) Article DOI: 10.1016/j.ejmech.2019.01.024 BindingDB Entry DOI: 10.7270/Q2Z03CPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50032527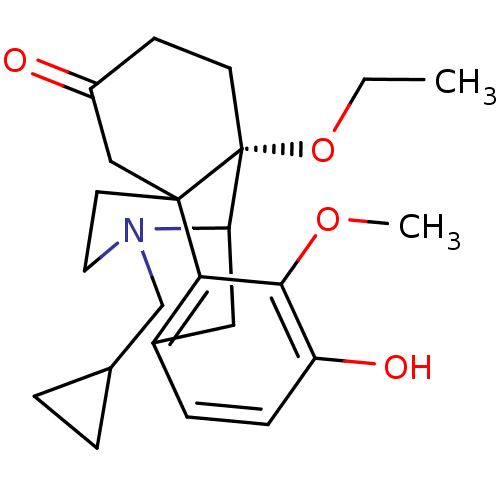 (17-cyclopropylmethyl-10-ethoxy-4-hydroxy-3-methoxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the ability to displace [3H]DAMGO radioligand binding from Opioid receptor mu 1 in guinea pig brain membr... | J Med Chem 38: 3071-7 (1995) BindingDB Entry DOI: 10.7270/Q2QJ7HZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length PARP1 after 4 mins by [32P]NAD+ incorporation assay | Eur J Med Chem 165: 198-215 (2019) Article DOI: 10.1016/j.ejmech.2019.01.024 BindingDB Entry DOI: 10.7270/Q2Z03CPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002913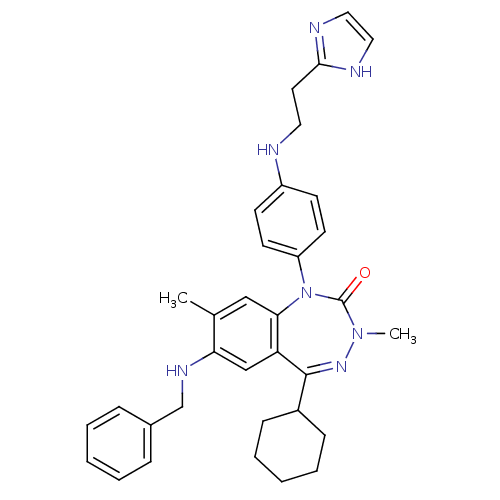 (CHEMBL389783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410042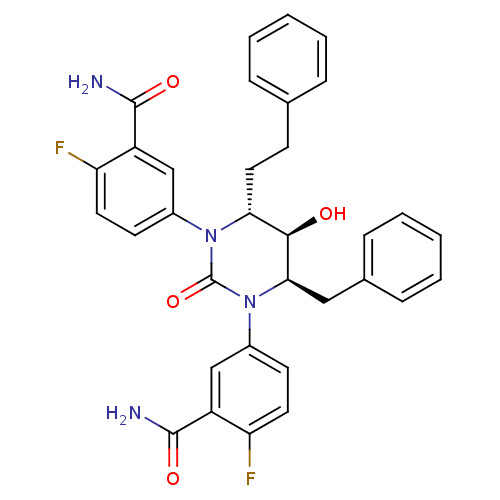 (CHEMBL366431) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50326659 (3,10-dimethoxy-17-methyl-(10S)-17-azatetracyclo[7....) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]-U-69,593 from kappa receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410032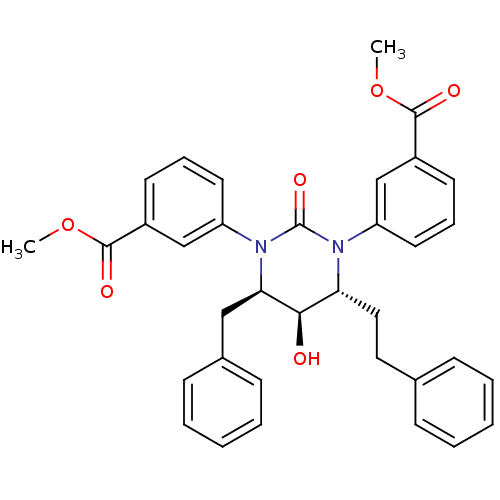 (CHEMBL361980) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410045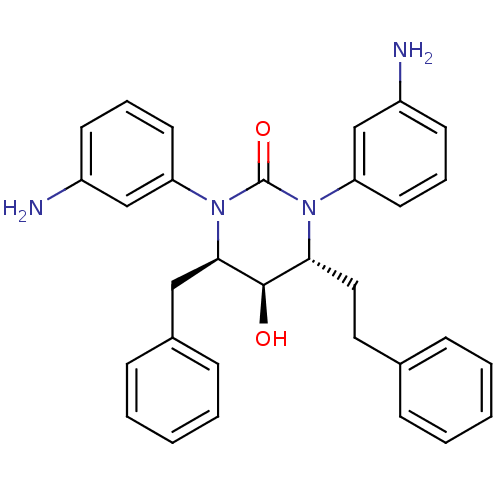 (CHEMBL179250) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263725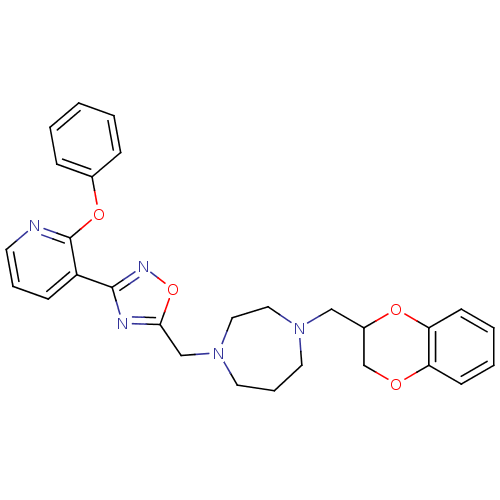 (1-((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methyl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic Alpha-2C receptor expressed in CHO cells assessed as inhibition of NE-induced calcium mobilizatio... | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002915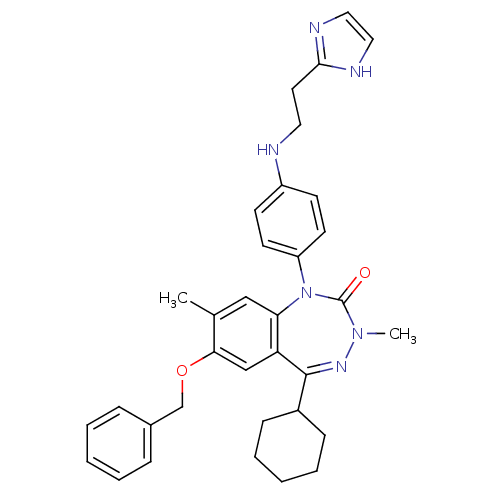 (CHEMBL389787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263725 (1-((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methyl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263621 (2-(3-cyanophenoxy)-N-(2-(4-((2,3-dihydrobenzo[b][1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic Alpha-2C receptor expressed in CHO cells assessed as inhibition of NE-induced calcium mobilizatio... | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263774 ((S)-N-{2-[4-(2,3-dihydro-benzo[1,4]dioxin-2-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic Alpha-2C receptor expressed in CHO cells assessed as inhibition of NE-induced calcium mobilizatio... | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263724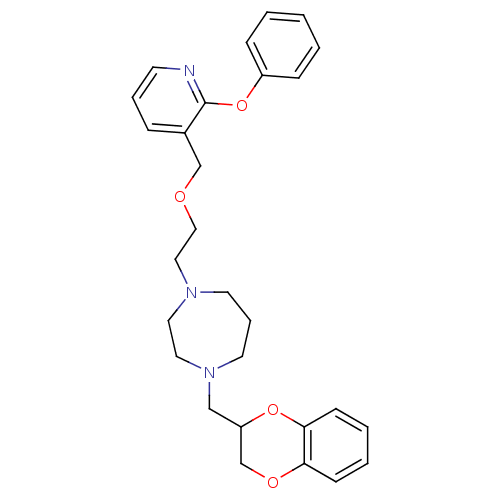 (1-((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methyl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410027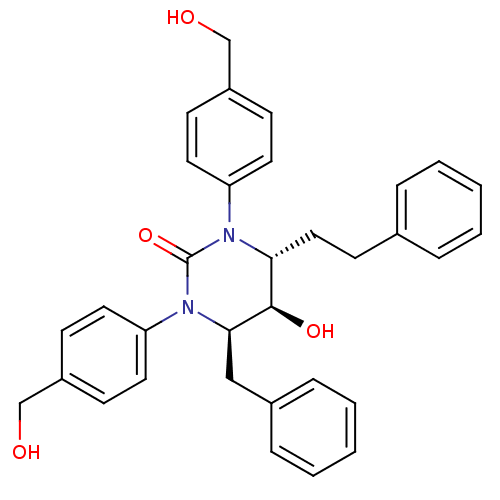 (CHEMBL180816) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263620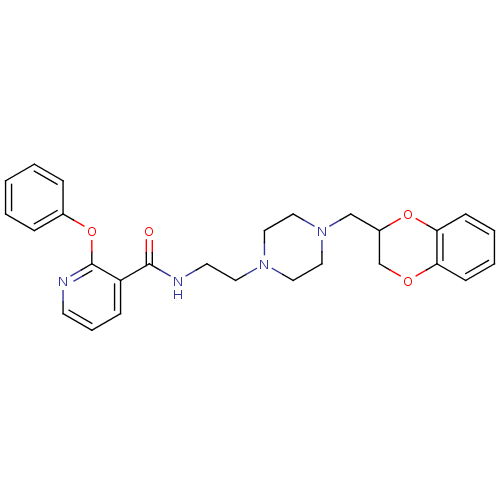 (CHEMBL478026 | N-(2-(4-((2,3-dihydrobenzo[b][1,4]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50316226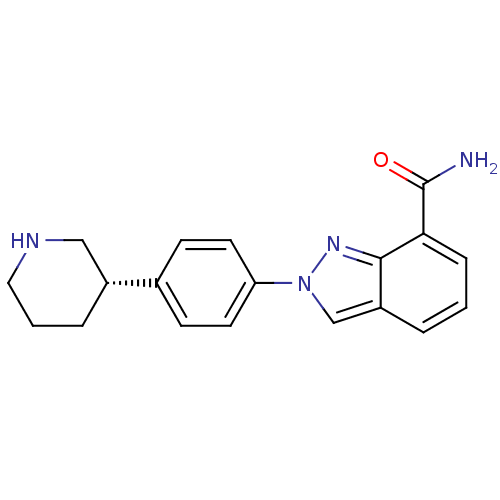 ((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University Curated by ChEMBL | Assay Description Inhibition of PARP1 (unknown origin) | Eur J Med Chem 165: 198-215 (2019) Article DOI: 10.1016/j.ejmech.2019.01.024 BindingDB Entry DOI: 10.7270/Q2Z03CPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002923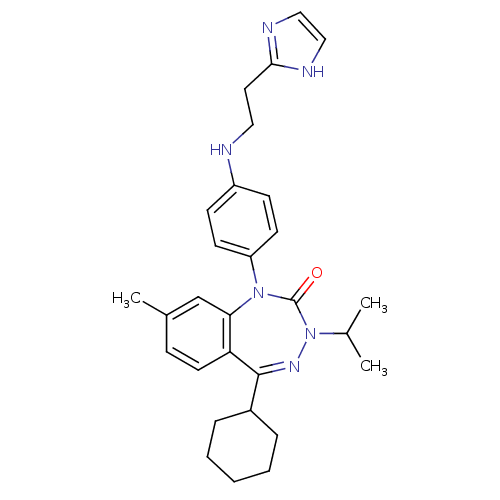 (CHEMBL243698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263673 (6-butoxy-N-(2-(4-((2,3-dihydrobenzo[b][1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic Alpha-2C receptor expressed in CHO cells assessed as inhibition of NE-induced calcium mobilizatio... | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002924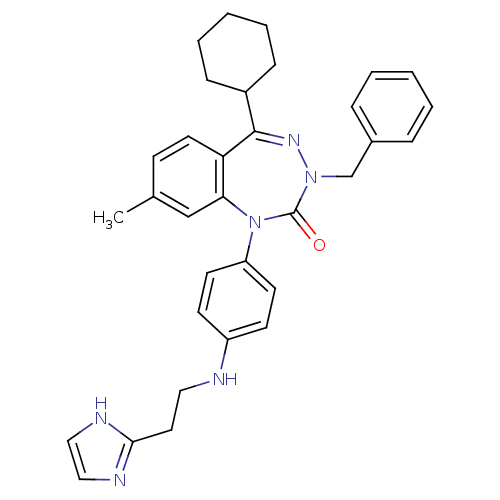 (CHEMBL242567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50316226 ((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University Curated by ChEMBL | Assay Description Inhibition of PARP2 (unknown origin) | Eur J Med Chem 165: 198-215 (2019) Article DOI: 10.1016/j.ejmech.2019.01.024 BindingDB Entry DOI: 10.7270/Q2Z03CPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50032530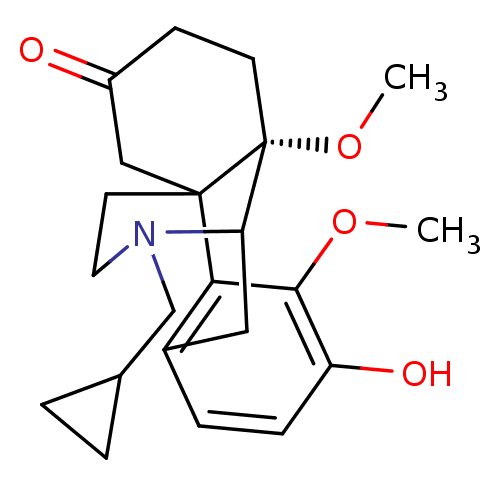 (17-cyclopropylmethyl-4-hydroxy-3,10-dimethoxy-17-a...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the ability to displace [3H]U-69593 radioligand binding from Opioid receptor kappa 1 in guinea pig brain ... | J Med Chem 38: 3071-7 (1995) BindingDB Entry DOI: 10.7270/Q2QJ7HZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263672 (2-butoxy-N-(2-(4-((2,3-dihydrobenzo[b][1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic Alpha-2C receptor expressed in CHO cells assessed as inhibition of NE-induced calcium mobilizatio... | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410034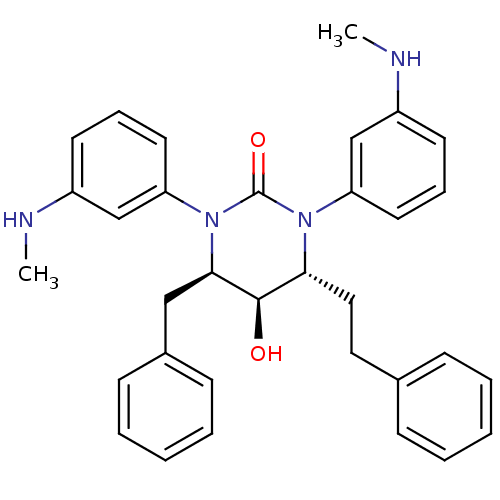 (CHEMBL181166) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263619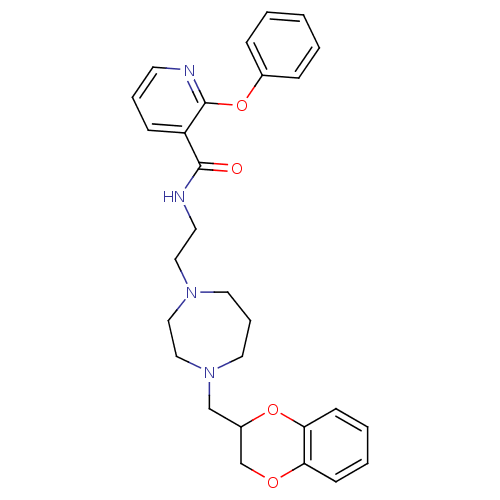 (CHEMBL477816 | N-(2-(4-((2,3-dihydrobenzo[b][1,4]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2C receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50263725 (1-((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methyl)-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic alpha2B receptor expressed in CHO cells assessed as inhibition of NE-induced calcium mobilization... | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50032527 (17-cyclopropylmethyl-10-ethoxy-4-hydroxy-3-methoxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vivo binding affinity was evaluated by measuring the ability to displace [3H]U-69593 radioligand binding from Opioid receptor kappa 1 in guinea pi... | J Med Chem 38: 3071-7 (1995) BindingDB Entry DOI: 10.7270/Q2QJ7HZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50032530 (17-cyclopropylmethyl-4-hydroxy-3,10-dimethoxy-17-a...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the ability to displace [3H]DAMGO radioligand binding from Opioid receptor mu 1 in guinea pig brain membr... | J Med Chem 38: 3071-7 (1995) BindingDB Entry DOI: 10.7270/Q2QJ7HZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002914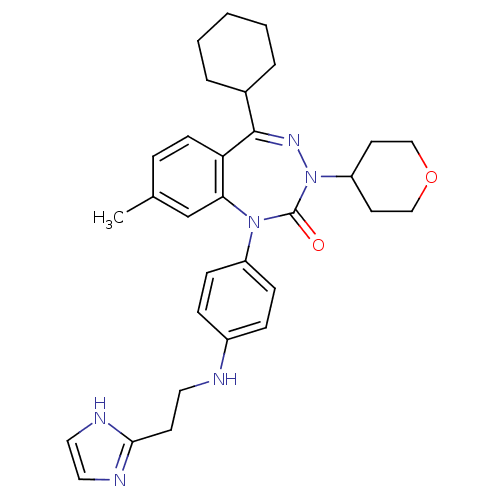 (CHEMBL244956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50263724 (1-((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methyl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic Alpha-2C receptor expressed in CHO cells assessed as inhibition of NE-induced calcium mobilizatio... | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002922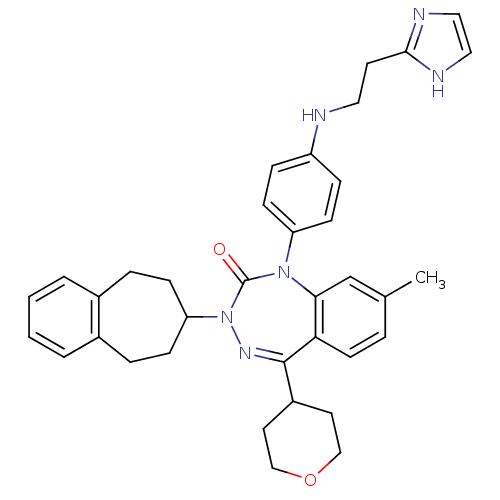 (CHEMBL224729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002925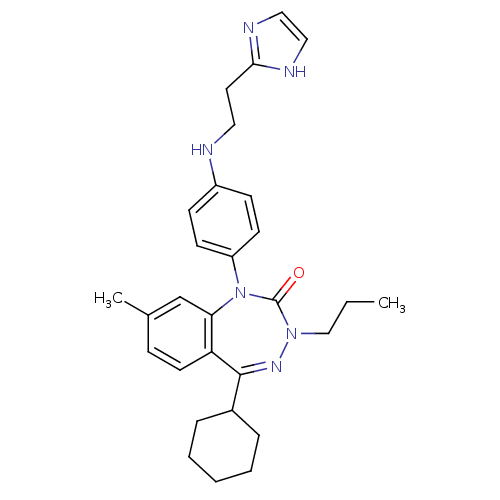 (CHEMBL244074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50032526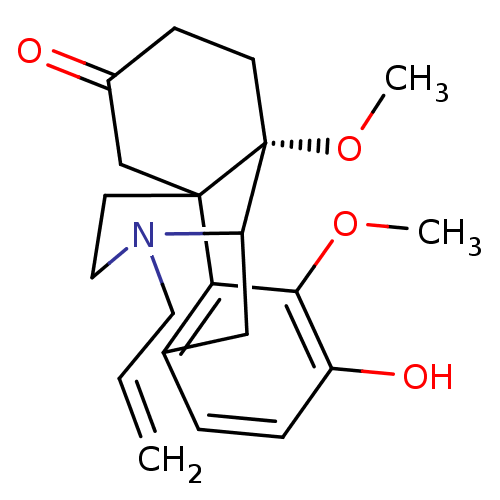 (17-allyl-4-hydroxy-3,10-dimethoxy-17-azatetracyclo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the ability to displace [3H]DAMGO radioligand binding from Opioid receptor mu 1 in guinea pig brain membr... | J Med Chem 38: 3071-7 (1995) BindingDB Entry DOI: 10.7270/Q2QJ7HZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50410035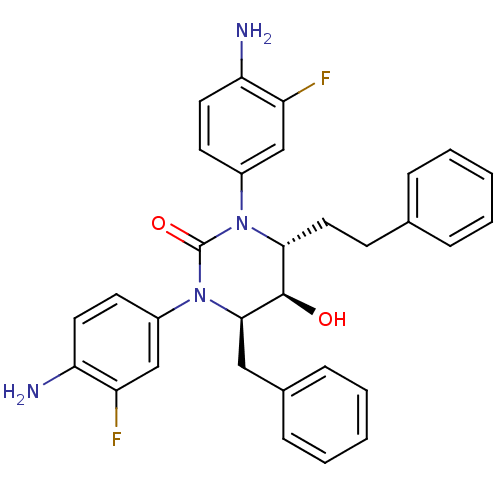 (CHEMBL181514) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clarkson University Curated by ChEMBL | Assay Description Antiviral activity potency was assessed by measuring effect on the accumulation of viral RNA transcripts 3 days after infection of MT-2 cells with HI... | Bioorg Med Chem Lett 15: 3767-70 (2005) Article DOI: 10.1016/j.bmcl.2005.05.087 BindingDB Entry DOI: 10.7270/Q2SQ91KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50263620 (CHEMBL478026 | N-(2-(4-((2,3-dihydrobenzo[b][1,4]d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity at human recombinant adrenergic alpha2B receptor expressed in CHO cells by radioligand binding assay | Bioorg Med Chem Lett 18: 5689-93 (2008) Article DOI: 10.1016/j.bmcl.2008.08.055 BindingDB Entry DOI: 10.7270/Q2N58M5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002912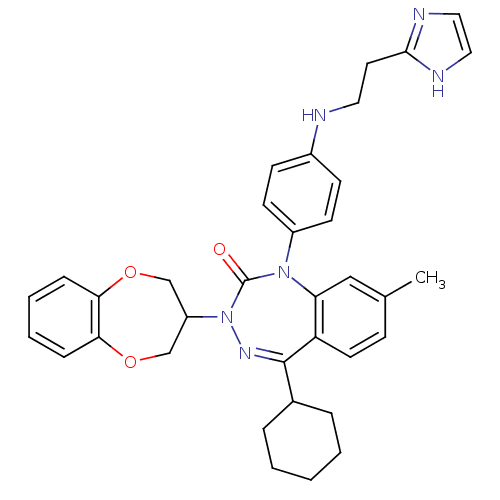 (CHEMBL388911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1394 total ) | Next | Last >> |