 Found 255 hits with Last Name = 'patel' and Initial = 'hs'
Found 255 hits with Last Name = 'patel' and Initial = 'hs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384584
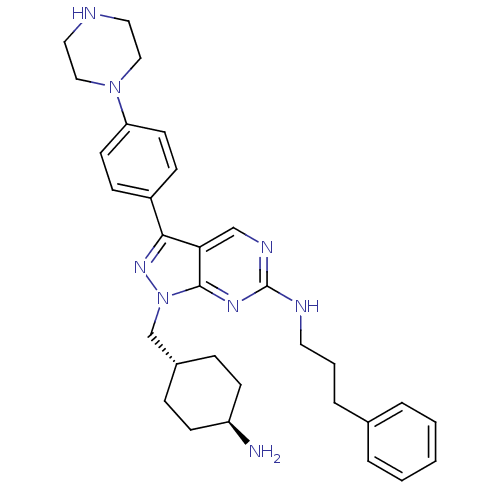
(CHEMBL2036807 | US9744172, Compound UNC607A)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(cc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(41.81,-28.1,;42.85,-26.96,;44.35,-27.28,;45.39,-26.14,;44.91,-24.68,;45.94,-23.54,;45.47,-22.07,;46.38,-20.82,;45.47,-19.56,;45.94,-18.1,;47.45,-17.78,;47.93,-16.32,;46.9,-15.17,;45.38,-15.5,;44.91,-16.96,;47.36,-13.71,;48.87,-13.39,;49.35,-11.94,;48.32,-10.79,;46.81,-11.11,;46.33,-12.57,;43.99,-20.04,;42.66,-19.28,;41.33,-20.05,;41.33,-21.6,;39.99,-22.36,;38.66,-21.59,;38.66,-20.05,;37.33,-19.28,;37.33,-17.74,;38.67,-16.98,;38.67,-15.44,;37.34,-14.67,;36,-15.45,;36,-16.98,;42.66,-22.37,;43.99,-21.6,;43.41,-24.35,;42.38,-25.49,)| Show InChI InChI=1S/C31H40N8/c32-26-12-8-24(9-13-26)22-39-30-28(21-35-31(36-30)34-16-4-7-23-5-2-1-3-6-23)29(37-39)25-10-14-27(15-11-25)38-19-17-33-18-20-38/h1-3,5-6,10-11,14-15,21,24,26,33H,4,7-9,12-13,16-20,22,32H2,(H,34,35,36)/t24-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384583
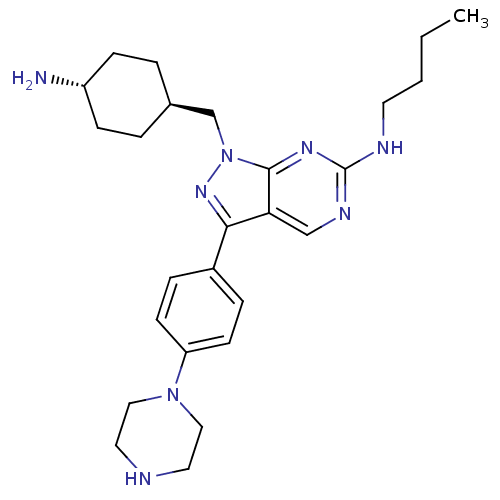
(CHEMBL2036806)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(19.9,-18.03,;19.9,-19.57,;21.23,-20.34,;21.23,-21.88,;22.56,-22.65,;23.9,-21.88,;23.9,-20.34,;25.23,-19.57,;26.56,-20.33,;28.04,-19.85,;28.95,-21.11,;28.04,-22.36,;28.51,-23.83,;27.48,-24.97,;27.96,-26.43,;26.92,-27.57,;25.42,-27.25,;24.38,-28.39,;24.95,-25.78,;25.98,-24.64,;26.56,-21.88,;25.23,-22.65,;28.51,-18.39,;30.02,-18.07,;30.5,-16.61,;29.47,-15.46,;27.95,-15.79,;27.48,-17.25,;29.93,-14,;31.44,-13.68,;31.92,-12.22,;30.89,-11.08,;29.38,-11.39,;28.9,-12.86,)| Show InChI InChI=1S/C26H38N8/c1-2-3-12-29-26-30-17-23-24(20-6-10-22(11-7-20)33-15-13-28-14-16-33)32-34(25(23)31-26)18-19-4-8-21(27)9-5-19/h6-7,10-11,17,19,21,28H,2-5,8-9,12-16,18,27H2,1H3,(H,29,30,31)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384582
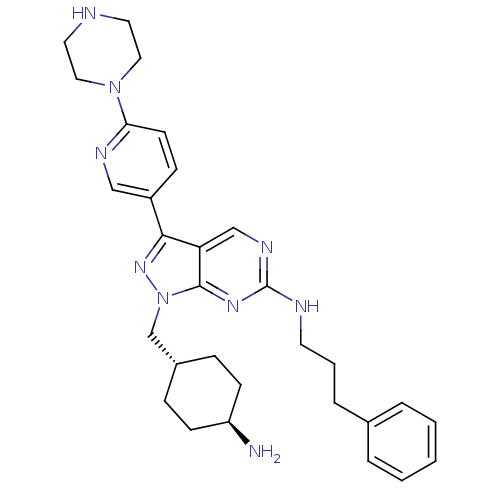
(CHEMBL2036805)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(nc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(11.21,-28.56,;12.24,-27.41,;13.75,-27.74,;14.79,-26.59,;14.31,-25.14,;15.34,-24,;14.86,-22.53,;15.77,-21.28,;14.86,-20.02,;15.34,-18.56,;14.31,-17.42,;14.78,-15.96,;16.29,-15.63,;17.32,-16.78,;16.84,-18.24,;16.76,-14.17,;18.27,-13.85,;18.74,-12.39,;17.71,-11.25,;16.21,-11.56,;15.72,-13.03,;13.39,-20.5,;12.05,-19.74,;10.72,-20.51,;10.72,-22.05,;9.39,-22.82,;8.05,-22.05,;8.05,-20.51,;6.72,-19.74,;6.72,-18.2,;8.06,-17.44,;8.06,-15.9,;6.73,-15.13,;5.39,-15.9,;5.4,-17.44,;12.05,-22.82,;13.39,-22.05,;12.8,-24.81,;11.77,-25.95,)| Show InChI InChI=1S/C30H39N9/c31-25-11-8-23(9-12-25)21-39-29-26(20-35-30(36-29)33-14-4-7-22-5-2-1-3-6-22)28(37-39)24-10-13-27(34-19-24)38-17-15-32-16-18-38/h1-3,5-6,10,13,19-20,23,25,32H,4,7-9,11-12,14-18,21,31H2,(H,33,35,36)/t23-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384585
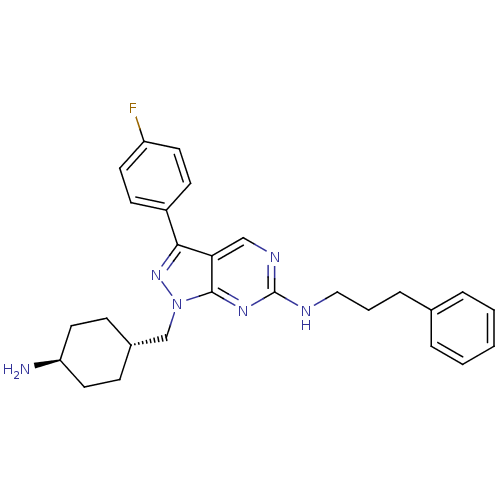
(CHEMBL2036809)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(F)cc3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(-.82,-49.25,;.22,-48.11,;1.72,-48.43,;2.76,-47.29,;2.28,-45.83,;3.31,-44.69,;2.84,-43.22,;3.75,-41.97,;2.84,-40.71,;3.31,-39.25,;4.82,-38.93,;5.3,-37.47,;4.27,-36.32,;4.74,-34.86,;2.75,-36.65,;2.28,-38.11,;1.36,-41.19,;.03,-40.43,;-1.3,-41.2,;-1.3,-42.74,;-2.64,-43.51,;-3.97,-42.74,;-3.97,-41.2,;-5.3,-40.43,;-5.3,-38.89,;-3.96,-38.13,;-3.96,-36.59,;-5.29,-35.82,;-6.63,-36.59,;-6.63,-38.13,;.03,-43.51,;1.36,-42.74,;.78,-45.5,;-.25,-46.64,)| Show InChI InChI=1S/C27H31FN6/c28-22-12-10-21(11-13-22)25-24-17-31-27(30-16-4-7-19-5-2-1-3-6-19)32-26(24)34(33-25)18-20-8-14-23(29)15-9-20/h1-3,5-6,10-13,17,20,23H,4,7-9,14-16,18,29H2,(H,30,31,32)/t20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50276802
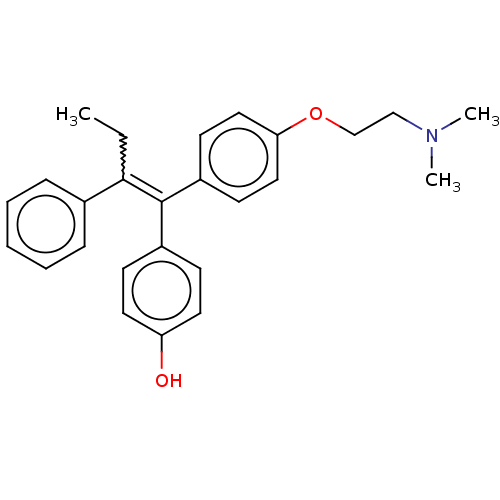
(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
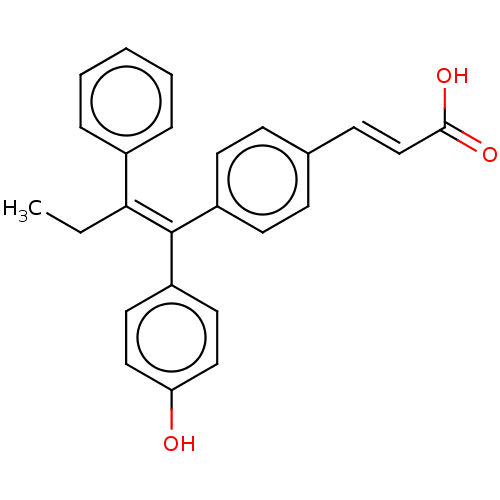
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384581
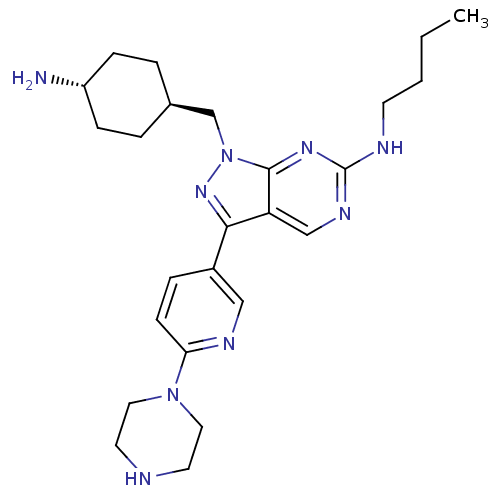
(CHEMBL2036804)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(nc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(-8.83,-18.78,;-8.83,-20.32,;-7.5,-21.09,;-7.5,-22.63,;-6.17,-23.41,;-4.83,-22.64,;-4.83,-21.09,;-3.5,-20.32,;-2.17,-21.08,;-.69,-20.61,;.22,-21.86,;-.69,-23.12,;-.22,-24.58,;-1.25,-25.72,;-.77,-27.18,;-1.81,-28.32,;-3.31,-28,;-4.35,-29.14,;-3.78,-26.53,;-2.76,-25.39,;-2.17,-22.64,;-3.5,-23.41,;-.22,-19.14,;-1.25,-18.01,;-.78,-16.54,;.73,-16.22,;1.77,-17.36,;1.29,-18.82,;1.2,-14.75,;2.71,-14.44,;3.19,-12.98,;2.16,-11.83,;.65,-12.15,;.17,-13.62,)| Show InChI InChI=1S/C25H37N9/c1-2-3-10-28-25-30-16-21-23(19-6-9-22(29-15-19)33-13-11-27-12-14-33)32-34(24(21)31-25)17-18-4-7-20(26)8-5-18/h6,9,15-16,18,20,27H,2-5,7-8,10-14,17,26H2,1H3,(H,28,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50276802

(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384576

(CHEMBL2036808)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(F)cc1 |r,wU:13.12,wD:16.16,(54.06,-16.98,;54.06,-18.52,;55.39,-19.29,;55.39,-20.83,;56.72,-21.6,;58.06,-20.83,;58.06,-19.29,;59.39,-18.52,;60.72,-19.28,;62.2,-18.8,;63.11,-20.06,;62.2,-21.31,;62.67,-22.78,;61.64,-23.92,;62.12,-25.38,;61.08,-26.52,;59.58,-26.2,;58.54,-27.34,;59.11,-24.73,;60.14,-23.59,;60.72,-20.83,;59.39,-21.6,;62.67,-17.34,;64.18,-17.02,;64.66,-15.56,;63.63,-14.41,;64.1,-12.95,;62.11,-14.74,;61.64,-16.2,)| Show InChI InChI=1S/C22H29FN6/c1-2-3-12-25-22-26-13-19-20(16-6-8-17(23)9-7-16)28-29(21(19)27-22)14-15-4-10-18(24)11-5-15/h6-9,13,15,18H,2-5,10-12,14,24H2,1H3,(H,25,26,27)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475356

(CHEMBL195080)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(OCCN(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C27H28N2O3/c1-4-24-26(19-8-6-5-7-9-19)27(23-15-10-20(30)18-25(23)28-24)32-22-13-11-21(12-14-22)31-17-16-29(2)3/h5-15,18,30H,4,16-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475363

(CHEMBL194774)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)N(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C28H26N2O3/c1-4-24-27(20-8-6-5-7-9-20)28(23-16-13-21(31)18-25(23)29-24)33-22-14-10-19(11-15-22)12-17-26(32)30(2)3/h5-18,31H,4H2,1-3H3/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475356

(CHEMBL195080)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(OCCN(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C27H28N2O3/c1-4-24-26(19-8-6-5-7-9-19)27(23-15-10-20(30)18-25(23)28-24)32-22-13-11-21(12-14-22)31-17-16-29(2)3/h5-15,18,30H,4,16-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475364
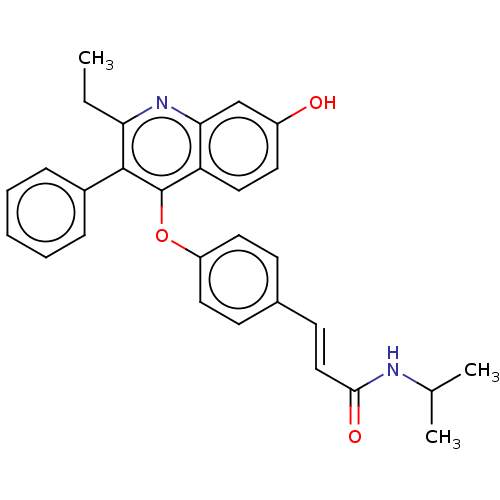
(CHEMBL370750)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)NC(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C29H28N2O3/c1-4-25-28(21-8-6-5-7-9-21)29(24-16-13-22(32)18-26(24)31-25)34-23-14-10-20(11-15-23)12-17-27(33)30-19(2)3/h5-19,32H,4H2,1-3H3,(H,30,33)/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475364

(CHEMBL370750)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)NC(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C29H28N2O3/c1-4-25-28(21-8-6-5-7-9-21)29(24-16-13-22(32)18-26(24)31-25)34-23-14-10-20(11-15-23)12-17-27(33)30-19(2)3/h5-19,32H,4H2,1-3H3,(H,30,33)/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475361
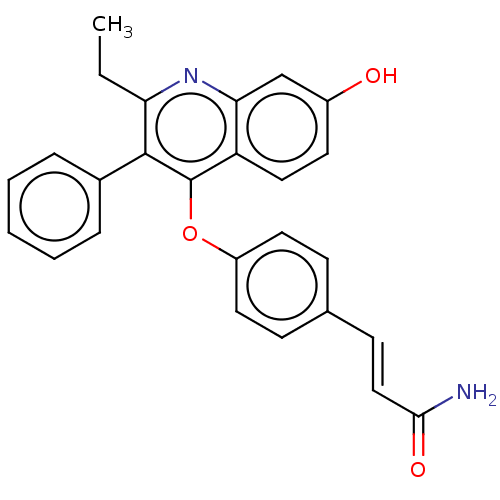
(CHEMBL363630)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(N)=O)cc2)c1-c1ccccc1 Show InChI InChI=1S/C26H22N2O3/c1-2-22-25(18-6-4-3-5-7-18)26(21-14-11-19(29)16-23(21)28-22)31-20-12-8-17(9-13-20)10-15-24(27)30/h3-16,29H,2H2,1H3,(H2,27,30)/b15-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475363

(CHEMBL194774)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)N(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C28H26N2O3/c1-4-24-27(20-8-6-5-7-9-20)28(23-16-13-21(31)18-25(23)29-24)33-22-14-10-19(11-15-22)12-17-26(32)30(2)3/h5-18,31H,4H2,1-3H3/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475361

(CHEMBL363630)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(N)=O)cc2)c1-c1ccccc1 Show InChI InChI=1S/C26H22N2O3/c1-2-22-25(18-6-4-3-5-7-18)26(21-14-11-19(29)16-23(21)28-22)31-20-12-8-17(9-13-20)10-15-24(27)30/h3-16,29H,2H2,1H3,(H2,27,30)/b15-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475360
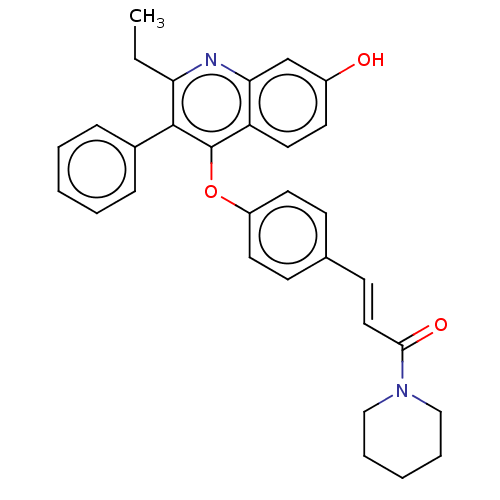
(CHEMBL193865)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)N3CCCCC3)cc2)c1-c1ccccc1 Show InChI InChI=1S/C31H30N2O3/c1-2-27-30(23-9-5-3-6-10-23)31(26-17-14-24(34)21-28(26)32-27)36-25-15-11-22(12-16-25)13-18-29(35)33-19-7-4-8-20-33/h3,5-6,9-18,21,34H,2,4,7-8,19-20H2,1H3/b18-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475360

(CHEMBL193865)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)N3CCCCC3)cc2)c1-c1ccccc1 Show InChI InChI=1S/C31H30N2O3/c1-2-27-30(23-9-5-3-6-10-23)31(26-17-14-24(34)21-28(26)32-27)36-25-15-11-22(12-16-25)13-18-29(35)33-19-7-4-8-20-33/h3,5-6,9-18,21,34H,2,4,7-8,19-20H2,1H3/b18-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475357
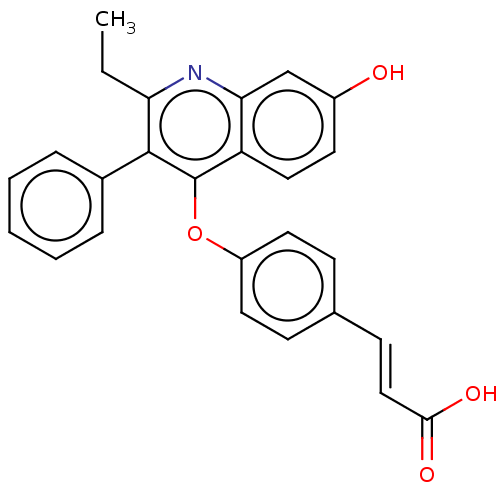
(CHEMBL370492)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(O)=O)cc2)c1-c1ccccc1 Show InChI InChI=1S/C26H21NO4/c1-2-22-25(18-6-4-3-5-7-18)26(21-14-11-19(28)16-23(21)27-22)31-20-12-8-17(9-13-20)10-15-24(29)30/h3-16,28H,2H2,1H3,(H,29,30)/b15-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475358
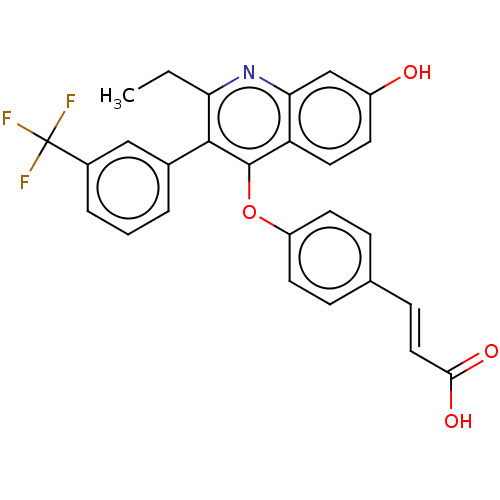
(CHEMBL192025)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(O)=O)cc2)c1-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H20F3NO4/c1-2-22-25(17-4-3-5-18(14-17)27(28,29)30)26(21-12-9-19(32)15-23(21)31-22)35-20-10-6-16(7-11-20)8-13-24(33)34/h3-15,32H,2H2,1H3,(H,33,34)/b13-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475357

(CHEMBL370492)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(O)=O)cc2)c1-c1ccccc1 Show InChI InChI=1S/C26H21NO4/c1-2-22-25(18-6-4-3-5-7-18)26(21-14-11-19(28)16-23(21)27-22)31-20-12-8-17(9-13-20)10-15-24(29)30/h3-16,28H,2H2,1H3,(H,29,30)/b15-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475358

(CHEMBL192025)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(O)=O)cc2)c1-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H20F3NO4/c1-2-22-25(17-4-3-5-18(14-17)27(28,29)30)26(21-12-9-19(32)15-23(21)31-22)35-20-10-6-16(7-11-20)8-13-24(33)34/h3-15,32H,2H2,1H3,(H,33,34)/b13-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475359

(CHEMBL194600)Show SMILES OC(=O)\C=C\c1ccc(Oc2c(-c3ccccc3)c(nc3cc(O)ccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C30H21NO4/c32-23-14-17-25-26(19-23)31-29(22-9-5-2-6-10-22)28(21-7-3-1-4-8-21)30(25)35-24-15-11-20(12-16-24)13-18-27(33)34/h1-19,32H,(H,33,34)/b18-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475362

(CHEMBL193730)Show SMILES CCc1nc2cc(O)ccc2c(Oc2cccc(\C=C\C(O)=O)c2)c1-c1ccccc1 Show InChI InChI=1S/C26H21NO4/c1-2-22-25(18-8-4-3-5-9-18)26(21-13-12-19(28)16-23(21)27-22)31-20-10-6-7-17(15-20)11-14-24(29)30/h3-16,28H,2H2,1H3,(H,29,30)/b14-11+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475362

(CHEMBL193730)Show SMILES CCc1nc2cc(O)ccc2c(Oc2cccc(\C=C\C(O)=O)c2)c1-c1ccccc1 Show InChI InChI=1S/C26H21NO4/c1-2-22-25(18-8-4-3-5-9-18)26(21-13-12-19(28)16-23(21)27-22)31-20-10-6-7-17(15-20)11-14-24(29)30/h3-16,28H,2H2,1H3,(H,29,30)/b14-11+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50475359

(CHEMBL194600)Show SMILES OC(=O)\C=C\c1ccc(Oc2c(-c3ccccc3)c(nc3cc(O)ccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C30H21NO4/c32-23-14-17-25-26(19-23)31-29(22-9-5-2-6-10-22)28(21-7-3-1-4-8-21)30(25)35-24-15-11-20(12-16-24)13-18-27(33)34/h1-19,32H,(H,33,34)/b18-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384584

(CHEMBL2036807 | US9744172, Compound UNC607A)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(cc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(41.81,-28.1,;42.85,-26.96,;44.35,-27.28,;45.39,-26.14,;44.91,-24.68,;45.94,-23.54,;45.47,-22.07,;46.38,-20.82,;45.47,-19.56,;45.94,-18.1,;47.45,-17.78,;47.93,-16.32,;46.9,-15.17,;45.38,-15.5,;44.91,-16.96,;47.36,-13.71,;48.87,-13.39,;49.35,-11.94,;48.32,-10.79,;46.81,-11.11,;46.33,-12.57,;43.99,-20.04,;42.66,-19.28,;41.33,-20.05,;41.33,-21.6,;39.99,-22.36,;38.66,-21.59,;38.66,-20.05,;37.33,-19.28,;37.33,-17.74,;38.67,-16.98,;38.67,-15.44,;37.34,-14.67,;36,-15.45,;36,-16.98,;42.66,-22.37,;43.99,-21.6,;43.41,-24.35,;42.38,-25.49,)| Show InChI InChI=1S/C31H40N8/c32-26-12-8-24(9-13-26)22-39-30-28(21-35-31(36-30)34-16-4-7-23-5-2-1-3-6-23)29(37-39)25-10-14-27(15-11-25)38-19-17-33-18-20-38/h1-3,5-6,10-11,14-15,21,24,26,33H,4,7-9,12-13,16-20,22,32H2,(H,34,35,36)/t24-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384583

(CHEMBL2036806)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(19.9,-18.03,;19.9,-19.57,;21.23,-20.34,;21.23,-21.88,;22.56,-22.65,;23.9,-21.88,;23.9,-20.34,;25.23,-19.57,;26.56,-20.33,;28.04,-19.85,;28.95,-21.11,;28.04,-22.36,;28.51,-23.83,;27.48,-24.97,;27.96,-26.43,;26.92,-27.57,;25.42,-27.25,;24.38,-28.39,;24.95,-25.78,;25.98,-24.64,;26.56,-21.88,;25.23,-22.65,;28.51,-18.39,;30.02,-18.07,;30.5,-16.61,;29.47,-15.46,;27.95,-15.79,;27.48,-17.25,;29.93,-14,;31.44,-13.68,;31.92,-12.22,;30.89,-11.08,;29.38,-11.39,;28.9,-12.86,)| Show InChI InChI=1S/C26H38N8/c1-2-3-12-29-26-30-17-23-24(20-6-10-22(11-7-20)33-15-13-28-14-16-33)32-34(25(23)31-26)18-19-4-8-21(27)9-5-19/h6-7,10-11,17,19,21,28H,2-5,8-9,12-16,18,27H2,1H3,(H,29,30,31)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384582

(CHEMBL2036805)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(nc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(11.21,-28.56,;12.24,-27.41,;13.75,-27.74,;14.79,-26.59,;14.31,-25.14,;15.34,-24,;14.86,-22.53,;15.77,-21.28,;14.86,-20.02,;15.34,-18.56,;14.31,-17.42,;14.78,-15.96,;16.29,-15.63,;17.32,-16.78,;16.84,-18.24,;16.76,-14.17,;18.27,-13.85,;18.74,-12.39,;17.71,-11.25,;16.21,-11.56,;15.72,-13.03,;13.39,-20.5,;12.05,-19.74,;10.72,-20.51,;10.72,-22.05,;9.39,-22.82,;8.05,-22.05,;8.05,-20.51,;6.72,-19.74,;6.72,-18.2,;8.06,-17.44,;8.06,-15.9,;6.73,-15.13,;5.39,-15.9,;5.4,-17.44,;12.05,-22.82,;13.39,-22.05,;12.8,-24.81,;11.77,-25.95,)| Show InChI InChI=1S/C30H39N9/c31-25-11-8-23(9-12-25)21-39-29-26(20-35-30(36-29)33-14-4-7-22-5-2-1-3-6-22)28(37-39)24-10-13-27(34-19-24)38-17-15-32-16-18-38/h1-3,5-6,10,13,19-20,23,25,32H,4,7-9,11-12,14-18,21,31H2,(H,33,35,36)/t23-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
pIC50 for 1 nM estradiol-induced Ishikawa cell proliferation |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384584

(CHEMBL2036807 | US9744172, Compound UNC607A)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(cc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(41.81,-28.1,;42.85,-26.96,;44.35,-27.28,;45.39,-26.14,;44.91,-24.68,;45.94,-23.54,;45.47,-22.07,;46.38,-20.82,;45.47,-19.56,;45.94,-18.1,;47.45,-17.78,;47.93,-16.32,;46.9,-15.17,;45.38,-15.5,;44.91,-16.96,;47.36,-13.71,;48.87,-13.39,;49.35,-11.94,;48.32,-10.79,;46.81,-11.11,;46.33,-12.57,;43.99,-20.04,;42.66,-19.28,;41.33,-20.05,;41.33,-21.6,;39.99,-22.36,;38.66,-21.59,;38.66,-20.05,;37.33,-19.28,;37.33,-17.74,;38.67,-16.98,;38.67,-15.44,;37.34,-14.67,;36,-15.45,;36,-16.98,;42.66,-22.37,;43.99,-21.6,;43.41,-24.35,;42.38,-25.49,)| Show InChI InChI=1S/C31H40N8/c32-26-12-8-24(9-13-26)22-39-30-28(21-35-31(36-30)34-16-4-7-23-5-2-1-3-6-23)29(37-39)25-10-14-27(15-11-25)38-19-17-33-18-20-38/h1-3,5-6,10-11,14-15,21,24,26,33H,4,7-9,12-13,16-20,22,32H2,(H,34,35,36)/t24-,26- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50276802

(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
pIC50 for 1 nM estradiol-induced Ishikawa cell proliferation |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384581

(CHEMBL2036804)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(nc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(-8.83,-18.78,;-8.83,-20.32,;-7.5,-21.09,;-7.5,-22.63,;-6.17,-23.41,;-4.83,-22.64,;-4.83,-21.09,;-3.5,-20.32,;-2.17,-21.08,;-.69,-20.61,;.22,-21.86,;-.69,-23.12,;-.22,-24.58,;-1.25,-25.72,;-.77,-27.18,;-1.81,-28.32,;-3.31,-28,;-4.35,-29.14,;-3.78,-26.53,;-2.76,-25.39,;-2.17,-22.64,;-3.5,-23.41,;-.22,-19.14,;-1.25,-18.01,;-.78,-16.54,;.73,-16.22,;1.77,-17.36,;1.29,-18.82,;1.2,-14.75,;2.71,-14.44,;3.19,-12.98,;2.16,-11.83,;.65,-12.15,;.17,-13.62,)| Show InChI InChI=1S/C25H37N9/c1-2-3-10-28-25-30-16-21-23(19-6-9-22(29-15-19)33-13-11-27-12-14-33)32-34(24(21)31-25)17-18-4-7-20(26)8-5-18/h6,9,15-16,18,20,27H,2-5,7-8,10-14,17,26H2,1H3,(H,28,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384576

(CHEMBL2036808)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(F)cc1 |r,wU:13.12,wD:16.16,(54.06,-16.98,;54.06,-18.52,;55.39,-19.29,;55.39,-20.83,;56.72,-21.6,;58.06,-20.83,;58.06,-19.29,;59.39,-18.52,;60.72,-19.28,;62.2,-18.8,;63.11,-20.06,;62.2,-21.31,;62.67,-22.78,;61.64,-23.92,;62.12,-25.38,;61.08,-26.52,;59.58,-26.2,;58.54,-27.34,;59.11,-24.73,;60.14,-23.59,;60.72,-20.83,;59.39,-21.6,;62.67,-17.34,;64.18,-17.02,;64.66,-15.56,;63.63,-14.41,;64.1,-12.95,;62.11,-14.74,;61.64,-16.2,)| Show InChI InChI=1S/C22H29FN6/c1-2-3-12-25-22-26-13-19-20(16-6-8-17(23)9-7-16)28-29(21(19)27-22)14-15-4-10-18(24)11-5-15/h6-9,13,15,18H,2-5,10-12,14,24H2,1H3,(H,25,26,27)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50384583

(CHEMBL2036806)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(19.9,-18.03,;19.9,-19.57,;21.23,-20.34,;21.23,-21.88,;22.56,-22.65,;23.9,-21.88,;23.9,-20.34,;25.23,-19.57,;26.56,-20.33,;28.04,-19.85,;28.95,-21.11,;28.04,-22.36,;28.51,-23.83,;27.48,-24.97,;27.96,-26.43,;26.92,-27.57,;25.42,-27.25,;24.38,-28.39,;24.95,-25.78,;25.98,-24.64,;26.56,-21.88,;25.23,-22.65,;28.51,-18.39,;30.02,-18.07,;30.5,-16.61,;29.47,-15.46,;27.95,-15.79,;27.48,-17.25,;29.93,-14,;31.44,-13.68,;31.92,-12.22,;30.89,-11.08,;29.38,-11.39,;28.9,-12.86,)| Show InChI InChI=1S/C26H38N8/c1-2-3-12-29-26-30-17-23-24(20-6-10-22(11-7-20)33-15-13-28-14-16-33)32-34(25(23)31-26)18-19-4-8-21(27)9-5-19/h6-7,10-11,17,19,21,28H,2-5,8-9,12-16,18,27H2,1H3,(H,29,30,31)/t19-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tyro3 using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384585

(CHEMBL2036809)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(F)cc3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(-.82,-49.25,;.22,-48.11,;1.72,-48.43,;2.76,-47.29,;2.28,-45.83,;3.31,-44.69,;2.84,-43.22,;3.75,-41.97,;2.84,-40.71,;3.31,-39.25,;4.82,-38.93,;5.3,-37.47,;4.27,-36.32,;4.74,-34.86,;2.75,-36.65,;2.28,-38.11,;1.36,-41.19,;.03,-40.43,;-1.3,-41.2,;-1.3,-42.74,;-2.64,-43.51,;-3.97,-42.74,;-3.97,-41.2,;-5.3,-40.43,;-5.3,-38.89,;-3.96,-38.13,;-3.96,-36.59,;-5.29,-35.82,;-6.63,-36.59,;-6.63,-38.13,;.03,-43.51,;1.36,-42.74,;.78,-45.5,;-.25,-46.64,)| Show InChI InChI=1S/C27H31FN6/c28-22-12-10-21(11-13-22)25-24-17-31-27(30-16-4-7-19-5-2-1-3-6-19)32-26(24)34(33-25)18-20-8-14-23(29)15-9-20/h1-3,5-6,10-13,17,20,23H,4,7-9,14-16,18,29H2,(H,30,31,32)/t20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475356

(CHEMBL195080)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(OCCN(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C27H28N2O3/c1-4-24-26(19-8-6-5-7-9-19)27(23-15-10-20(30)18-25(23)28-24)32-22-13-11-21(12-14-22)31-17-16-29(2)3/h5-15,18,30H,4,16-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
pIC50 for 1 nM estradiol-induced Ishikawa cell proliferation |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384582

(CHEMBL2036805)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(nc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(11.21,-28.56,;12.24,-27.41,;13.75,-27.74,;14.79,-26.59,;14.31,-25.14,;15.34,-24,;14.86,-22.53,;15.77,-21.28,;14.86,-20.02,;15.34,-18.56,;14.31,-17.42,;14.78,-15.96,;16.29,-15.63,;17.32,-16.78,;16.84,-18.24,;16.76,-14.17,;18.27,-13.85,;18.74,-12.39,;17.71,-11.25,;16.21,-11.56,;15.72,-13.03,;13.39,-20.5,;12.05,-19.74,;10.72,-20.51,;10.72,-22.05,;9.39,-22.82,;8.05,-22.05,;8.05,-20.51,;6.72,-19.74,;6.72,-18.2,;8.06,-17.44,;8.06,-15.9,;6.73,-15.13,;5.39,-15.9,;5.4,-17.44,;12.05,-22.82,;13.39,-22.05,;12.8,-24.81,;11.77,-25.95,)| Show InChI InChI=1S/C30H39N9/c31-25-11-8-23(9-12-25)21-39-29-26(20-35-30(36-29)33-14-4-7-22-5-2-1-3-6-22)28(37-39)24-10-13-27(34-19-24)38-17-15-32-16-18-38/h1-3,5-6,10,13,19-20,23,25,32H,4,7-9,11-12,14-18,21,31H2,(H,33,35,36)/t23-,25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475363

(CHEMBL194774)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)N(C)C)cc2)c1-c1ccccc1 Show InChI InChI=1S/C28H26N2O3/c1-4-24-27(20-8-6-5-7-9-20)28(23-16-13-21(31)18-25(23)29-24)33-22-14-10-19(11-15-22)12-17-26(32)30(2)3/h5-18,31H,4H2,1-3H3/b17-12+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
pIC50 for 1 nM estradiol-induced Ishikawa cell proliferation |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50475360

(CHEMBL193865)Show SMILES CCc1nc2cc(O)ccc2c(Oc2ccc(\C=C\C(=O)N3CCCCC3)cc2)c1-c1ccccc1 Show InChI InChI=1S/C31H30N2O3/c1-2-27-30(23-9-5-3-6-10-23)31(26-17-14-24(34)21-28(26)32-27)36-25-15-11-22(12-16-25)13-18-29(35)33-19-7-4-8-20-33/h3,5-6,9-18,21,34H,2,4,7-8,19-20H2,1H3/b18-13+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
pIC50 for 1 nM estradiol-induced Ishikawa cell proliferation |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384583

(CHEMBL2036806)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(19.9,-18.03,;19.9,-19.57,;21.23,-20.34,;21.23,-21.88,;22.56,-22.65,;23.9,-21.88,;23.9,-20.34,;25.23,-19.57,;26.56,-20.33,;28.04,-19.85,;28.95,-21.11,;28.04,-22.36,;28.51,-23.83,;27.48,-24.97,;27.96,-26.43,;26.92,-27.57,;25.42,-27.25,;24.38,-28.39,;24.95,-25.78,;25.98,-24.64,;26.56,-21.88,;25.23,-22.65,;28.51,-18.39,;30.02,-18.07,;30.5,-16.61,;29.47,-15.46,;27.95,-15.79,;27.48,-17.25,;29.93,-14,;31.44,-13.68,;31.92,-12.22,;30.89,-11.08,;29.38,-11.39,;28.9,-12.86,)| Show InChI InChI=1S/C26H38N8/c1-2-3-12-29-26-30-17-23-24(20-6-10-22(11-7-20)33-15-13-28-14-16-33)32-34(25(23)31-26)18-19-4-8-21(27)9-5-19/h6-7,10-11,17,19,21,28H,2-5,8-9,12-16,18,27H2,1H3,(H,29,30,31)/t19-,21- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28674
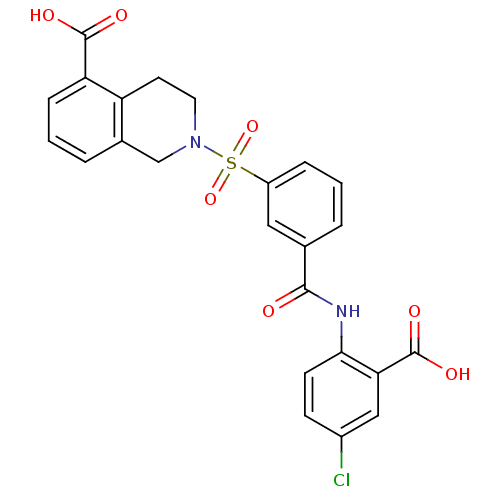
(2-({3-[(2-carboxy-4-chlorophenyl)carbamoyl]benzene...)Show SMILES OC(=O)c1cc(Cl)ccc1NC(=O)c1cccc(c1)S(=O)(=O)N1CCc2c(C1)cccc2C(O)=O Show InChI InChI=1S/C24H19ClN2O7S/c25-16-7-8-21(20(12-16)24(31)32)26-22(28)14-3-1-5-17(11-14)35(33,34)27-10-9-18-15(13-27)4-2-6-19(18)23(29)30/h1-8,11-12H,9-10,13H2,(H,26,28)(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | 1.26E+3 | n/a | n/a | 7.0 | 22 |
GSK
| Assay Description
Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... |
Bioorg Med Chem Lett 18: 5018-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.011
BindingDB Entry DOI: 10.7270/Q2WD3XWS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28691
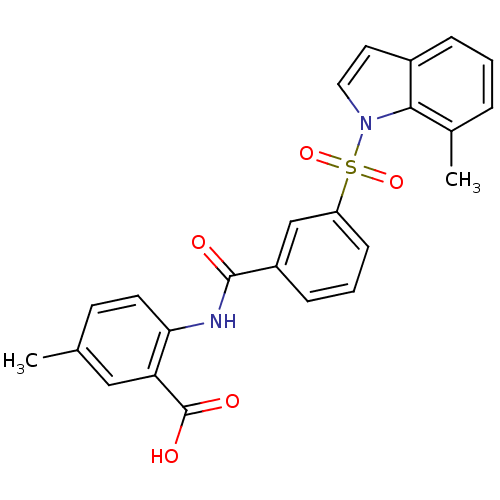
(5-methyl-2-({3-[(7-methyl-1H-indole-1-)sulfonyl]be...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)S(=O)(=O)n2ccc3cccc(C)c23)c(c1)C(O)=O Show InChI InChI=1S/C24H20N2O5S/c1-15-9-10-21(20(13-15)24(28)29)25-23(27)18-7-4-8-19(14-18)32(30,31)26-12-11-17-6-3-5-16(2)22(17)26/h3-14H,1-2H3,(H,25,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | 79 | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... |
Bioorg Med Chem Lett 18: 5018-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.011
BindingDB Entry DOI: 10.7270/Q2WD3XWS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
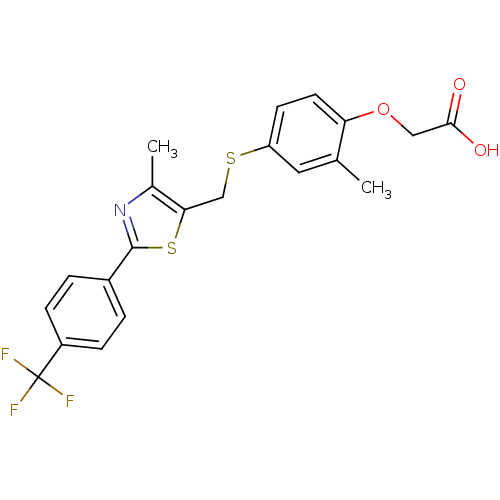
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | 3 | n/a | n/a | 7.0 | 22 |
GSK
| Assay Description
Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... |
Bioorg Med Chem Lett 18: 5018-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.011
BindingDB Entry DOI: 10.7270/Q2WD3XWS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28669

(2-{[3-(1,2,3,4-tetrahydroisoquinoline-2-sulfonyl)b...)Show SMILES OC(=O)c1cc(ccc1NC(=O)c1cccc(c1)S(=O)(=O)N1CCc2ccccc2C1)C(F)(F)F Show InChI InChI=1S/C24H19F3N2O5S/c25-24(26,27)18-8-9-21(20(13-18)23(31)32)28-22(30)16-6-3-7-19(12-16)35(33,34)29-11-10-15-4-1-2-5-17(15)14-29/h1-9,12-13H,10-11,14H2,(H,28,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | 158 | n/a | n/a | 7.0 | 22 |
GSK
| Assay Description
Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... |
Bioorg Med Chem Lett 18: 5018-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.011
BindingDB Entry DOI: 10.7270/Q2WD3XWS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28670
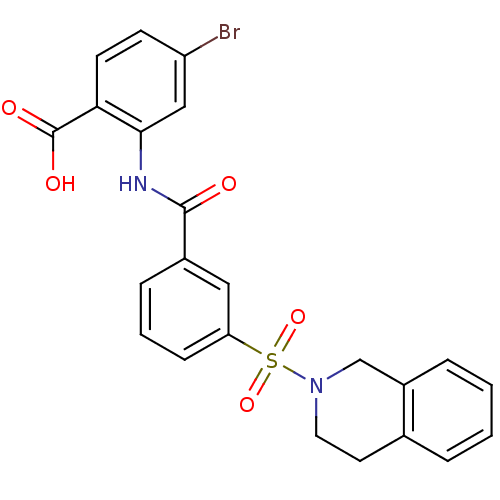
(4-bromo-2-{[3-(1,2,3,4-tetrahydroisoquinoline-2-su...)Show SMILES OC(=O)c1ccc(Br)cc1NC(=O)c1cccc(c1)S(=O)(=O)N1CCc2ccccc2C1 Show InChI InChI=1S/C23H19BrN2O5S/c24-18-8-9-20(23(28)29)21(13-18)25-22(27)16-6-3-7-19(12-16)32(30,31)26-11-10-15-4-1-2-5-17(15)14-26/h1-9,12-13H,10-11,14H2,(H,25,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | 158 | n/a | n/a | 7.0 | 22 |
GSK
| Assay Description
Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga... |
Bioorg Med Chem Lett 18: 5018-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.011
BindingDB Entry DOI: 10.7270/Q2WD3XWS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data