 Found 277 hits with Last Name = 'payne' and Initial = 'rj'
Found 277 hits with Last Name = 'payne' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543
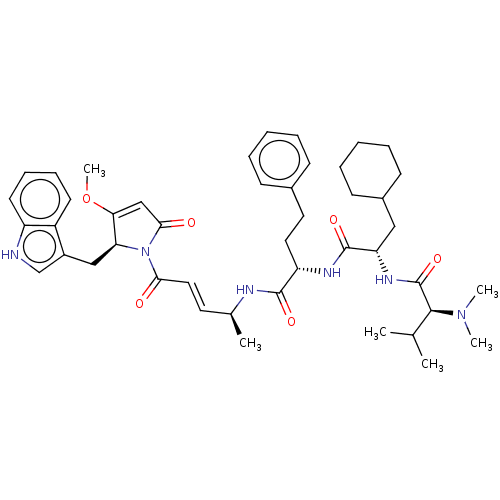
(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 861 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Salicylate synthase
(Mycobacterium tuberculosis) | BDBM92747
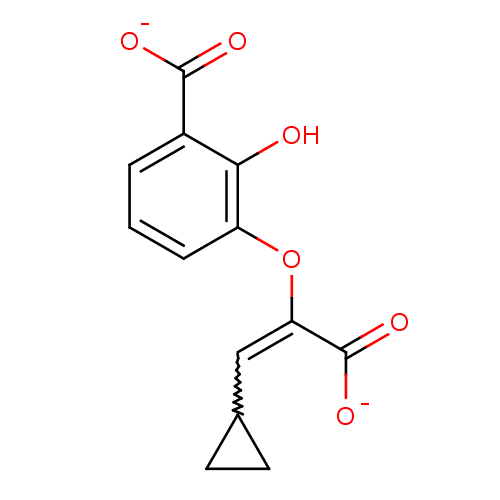
(cyclopropyl-AMT)Show SMILES Oc1c(OC(=CC2CC2)C([O-])=O)cccc1C([O-])=O |w:5.5| Show InChI InChI=1S/C13H12O6/c14-11-8(12(15)16)2-1-3-9(11)19-10(13(17)18)6-7-4-5-7/h1-3,6-7,14H,4-5H2,(H,15,16)(H,17,18)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Auckland
| Assay Description
The thermal stability of MbtI in the absence and presence of inhibitors was measured using a fluorescence-based thermal shift assay. |
Biochemistry 51: 4868-79 (2012)
Article DOI: 10.1021/bi3002067
BindingDB Entry DOI: 10.7270/Q28K77PG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603782
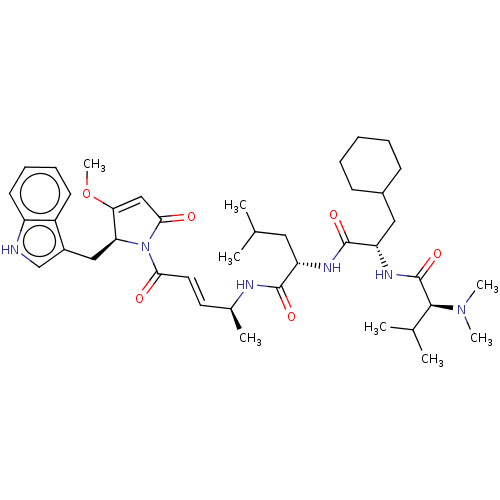
(CHEMBL4596414)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603779
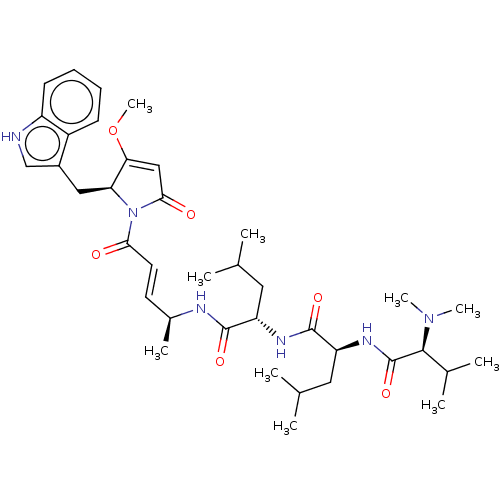
(CHEMBL3558756)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603794

(CHEMBL5184737)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc(cc1)C#N)C(=O)\C=C\[C@@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603788

(CHEMBL5171874)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccccc1)C(=O)\C=C\[C@@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603795

(CHEMBL5175270)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc(cc1)C#N)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603793

(CHEMBL5199542)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc(OC)cc1)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.278 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603792

(CHEMBL5176188)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc(OC)cc1)C(=O)\C=C\[C@@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.304 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603781

(CHEMBL4596941)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.367 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603789

(CHEMBL5183942)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccccc1)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.416 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603783
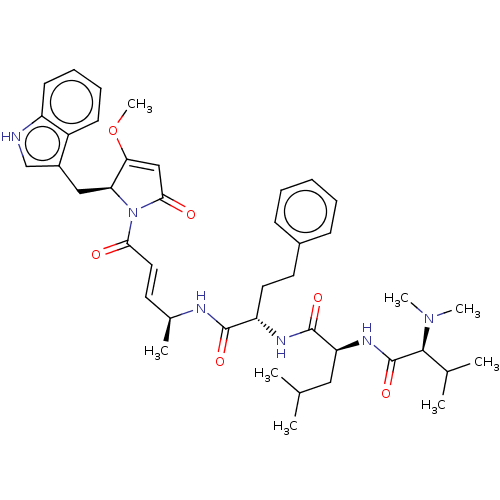
(CHEMBL4595806)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.623 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603790

(CHEMBL5191563)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)\C=C\[C@@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603796

(CHEMBL5204190)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc2ccccc2c1)C(=O)\C=C\[C@@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603787

(CHEMBL4597308)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C)C1CCCCC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603780

(CHEMBL4596992)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603791

(CHEMBL5179302)Show SMILES COC1=CC(=O)N(C1Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50603786

(CHEMBL4595664)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02063
BindingDB Entry DOI: 10.7270/Q2NZ8CQ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data