

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7084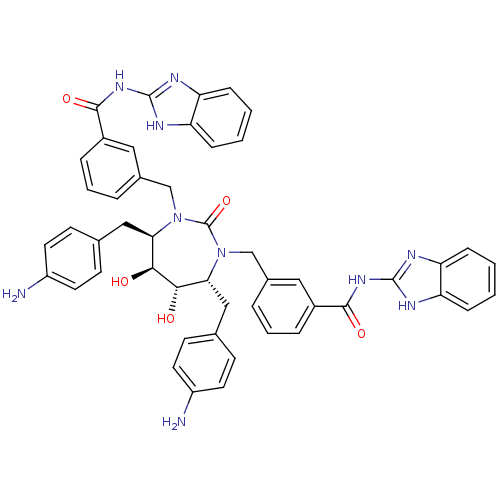 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-4,7-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | -64.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069201 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-(4-hydroxy-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124714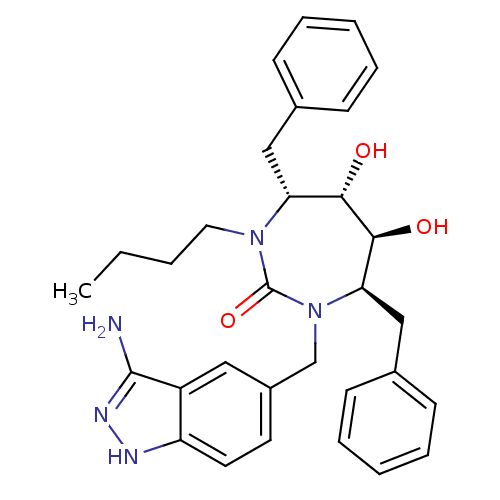 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM155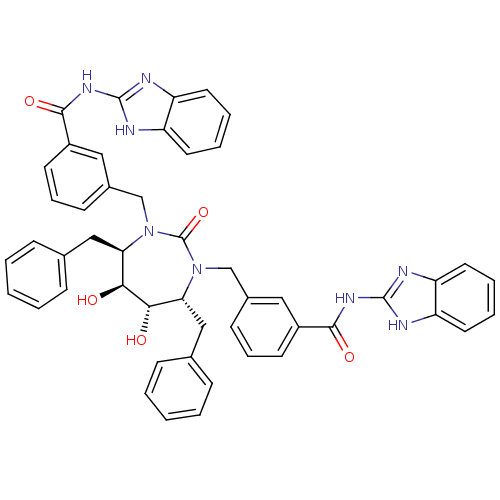 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0240 | -63.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7088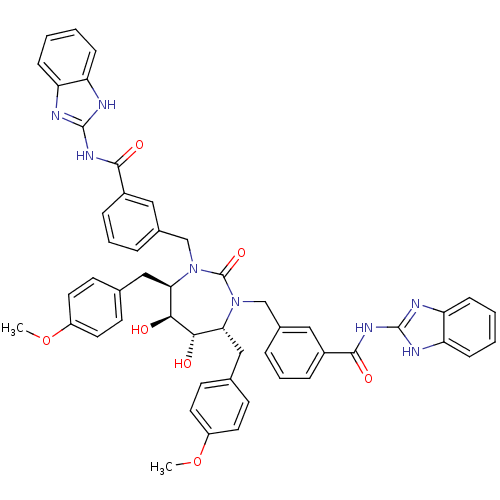 (SD146 Analog 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | -62.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM182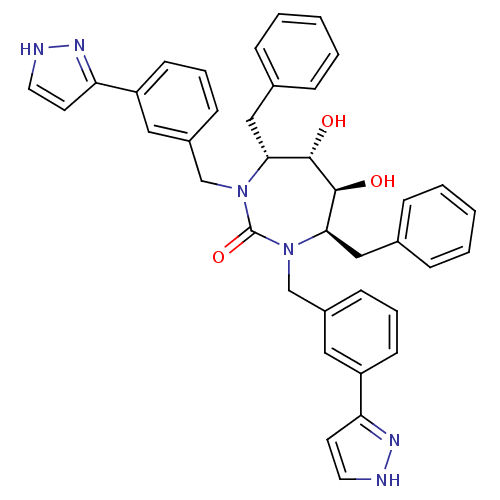 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065064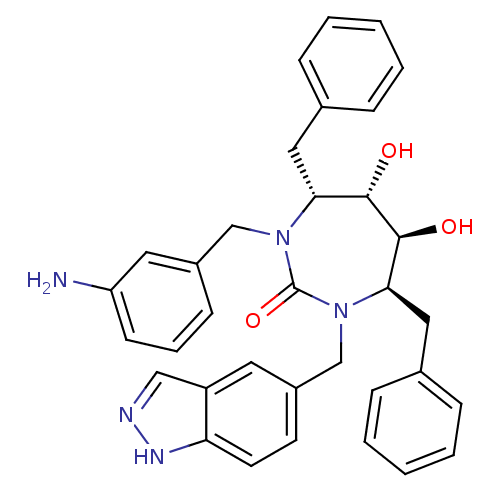 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124721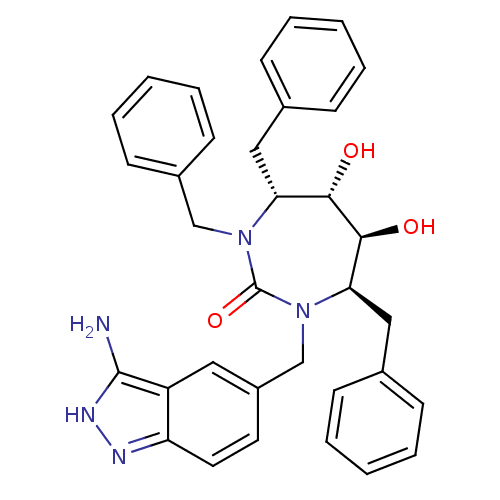 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7086 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | -62.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124716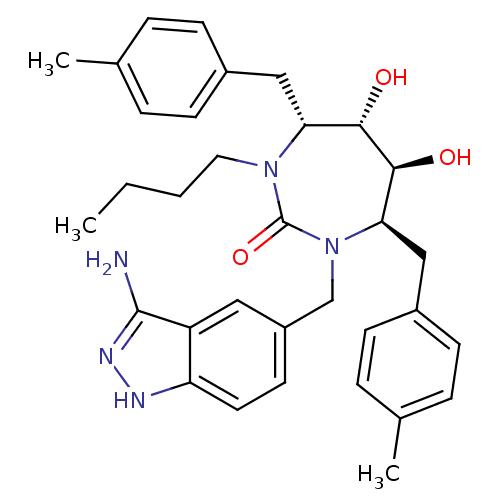 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7085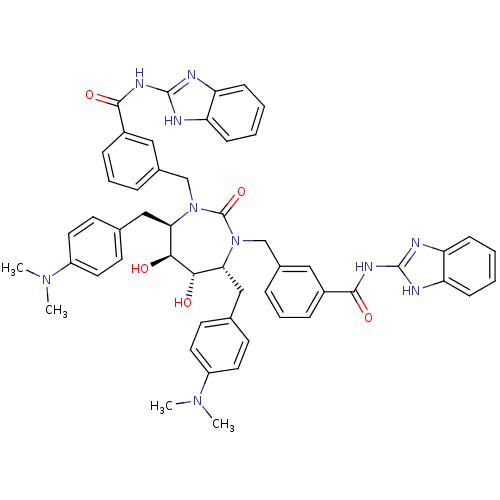 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | -61.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7090 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | -61.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7031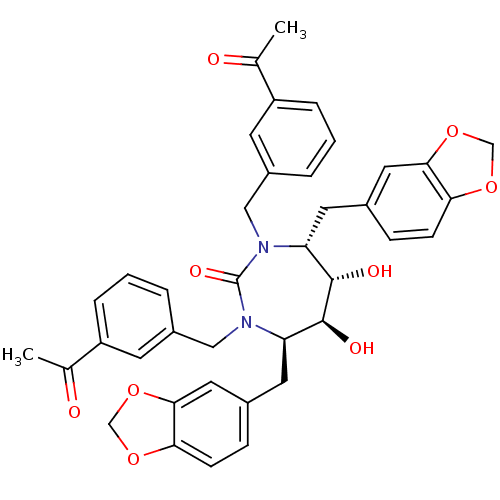 ((4R,5S,6S,7R)-4,7-bis(2H-1,3-benzodioxol-5-ylmethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | -61.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 39: 2156-69 (1996) Article DOI: 10.1021/jm960083n BindingDB Entry DOI: 10.7270/Q257197T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124715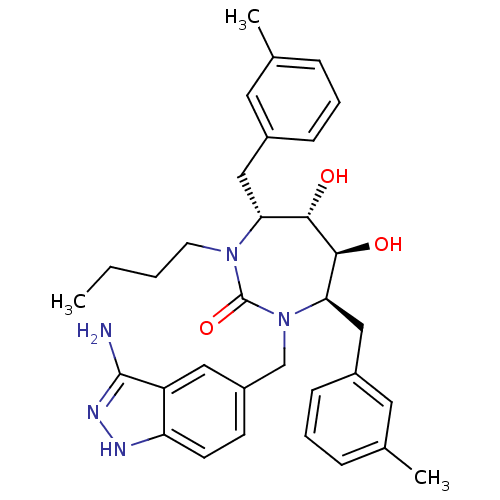 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7091 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | -61.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124722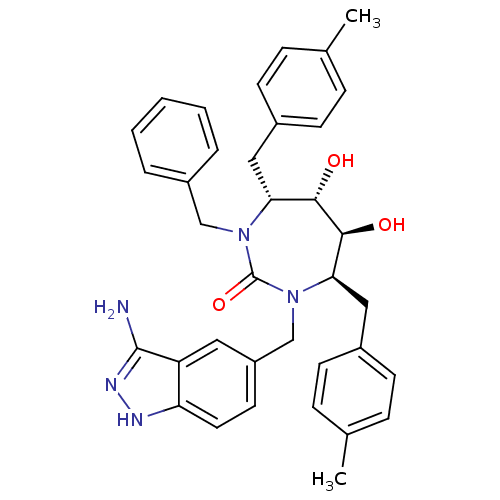 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50457085 (CHEMBL4203542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 mins followed by CGS-21680 addition m... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082401 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069204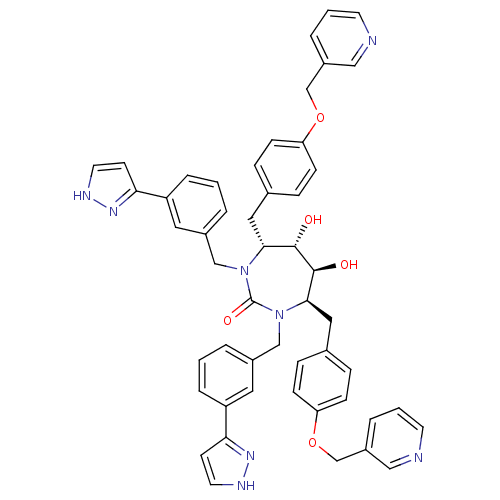 ((4R,5S,6S,7R)-5,6-Dihydroxy-1,3-bis-[3-(1H-pyrazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069199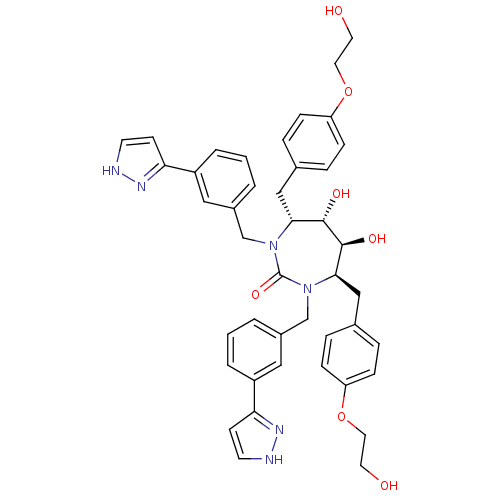 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-[4-(2-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50457098 (CHEMBL4217582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 mins followed by CGS-21680 addition m... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457098 (CHEMBL4217582) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069197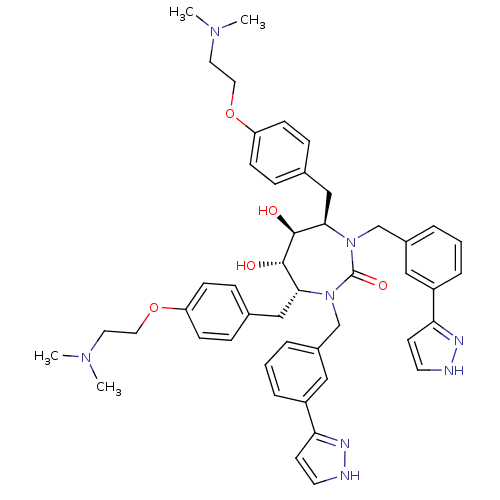 ((4R,5S,6S,7R)-4,7-Bis-[4-(2-dimethylamino-ethoxy)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124718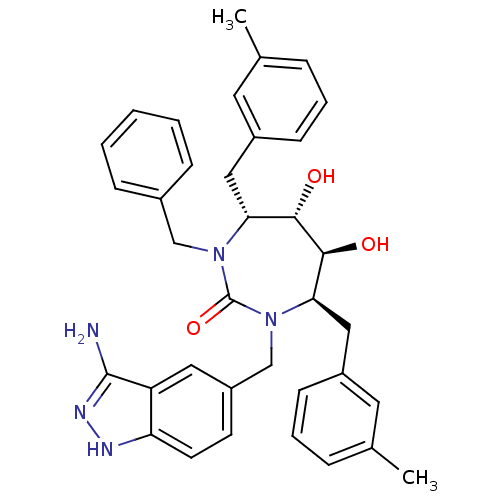 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7089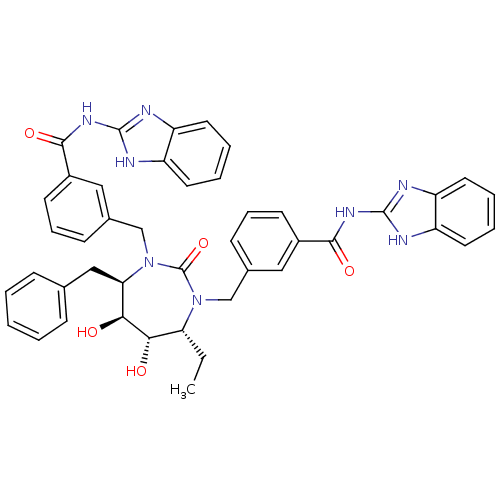 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0690 | -60.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457084 (CHEMBL4217248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069196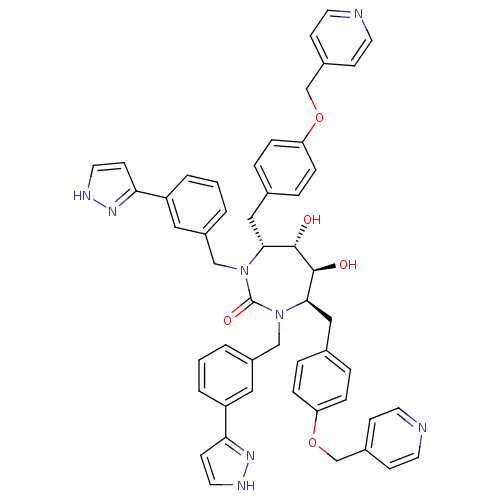 ((4R,5S,6S,7R)-5,6-Dihydroxy-1,3-bis-[3-(1H-pyrazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124723 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069203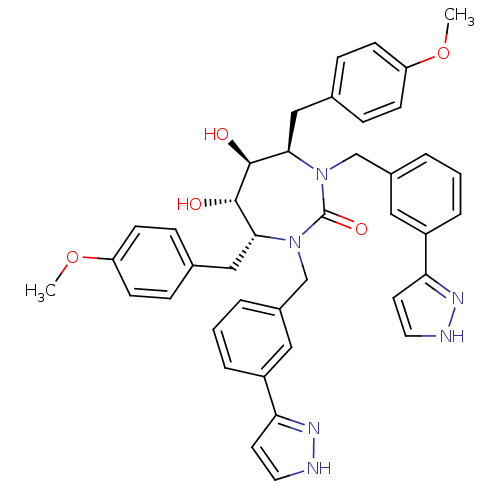 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-(4-methoxy-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7034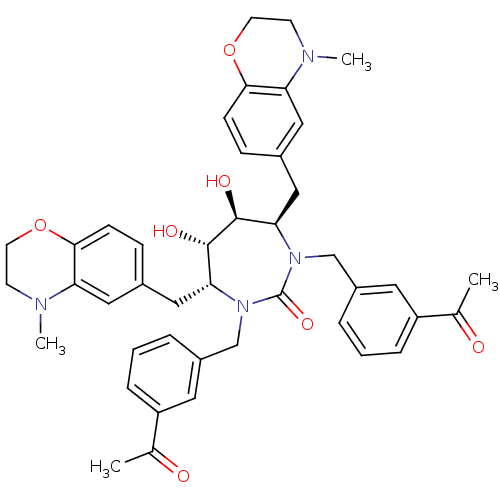 ((4R,5S,6S,7R)-1,3-bis[(3-acetylphenyl)methyl]-5,6-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | -59.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 39: 2156-69 (1996) Article DOI: 10.1021/jm960083n BindingDB Entry DOI: 10.7270/Q257197T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082400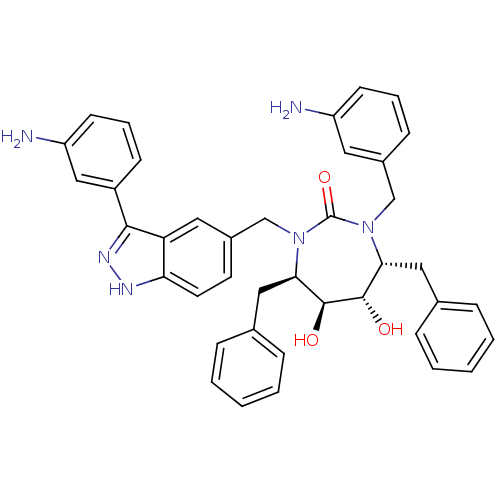 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-3-[3-(3-amino-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124717 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7075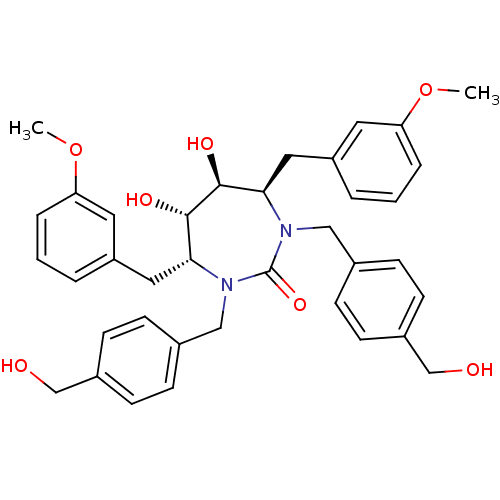 ((4R,5S,6S,7R)-5,6-dihydroxy-1,3-bis({[4-(hydroxyme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -59.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082397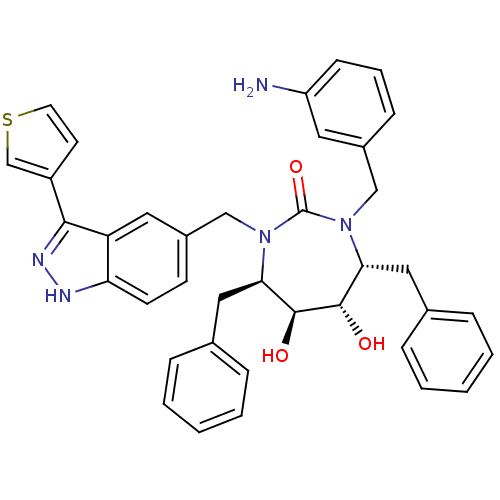 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069206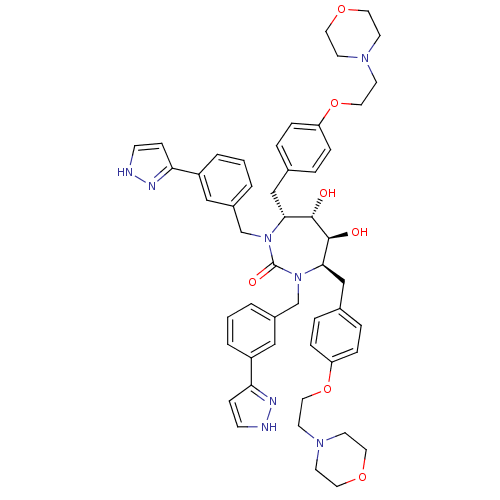 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-[4-(2-morpholi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457085 (CHEMBL4203542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM139889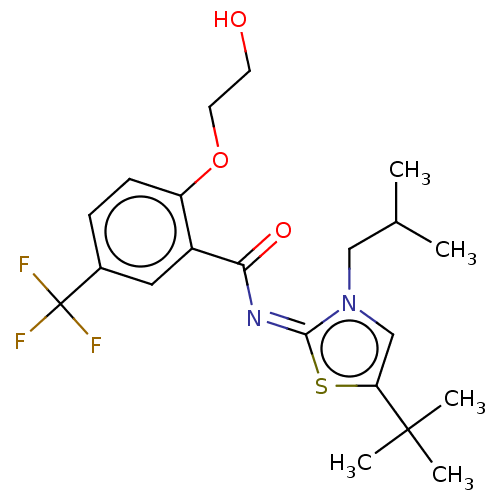 (US8895592, 19) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The CB1 and CB2 radioligand binding assays described herein are utilized to ascertain the selectivity of compounds of the present application for bin... | US Patent US8895592 (2014) BindingDB Entry DOI: 10.7270/Q2CV4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082403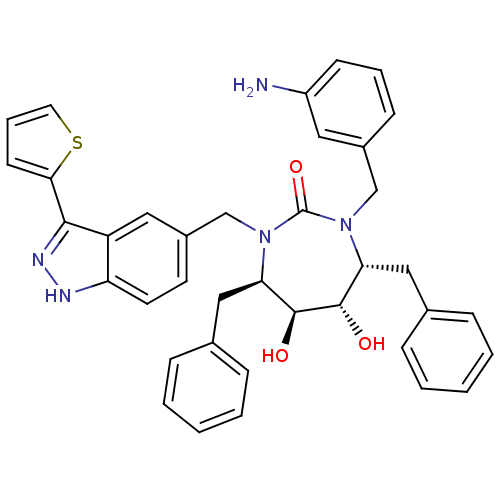 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124719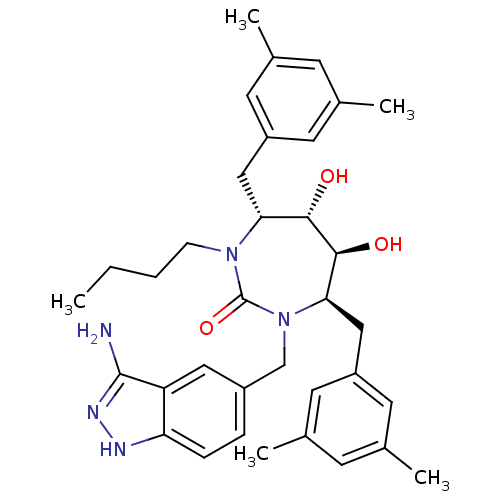 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50235055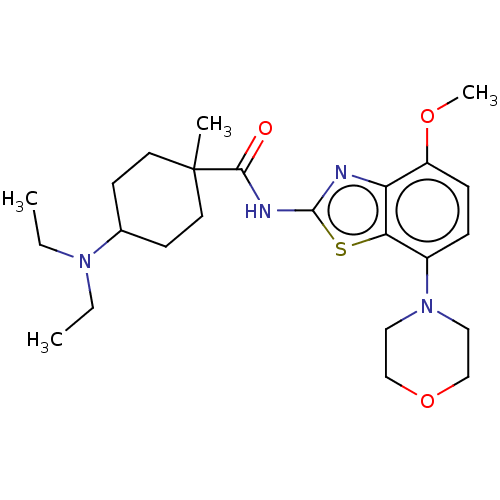 (CHEMBL4095355) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor expressed in HEK293 cell membranes assessed as decrease in CGS-21680/forskolin-induced cAMP level... | J Med Chem 60: 681-694 (2017) Article DOI: 10.1021/acs.jmedchem.6b01584 BindingDB Entry DOI: 10.7270/Q23J3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457093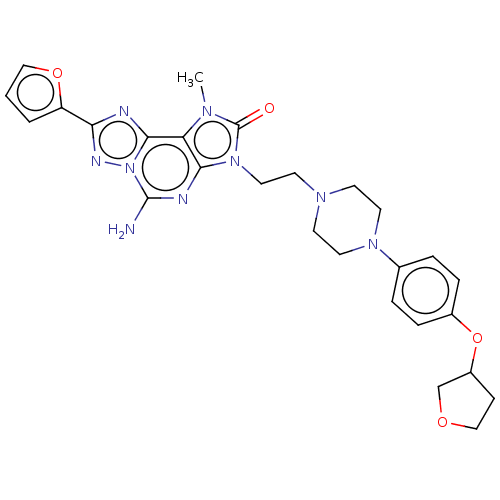 (CHEMBL4212821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 90 mins radioligand binding assay | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124720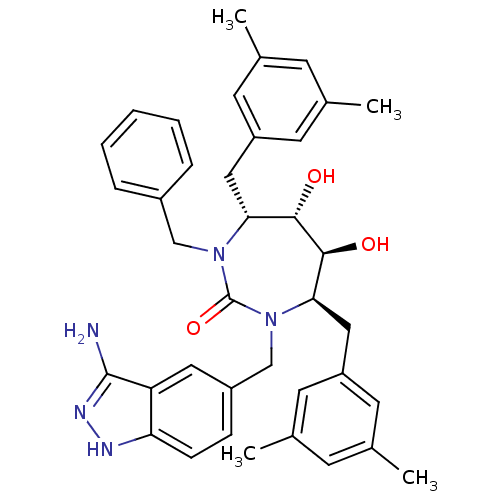 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457099 (CHEMBL4213177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457093 (CHEMBL4212821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7078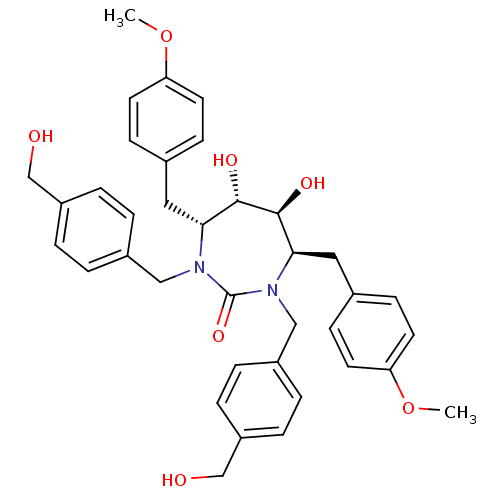 ((4R,5S,6S,7R)-5,6-dihydroxy-1,3-bis({[4-(hydroxyme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | -57.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082396 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457097 (CHEMBL4205884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082398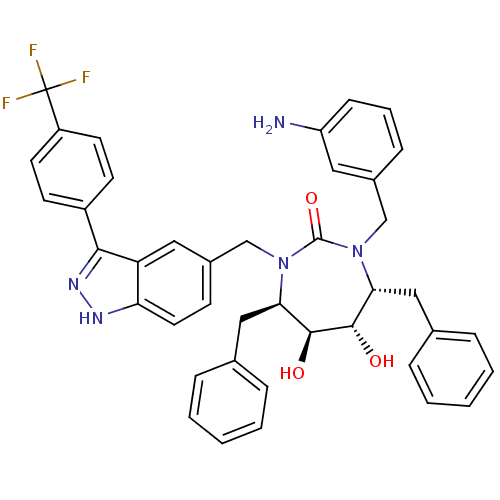 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50235053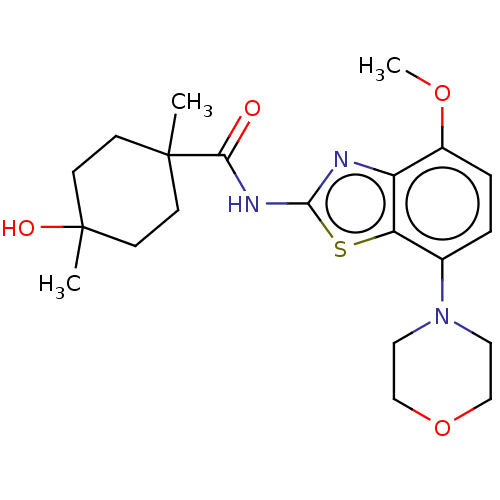 (CHEMBL4064207) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor expressed in HEK293 cell membranes assessed as decrease in CGS-21680/forskolin-induced cAMP level... | J Med Chem 60: 681-694 (2017) Article DOI: 10.1021/acs.jmedchem.6b01584 BindingDB Entry DOI: 10.7270/Q23J3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457092 (CHEMBL4207914) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5739 total ) | Next | Last >> |