

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50405732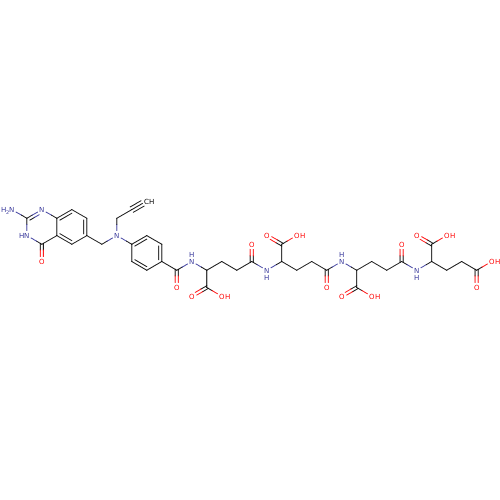 (CHEMBL290277) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pedagogical University Curated by ChEMBL | Assay Description Compound was tested for inhibition of human (WI-L2) thymidylate synthase | J Med Chem 32: 160-5 (1989) BindingDB Entry DOI: 10.7270/Q2RR20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50405733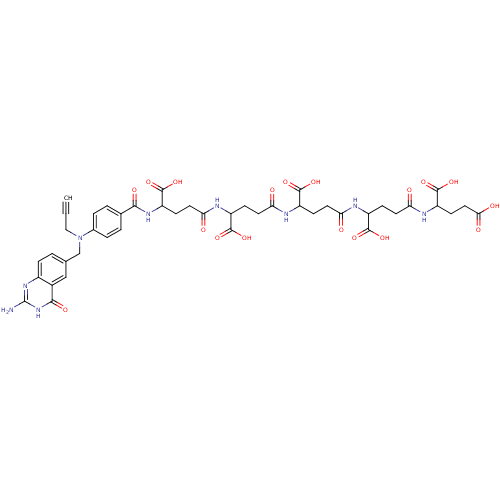 (CHEMBL406575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pedagogical University Curated by ChEMBL | Assay Description Compound was tested for inhibition of human (WI-L2) thymidylate synthase | J Med Chem 32: 160-5 (1989) BindingDB Entry DOI: 10.7270/Q2RR20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50405731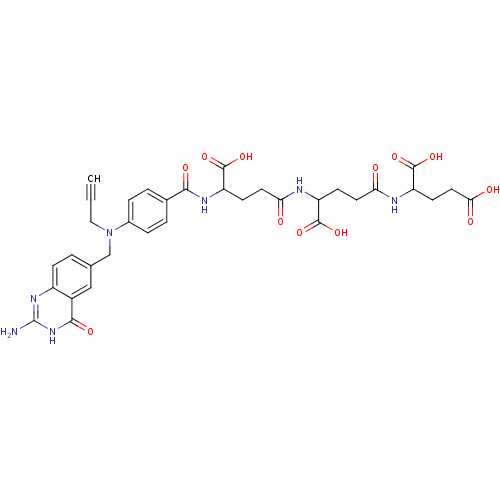 (CHEMBL295708) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pedagogical University Curated by ChEMBL | Assay Description Compound was tested for inhibition of human (WI-L2) thymidylate synthase | J Med Chem 32: 160-5 (1989) BindingDB Entry DOI: 10.7270/Q2RR20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50405730 (CHEMBL295681) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pedagogical University Curated by ChEMBL | Assay Description Compound was tested for inhibition of human (WI-L2) thymidylate synthase | J Med Chem 32: 160-5 (1989) BindingDB Entry DOI: 10.7270/Q2RR20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406713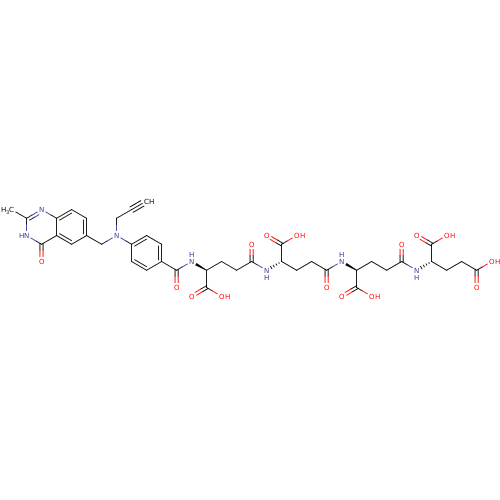 (CHEMBL1202139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406717 (CHEMBL1202137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406714 (CHEMBL264807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406721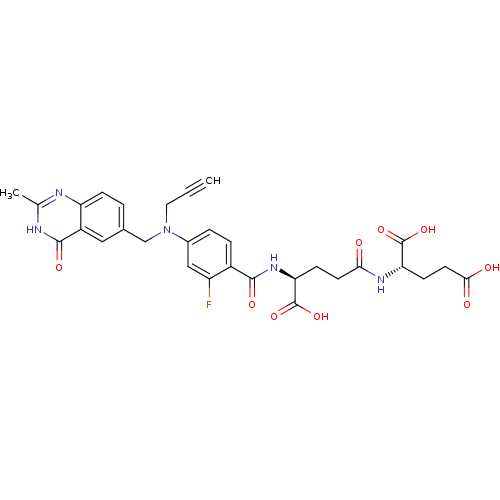 (CHEMBL171226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406716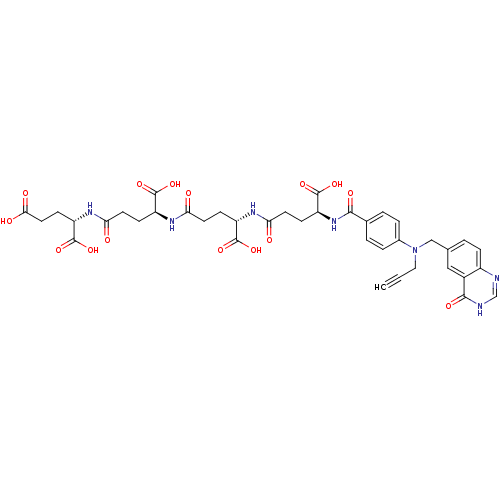 (CHEMBL1202138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406718 (CHEMBL1202140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406719 (CHEMBL436448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049164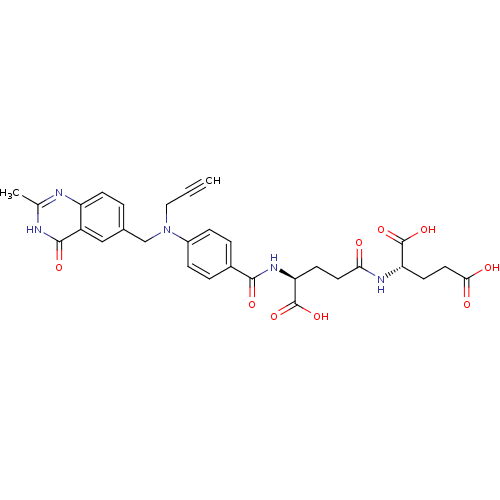 ((S)-2-((S)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50028408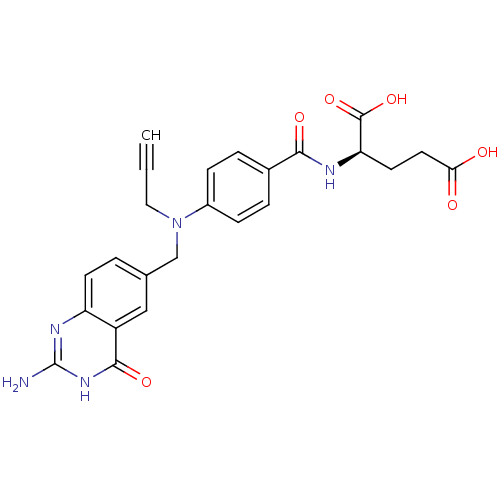 ((R)-2-{4-[(2-Amino-4-oxo-1,4-dihydro-quinazolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for binding affinity against thymidylate synthase(TS) | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406711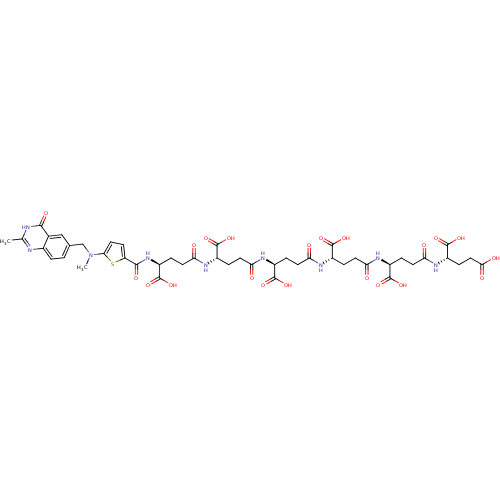 (CHEMBL405513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406712 (CHEMBL268593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406720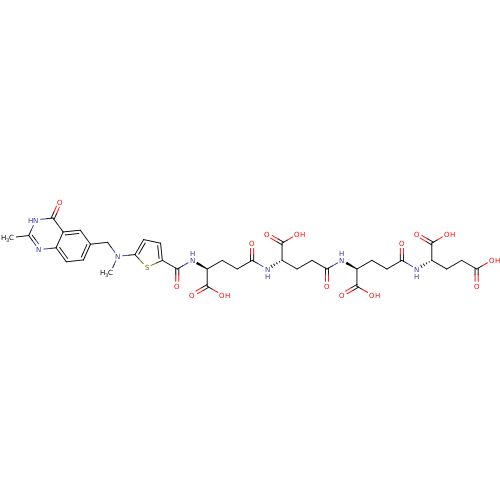 (CHEMBL172160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50027900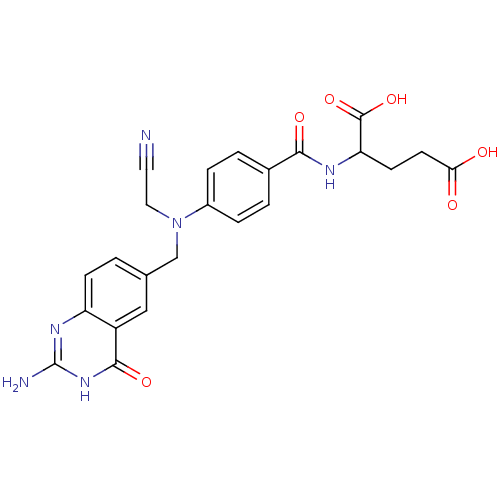 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pedagogical University Curated by ChEMBL | Assay Description Compound was tested for inhibition of human (WI-L2) thymidylate synthase | J Med Chem 32: 160-5 (1989) BindingDB Entry DOI: 10.7270/Q2RR20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406715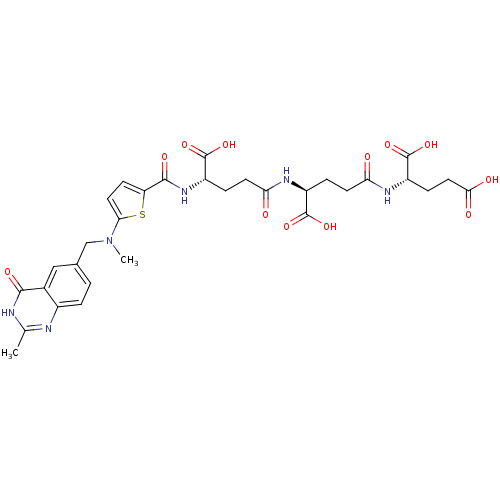 (CHEMBL355321) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033900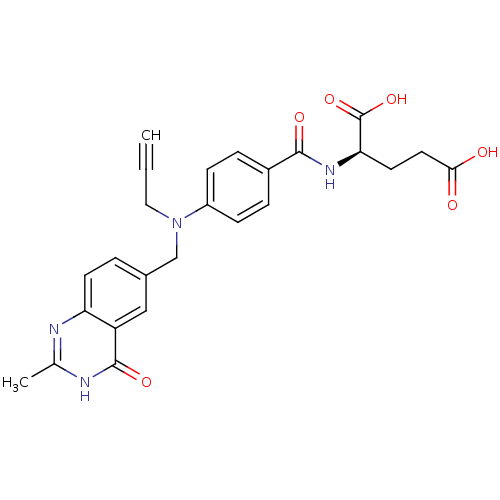 ((R)-2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for binding affinity against thymidylate synthase(TS) | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006689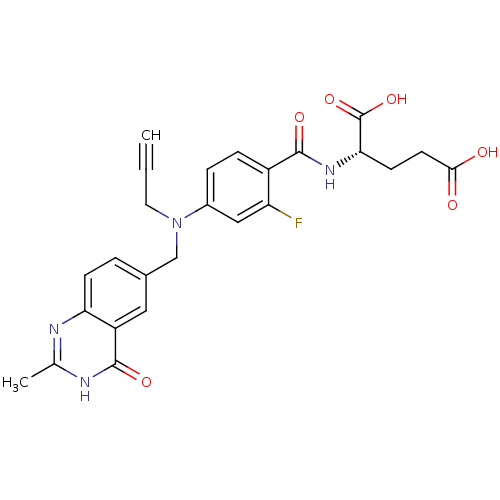 ((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406722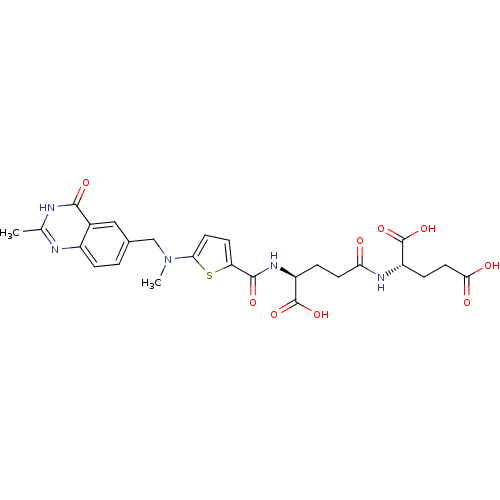 (CHEMBL353302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037311 ((R)-2-({5-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for binding affinity against thymidylate synthase(TS) | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014480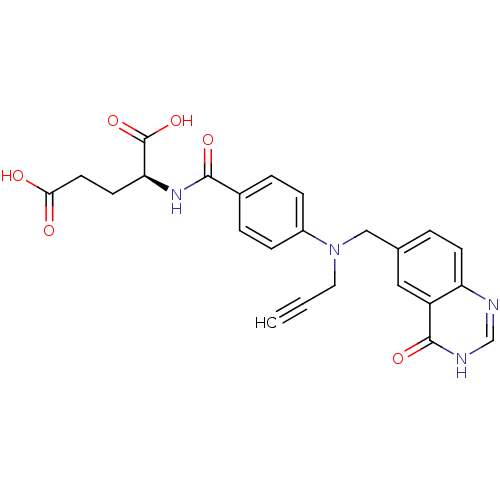 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004372 (2-{4-[(4-Chloro-2-methyl-quinolin-6-ylmethyl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18795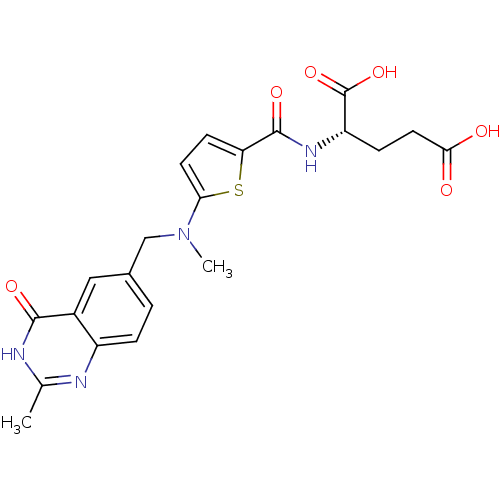 ((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 418 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells that overproduce thymidylate syntha... | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037303 ((R)-2-((R)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037316 ((R)-2-((R)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037313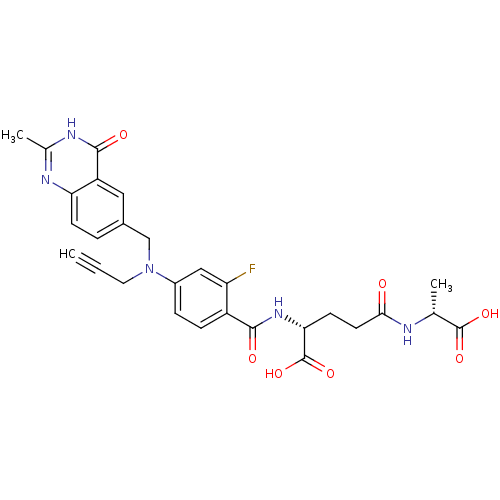 ((R)-4-((R)-1-Carboxy-ethylcarbamoyl)-2-{2-fluoro-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037314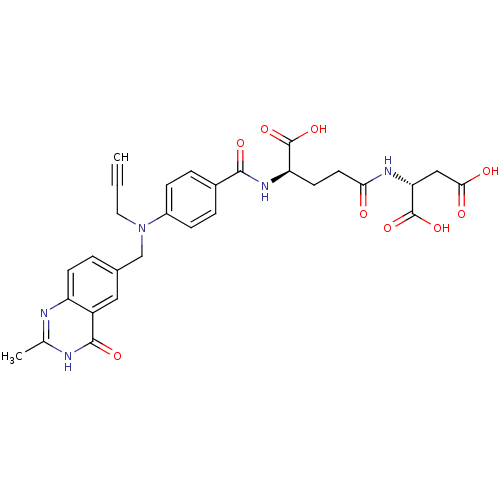 ((R)-2-((R)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037319 ((R)-4-(Carboxymethyl-carbamoyl)-2-{4-[(2-methyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037306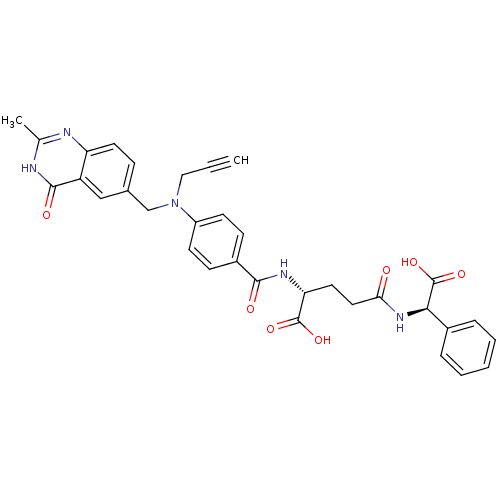 ((R)-4-[((R)-Carboxy-phenyl-methyl)-carbamoyl]-2-{4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037305 ((R)-4-(3-Carboxy-propylcarbamoyl)-2-{4-[(2-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037308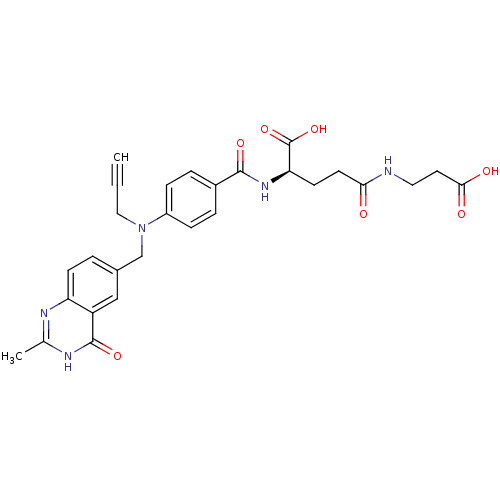 ((R)-4-(2-Carboxy-ethylcarbamoyl)-2-{4-[(2-methyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037302 ((R)-2-((R)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037309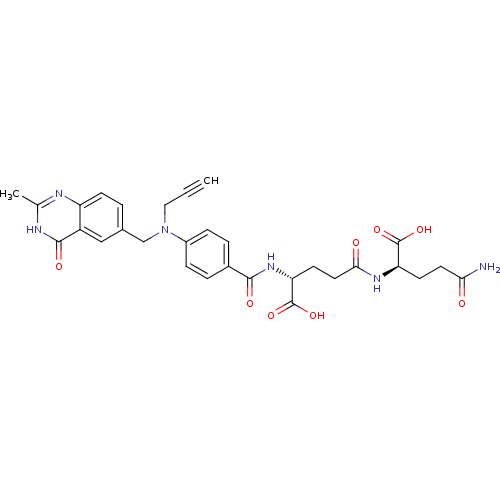 ((R)-4-((R)-3-Carbamoyl-1-carboxy-propylcarbamoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037315 ((R)-4-((R)-1-Carboxy-propylcarbamoyl)-2-{4-[(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037307 ((R)-4-((R)-1-Carboxy-ethylcarbamoyl)-2-{4-[(2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037321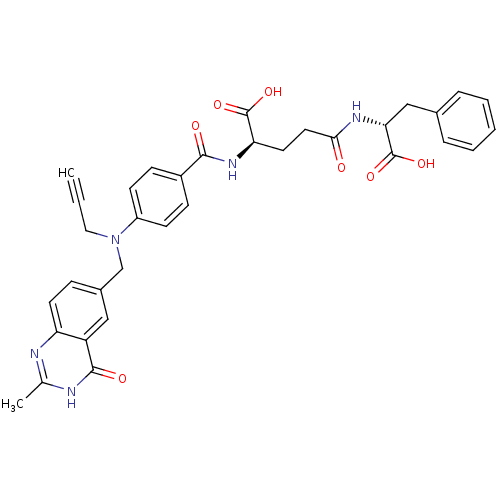 ((R)-4-((R)-1-Carboxy-2-phenyl-ethylcarbamoyl)-2-{4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037310 ((R)-2-((R)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037320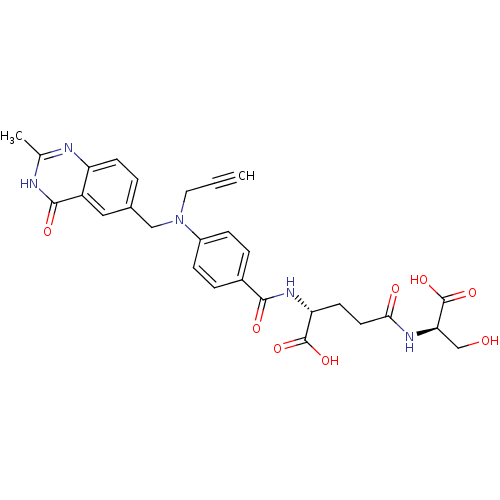 ((R)-4-((R)-1-Carboxy-2-hydroxy-ethylcarbamoyl)-2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50368894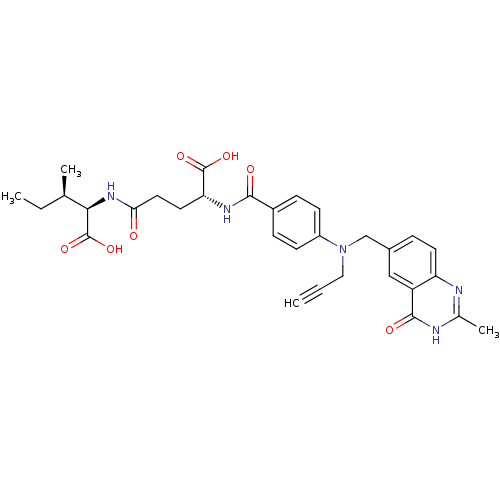 (CHEMBL1790836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037322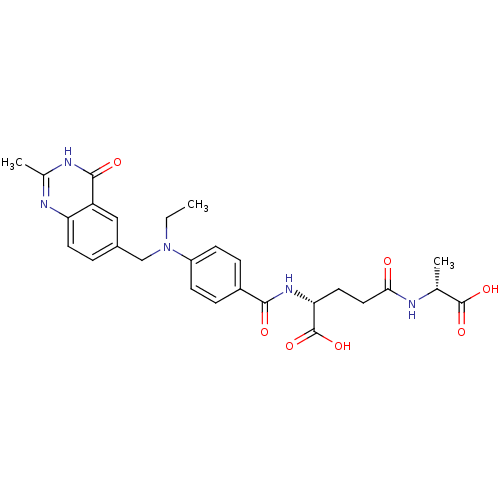 ((R)-4-((R)-1-Carboxy-ethylcarbamoyl)-2-{4-[ethyl-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037317 ((R)-4-Methylcarbamoyl-2-{4-[(2-methyl-4-oxo-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037312 ((R)-4-Ethylcarbamoyl-2-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037304 ((R)-4-Butylcarbamoyl-2-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037301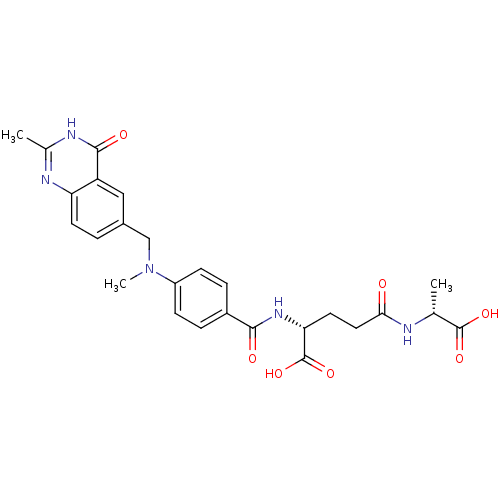 ((R)-4-((R)-1-Carboxy-ethylcarbamoyl)-2-{4-[methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50037318 ((R)-4-Benzylcarbamoyl-2-{4-[(2-methyl-4-oxo-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein complementing XP-A cells (Homo sapiens) | BDBM50241713 (CHEMBL4065804) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Fibrinogen binding to Fibrinogen receptor | J Med Chem 60: 8055-8070 (2017) Article DOI: 10.1021/acs.jmedchem.7b00780 BindingDB Entry DOI: 10.7270/Q2FF3VJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein complementing XP-A cells (Homo sapiens) | BDBM50241670 (CHEMBL4085647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description The compound was tested for the inhibition of fibrinogen receptor | J Med Chem 60: 8055-8070 (2017) Article DOI: 10.1021/acs.jmedchem.7b00780 BindingDB Entry DOI: 10.7270/Q2FF3VJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |