 Found 37 hits with Last Name = 'patel' and Initial = 'mg'
Found 37 hits with Last Name = 'patel' and Initial = 'mg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495
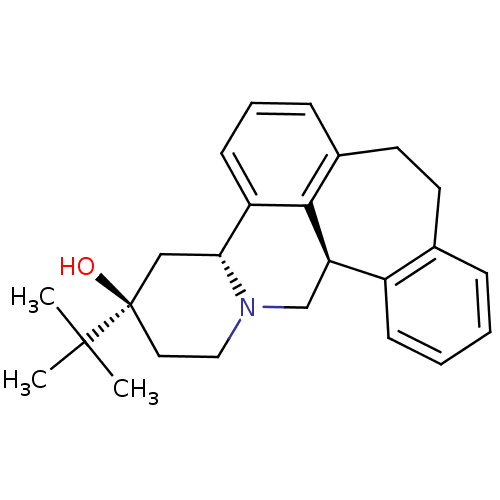
((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291683
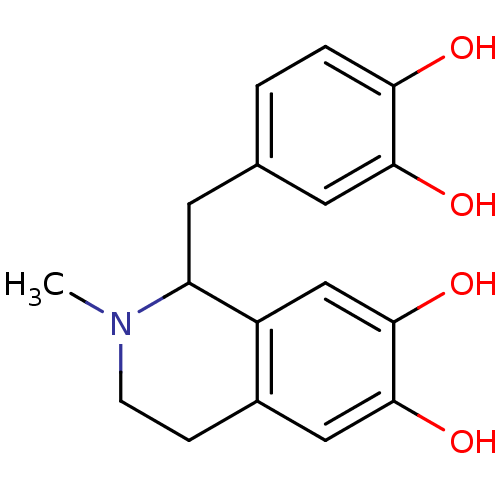
(1-(3,4-Dihydroxy-benzyl)-2-methyl-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C17H19NO4/c1-18-5-4-11-8-16(21)17(22)9-12(11)13(18)6-10-2-3-14(19)15(20)7-10/h2-3,7-9,13,19-22H,4-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681
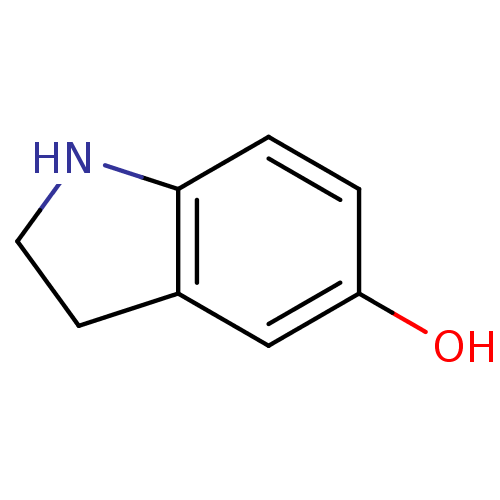
(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614
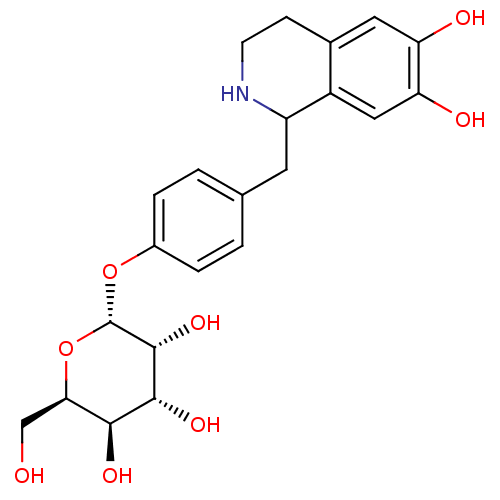
(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291683

(1-(3,4-Dihydroxy-benzyl)-2-methyl-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C17H19NO4/c1-18-5-4-11-8-16(21)17(22)9-12(11)13(18)6-10-2-3-14(19)15(20)7-10/h2-3,7-9,13,19-22H,4-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027331
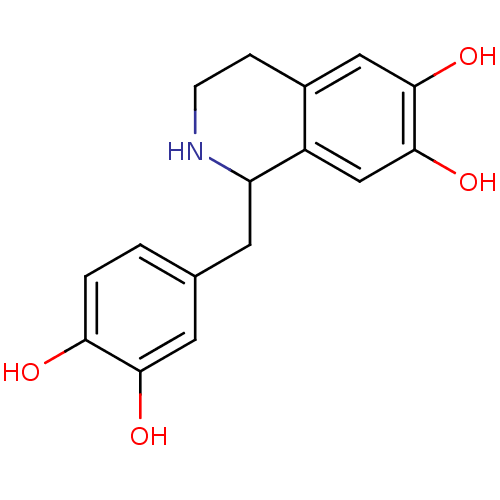
(1-(3,4-dihydroxybenzyl)-1,2,3,4-tetrahydroisoquino...)Show InChI InChI=1S/C16H17NO4/c18-13-2-1-9(6-14(13)19)5-12-11-8-16(21)15(20)7-10(11)3-4-17-12/h1-2,6-8,12,17-21H,3-5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50242856
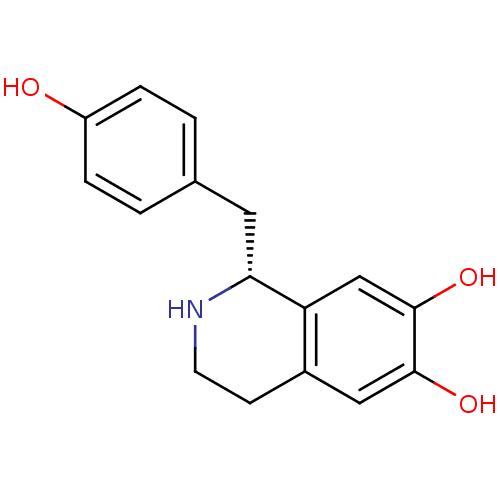
((1R)-1-(4-hydroxybenzyl)-1,2,3,4-tetrahydroisoquin...)Show InChI InChI=1S/C16H17NO3/c18-12-3-1-10(2-4-12)7-14-13-9-16(20)15(19)8-11(13)5-6-17-14/h1-4,8-9,14,17-20H,5-7H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027331

(1-(3,4-dihydroxybenzyl)-1,2,3,4-tetrahydroisoquino...)Show InChI InChI=1S/C16H17NO4/c18-13-2-1-9(6-14(13)19)5-12-11-8-16(21)15(20)7-10(11)3-4-17-12/h1-2,6-8,12,17-21H,3-5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50242856

((1R)-1-(4-hydroxybenzyl)-1,2,3,4-tetrahydroisoquin...)Show InChI InChI=1S/C16H17NO3/c18-12-3-1-10(2-4-12)7-14-13-9-16(20)15(19)8-11(13)5-6-17-14/h1-4,8-9,14,17-20H,5-7H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50120316
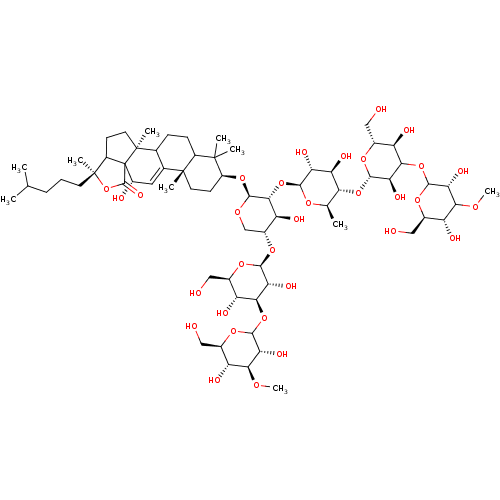
(CHEMBL2367887 | Triterpene Glycoside analogue)Show SMILES CO[C@H]1[C@H](O)[C@@H](CO)OC(O[C@H]2[C@H](O)[C@@H](CO)O[C@@H](O[C@@H]3CO[C@@H](O[C@H]4CC[C@@]5(C)C(CCC6C5=C[C@H](O)C57C(CC[C@@]65C)[C@](C)(CCCC(C)C)OC7=O)C4(C)C)[C@H](O[C@@H]4O[C@H](C)[C@@H](O[C@@H]5O[C@H](CO)[C@@H](O)C(OC6O[C@H](CO)[C@@H](O)C(OC)[C@H]6O)[C@H]5O)[C@H](O)[C@H]4O)[C@H]3O)[C@@H]2O)[C@@H]1O |t:35| Show InChI InChI=1S/C67H110O32/c1-26(2)12-11-17-66(8)36-15-19-65(7)28-13-14-35-63(4,5)38(16-18-64(35,6)29(28)20-37(72)67(36,65)62(84)99-66)94-61-55(43(77)34(25-87-61)93-57-48(82)53(41(75)32(23-70)89-57)96-58-46(80)51(85-9)39(73)30(21-68)90-58)98-56-45(79)44(78)50(27(3)88-56)95-60-49(83)54(42(76)33(24-71)92-60)97-59-47(81)52(86-10)40(74)31(22-69)91-59/h20,26-28,30-61,68-83H,11-19,21-25H2,1-10H3/t27-,28?,30-,31-,32-,33-,34-,35?,36?,37+,38+,39-,40-,41-,42-,43+,44-,45-,46-,47-,48-,49-,50-,51+,52?,53+,54?,55-,56+,57+,58?,59?,60+,61+,64-,65+,66+,67?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human CCR5 chemokine receptor (CCR5) expressed in CHO cells |
Bioorg Med Chem Lett 12: 3203-5 (2002)
BindingDB Entry DOI: 10.7270/Q2MG7NVR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291684

(1-Methyl-1,2,3,4-tetrahydro-isoquinoline-6,7-diol ...)Show InChI InChI=1S/C10H13NO2/c1-6-8-5-10(13)9(12)4-7(8)2-3-11-6/h4-6,11-13H,2-3H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291684

(1-Methyl-1,2,3,4-tetrahydro-isoquinoline-6,7-diol ...)Show InChI InChI=1S/C10H13NO2/c1-6-8-5-10(13)9(12)4-7(8)2-3-11-6/h4-6,11-13H,2-3H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681

(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614

(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50120315
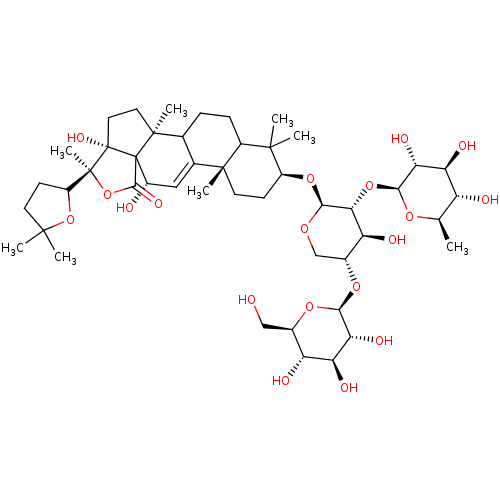
(CHEMBL2367888 | Triterpene Glycoside analogue)Show SMILES C[C@H]1O[C@@H](O[C@@H]2[C@@H](O)[C@@H](CO[C@H]2O[C@H]2CC[C@@]3(C)C(CCC4C3=C[C@H](O)C35C(=O)O[C@@](C)(C6CCC(C)(C)O6)[C@@]3(O)CC[C@@]45C)C2(C)C)O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O |t:24| Show InChI InChI=1S/C47H74O19/c1-20-29(50)32(53)34(55)37(60-20)64-36-31(52)24(62-38-35(56)33(54)30(51)23(18-48)61-38)19-59-39(36)63-27-12-14-43(6)22-17-26(49)47-40(57)66-45(8,28-11-13-41(2,3)65-28)46(47,58)16-15-44(47,7)21(22)9-10-25(43)42(27,4)5/h17,20-21,23-39,48-56,58H,9-16,18-19H2,1-8H3/t20-,21?,23-,24-,25?,26+,27+,28?,29-,30-,31+,32+,33+,34-,35-,36-,37+,38+,39+,43-,44+,45+,46+,47?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human CCR5 chemokine receptor (CCR5) expressed in CHO cells |
Bioorg Med Chem Lett 12: 3203-5 (2002)
BindingDB Entry DOI: 10.7270/Q2MG7NVR |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50292438
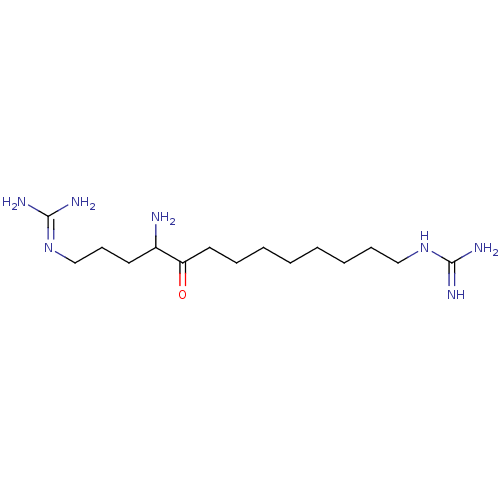
(1,-(4-amino-5-oxotridecane-1,13-diyl)diguanidine |...)Show SMILES [#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C15H33N7O/c16-12(8-7-11-22-15(19)20)13(23)9-5-3-1-2-4-6-10-21-14(17)18/h12H,1-11,16H2,(H4,17,18,21)(H4,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of QNB from muscarinic M2 receptor |
J Nat Prod 58: 843-847 (1995)
Article DOI: 10.1021/np50120a004
BindingDB Entry DOI: 10.7270/Q2FJ2GS1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50292438

(1,-(4-amino-5-oxotridecane-1,13-diyl)diguanidine |...)Show SMILES [#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C15H33N7O/c16-12(8-7-11-22-15(19)20)13(23)9-5-3-1-2-4-6-10-21-14(17)18/h12H,1-11,16H2,(H4,17,18,21)(H4,19,20,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of QNB from muscarinic M1 receptor |
J Nat Prod 58: 843-847 (1995)
Article DOI: 10.1021/np50120a004
BindingDB Entry DOI: 10.7270/Q2FJ2GS1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50292438

(1,-(4-amino-5-oxotridecane-1,13-diyl)diguanidine |...)Show SMILES [#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C15H33N7O/c16-12(8-7-11-22-15(19)20)13(23)9-5-3-1-2-4-6-10-21-14(17)18/h12H,1-11,16H2,(H4,17,18,21)(H4,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of QNB from muscarinic M4 receptor |
J Nat Prod 58: 843-847 (1995)
Article DOI: 10.1021/np50120a004
BindingDB Entry DOI: 10.7270/Q2FJ2GS1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50292439
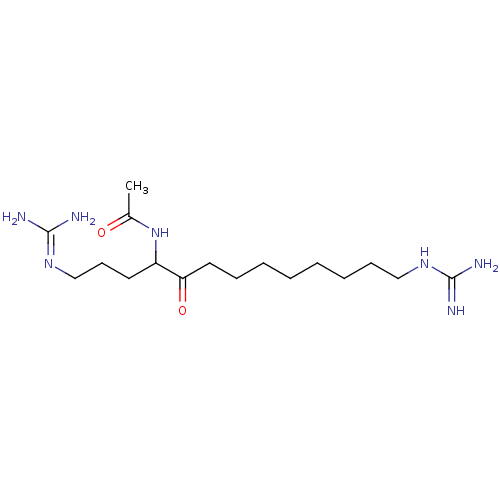
(CHEMBL479224 | N-(1,13-diguanidino-5-oxotridecan-4...)Show SMILES [#6]-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C17H35N7O2/c1-13(25)24-14(9-8-12-23-17(20)21)15(26)10-6-4-2-3-5-7-11-22-16(18)19/h14H,2-12H2,1H3,(H,24,25)(H4,18,19,22)(H4,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of QNB from muscarinic M4 receptor |
J Nat Prod 58: 843-847 (1995)
Article DOI: 10.1021/np50120a004
BindingDB Entry DOI: 10.7270/Q2FJ2GS1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50292439

(CHEMBL479224 | N-(1,13-diguanidino-5-oxotridecan-4...)Show SMILES [#6]-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C17H35N7O2/c1-13(25)24-14(9-8-12-23-17(20)21)15(26)10-6-4-2-3-5-7-11-22-16(18)19/h14H,2-12H2,1H3,(H,24,25)(H4,18,19,22)(H4,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of QNB from muscarinic M2 receptor |
J Nat Prod 58: 843-847 (1995)
Article DOI: 10.1021/np50120a004
BindingDB Entry DOI: 10.7270/Q2FJ2GS1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681

(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [3H]spiperone to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50292439

(CHEMBL479224 | N-(1,13-diguanidino-5-oxotridecan-4...)Show SMILES [#6]-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C17H35N7O2/c1-13(25)24-14(9-8-12-23-17(20)21)15(26)10-6-4-2-3-5-7-11-22-16(18)19/h14H,2-12H2,1H3,(H,24,25)(H4,18,19,22)(H4,20,21,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of QNB from muscarinic M1 receptor |
J Nat Prod 58: 843-847 (1995)
Article DOI: 10.1021/np50120a004
BindingDB Entry DOI: 10.7270/Q2FJ2GS1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50290331
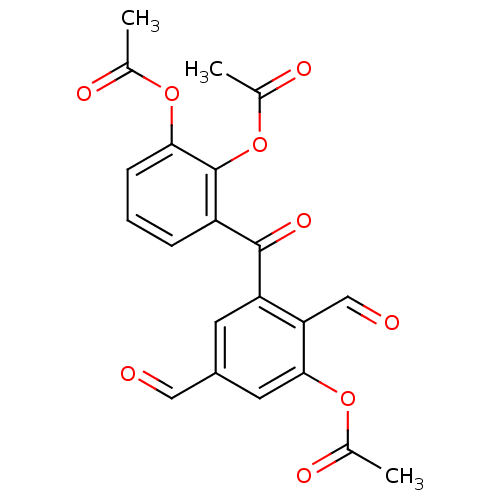
(Acetic acid 2-acetoxy-6-(3-acetoxy-2,5-diformyl-be...)Show SMILES CC(=O)Oc1cccc(C(=O)c2cc(C=O)cc(OC(C)=O)c2C=O)c1OC(C)=O Show InChI InChI=1S/C21H16O9/c1-11(24)28-18-6-4-5-15(21(18)30-13(3)26)20(27)16-7-14(9-22)8-19(17(16)10-23)29-12(2)25/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
Bioorg Med Chem Lett 7: 2547-2550 (1997)
Article DOI: 10.1016/S0960-894X(97)10012-9
BindingDB Entry DOI: 10.7270/Q2RN37WP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614

(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [3H]spiperone to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50292388
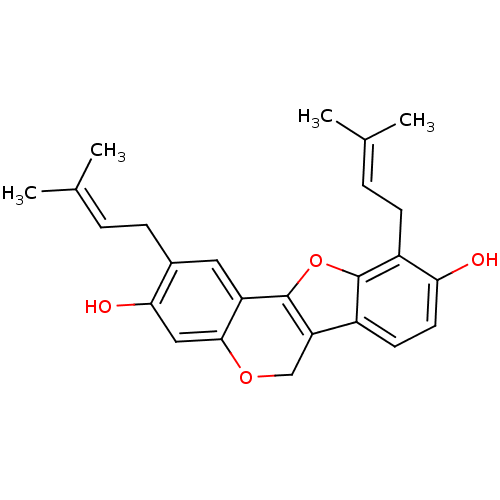
(3,9-dihydroxy-2,10-diprenylpterocap-6a-ene | CHEMB...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2-c3oc4c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])ccc4c3-[#6]-[#8]-c2cc1-[#8] Show InChI InChI=1S/C25H26O4/c1-14(2)5-7-16-11-19-23(12-22(16)27)28-13-20-17-9-10-21(26)18(8-6-15(3)4)24(17)29-25(19)20/h5-6,9-12,26-27H,7-8,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLA2 in fMLP and A23187 ionophore-stimulated human HL60 cells assessed as effect on [3H]arachidonic acid release |
J Nat Prod 60: 537-539 (1997)
Article DOI: 10.1021/np960533e
BindingDB Entry DOI: 10.7270/Q21G0M8S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50290330
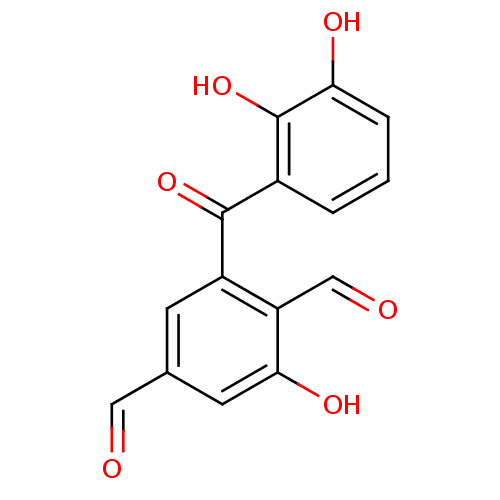
(2-(2,3-Dihydroxy-benzoyl)-6-hydroxy-benzene-1,4-di...)Show InChI InChI=1S/C15H10O6/c16-6-8-4-10(11(7-17)13(19)5-8)14(20)9-2-1-3-12(18)15(9)21/h1-7,18-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
Bioorg Med Chem Lett 7: 2547-2550 (1997)
Article DOI: 10.1016/S0960-894X(97)10012-9
BindingDB Entry DOI: 10.7270/Q2RN37WP |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50241416
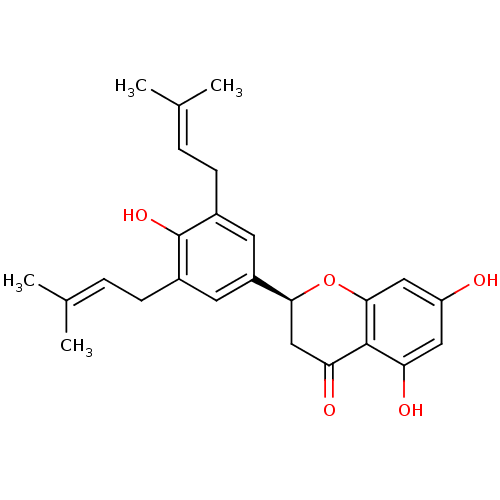
((2S)-5,7-dihydroxy-2-[4-hydroxy-3,5-bis(3-methylbu...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(cc(-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2c(-[#8])cc(-[#8])cc2-[#8]-1 |r| Show InChI InChI=1S/C25H28O5/c1-14(2)5-7-16-9-18(10-17(25(16)29)8-6-15(3)4)22-13-21(28)24-20(27)11-19(26)12-23(24)30-22/h5-6,9-12,22,26-27,29H,7-8,13H2,1-4H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLA2 in fMLP and A23187 ionophore-stimulated human HL60 cells assessed as effect on [3H]arachidonic acid release |
J Nat Prod 60: 537-539 (1997)
Article DOI: 10.1021/np960533e
BindingDB Entry DOI: 10.7270/Q21G0M8S |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50292387

(4'-hydroxy-6,3',5'-triprenylisoflavonone | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(cc(-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8])-[#6]-1-[#6]-[#6](=O)-c2c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc2-[#8]-1 Show InChI InChI=1S/C30H36O5/c1-17(2)7-10-20-13-22(14-21(29(20)33)11-8-18(3)4)26-16-25(32)28-27(35-26)15-24(31)23(30(28)34)12-9-19(5)6/h7-9,13-15,26,31,33-34H,10-12,16H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLA2 in fMLP and A23187 ionophore-stimulated human HL60 cells assessed as effect on [3H]arachidonic acid release |
J Nat Prod 60: 537-539 (1997)
Article DOI: 10.1021/np960533e
BindingDB Entry DOI: 10.7270/Q21G0M8S |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681

(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [3H]spiperone to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614

(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [3H]spiperone to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data