 Found 501 hits with Last Name = 'pearce' and Initial = 'd'
Found 501 hits with Last Name = 'pearce' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295778
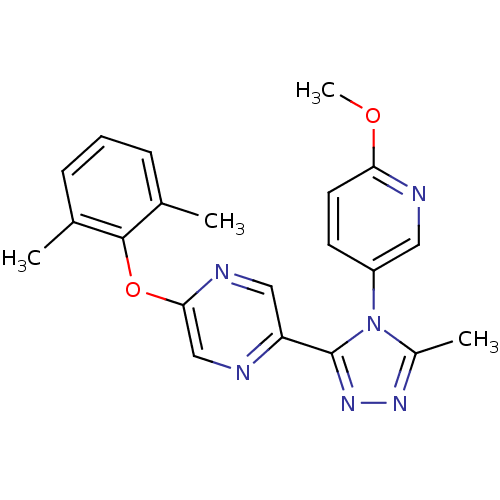
(2-(2,6-dimethylphenoxy)-5-(4-(6-methoxypyridin-3-y...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2c(C)cccc2C)cn1 Show InChI InChI=1S/C21H20N6O2/c1-13-6-5-7-14(2)20(13)29-19-12-22-17(11-24-19)21-26-25-15(3)27(21)16-8-9-18(28-4)23-10-16/h5-12H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50262270
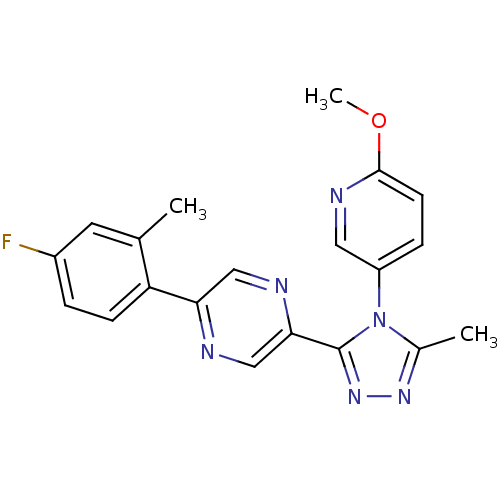
(2-(4-fluoro-2-methylphenyl)-5-(4-(6-methoxypyridin...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(cn1)-c1ccc(F)cc1C Show InChI InChI=1S/C20H17FN6O/c1-12-8-14(21)4-6-16(12)17-10-23-18(11-22-17)20-26-25-13(2)27(20)15-5-7-19(28-3)24-9-15/h4-11H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295779
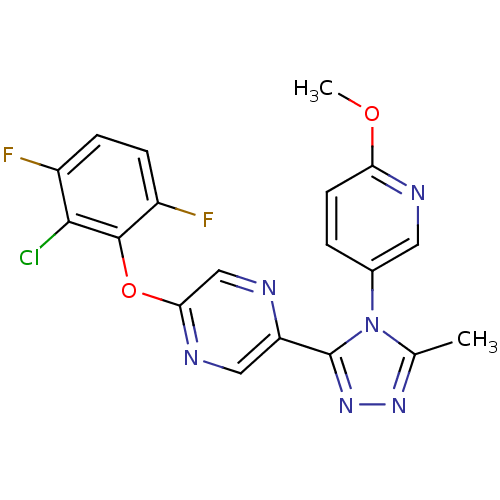
(2-(2-chloro-3,6-difluorophenoxy)-5-(4-(6-methoxypy...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2c(F)ccc(F)c2Cl)cn1 Show InChI InChI=1S/C19H13ClF2N6O2/c1-10-26-27-19(28(10)11-3-6-15(29-2)24-7-11)14-8-25-16(9-23-14)30-18-13(22)5-4-12(21)17(18)20/h3-9H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295777
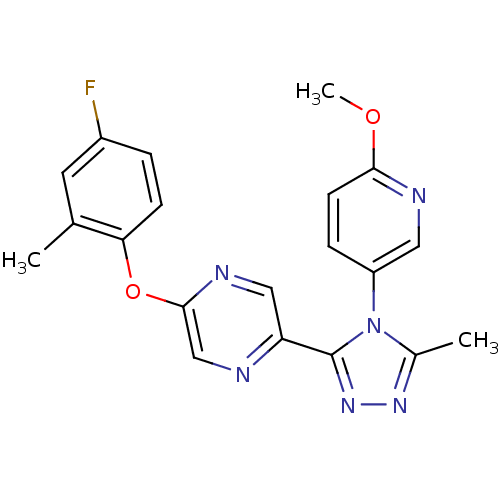
(2-(4-fluoro-2-methylphenoxy)-5-(4-(6-methoxypyridi...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccc(F)cc2C)cn1 Show InChI InChI=1S/C20H17FN6O2/c1-12-8-14(21)4-6-17(12)29-19-11-22-16(10-24-19)20-26-25-13(2)27(20)15-5-7-18(28-3)23-9-15/h4-11H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295772
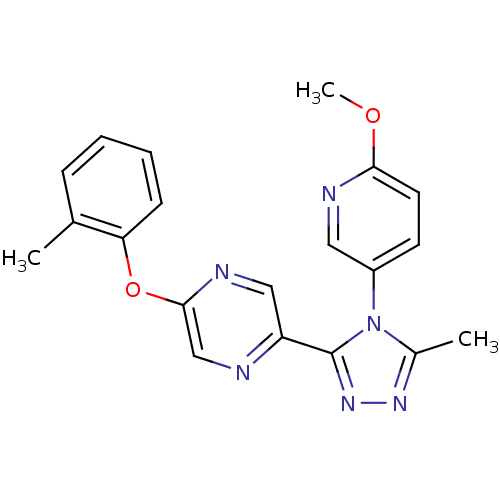
(2-(4-(6-methoxypyridin-3-yl)-5-methyl-4H-1,2,4-tri...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccccc2C)cn1 Show InChI InChI=1S/C20H18N6O2/c1-13-6-4-5-7-17(13)28-19-12-21-16(11-23-19)20-25-24-14(2)26(20)15-8-9-18(27-3)22-10-15/h4-12H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295776
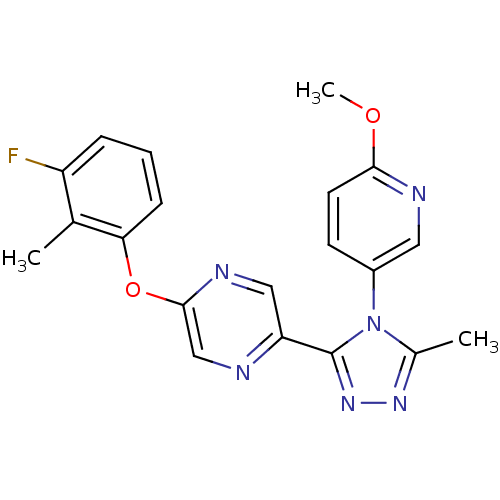
(2-(3-fluoro-2-methylphenoxy)-5-(4-(6-methoxypyridi...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2cccc(F)c2C)cn1 Show InChI InChI=1S/C20H17FN6O2/c1-12-15(21)5-4-6-17(12)29-19-11-22-16(10-24-19)20-26-25-13(2)27(20)14-7-8-18(28-3)23-9-14/h4-11H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257637
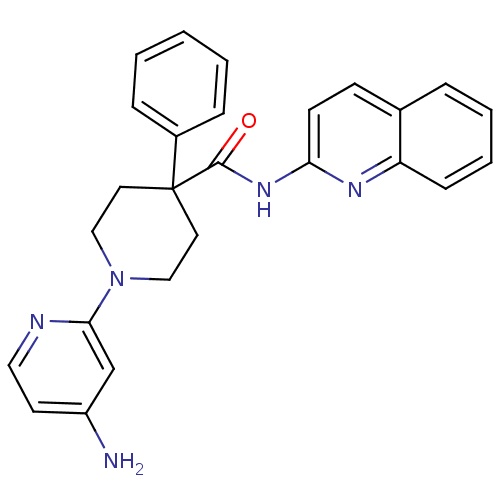
(1-(4-aminopyridin-2-yl)-4-phenyl-N-(quinolin-2-yl)...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)(C(=O)Nc1ccc2ccccc2n1)c1ccccc1 Show InChI InChI=1S/C26H25N5O/c27-21-12-15-28-24(18-21)31-16-13-26(14-17-31,20-7-2-1-3-8-20)25(32)30-23-11-10-19-6-4-5-9-22(19)29-23/h1-12,15,18H,13-14,16-17H2,(H2,27,28)(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257723

(2-(3-phenyl-3H-spiro[isobenzofuran-1,4'-piperidine...)Show SMILES Nc1ccnc(c1)N1CCC2(CC1)OC(c1ccccc21)c1ccccc1 Show InChI InChI=1S/C23H23N3O/c24-18-10-13-25-21(16-18)26-14-11-23(12-15-26)20-9-5-4-8-19(20)22(27-23)17-6-2-1-3-7-17/h1-10,13,16,22H,11-12,14-15H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295771
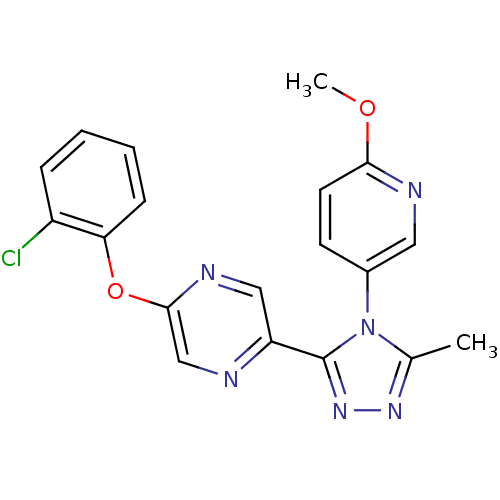
(2-(2-chlorophenoxy)-5-(4-(6-methoxypyridin-3-yl)-5...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccccc2Cl)cn1 Show InChI InChI=1S/C19H15ClN6O2/c1-12-24-25-19(26(12)13-7-8-17(27-2)22-9-13)15-10-23-18(11-21-15)28-16-6-4-3-5-14(16)20/h3-11H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257639
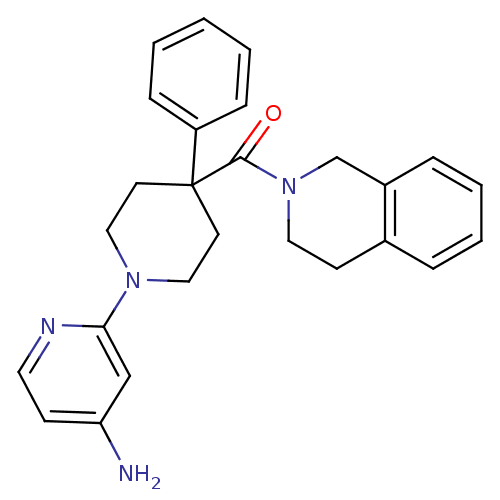
((1-(4-aminopyridin-2-yl)-4-phenylpiperidin-4-yl)(3...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)(C(=O)N1CCc2ccccc2C1)c1ccccc1 Show InChI InChI=1S/C26H28N4O/c27-23-10-14-28-24(18-23)29-16-12-26(13-17-29,22-8-2-1-3-9-22)25(31)30-15-11-20-6-4-5-7-21(20)19-30/h1-10,14,18H,11-13,15-17,19H2,(H2,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295780
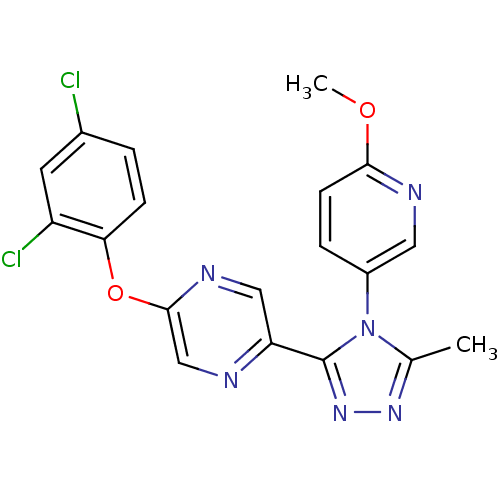
(2-(2,4-dichlorophenoxy)-5-(4-(6-methoxypyridin-3-y...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccc(Cl)cc2Cl)cn1 Show InChI InChI=1S/C19H14Cl2N6O2/c1-11-25-26-19(27(11)13-4-6-17(28-2)23-8-13)15-9-24-18(10-22-15)29-16-5-3-12(20)7-14(16)21/h3-10H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295773

(2-(2-ethylphenoxy)-5-(4-(6-methoxypyridin-3-yl)-5-...)Show SMILES CCc1ccccc1Oc1cnc(cn1)-c1nnc(C)n1-c1ccc(OC)nc1 Show InChI InChI=1S/C21H20N6O2/c1-4-15-7-5-6-8-18(15)29-20-13-22-17(12-24-20)21-26-25-14(2)27(21)16-9-10-19(28-3)23-11-16/h5-13H,4H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257684
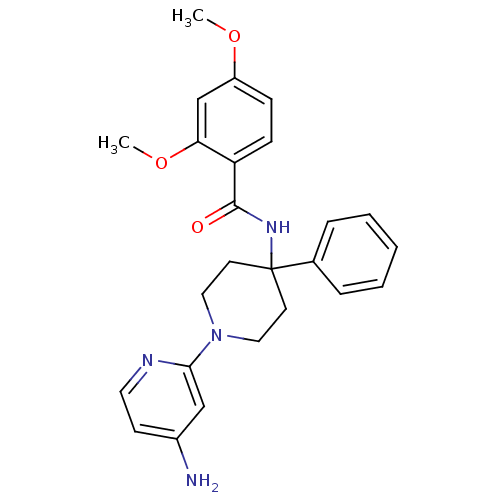
(CHEMBL495346 | N-(1-(4-aminopyridin-2-yl)-4-phenyl...)Show SMILES COc1ccc(C(=O)NC2(CCN(CC2)c2cc(N)ccn2)c2ccccc2)c(OC)c1 Show InChI InChI=1S/C25H28N4O3/c1-31-20-8-9-21(22(17-20)32-2)24(30)28-25(18-6-4-3-5-7-18)11-14-29(15-12-25)23-16-19(26)10-13-27-23/h3-10,13,16-17H,11-12,14-15H2,1-2H3,(H2,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257638
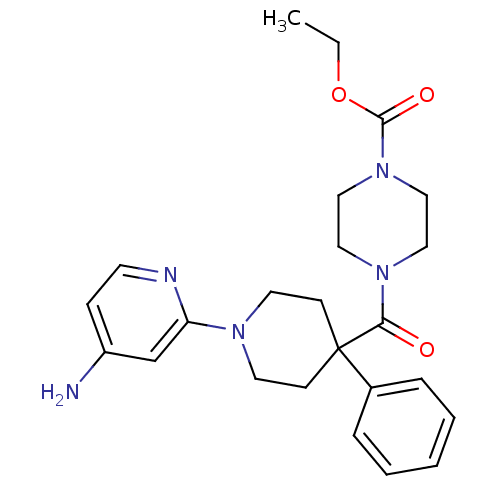
(CHEMBL493743 | ethyl 4-(1-(4-aminopyridin-2-yl)-4-...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)C1(CCN(CC1)c1cc(N)ccn1)c1ccccc1 Show InChI InChI=1S/C24H31N5O3/c1-2-32-23(31)29-16-14-28(15-17-29)22(30)24(19-6-4-3-5-7-19)9-12-27(13-10-24)21-18-20(25)8-11-26-21/h3-8,11,18H,2,9-10,12-17H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295775
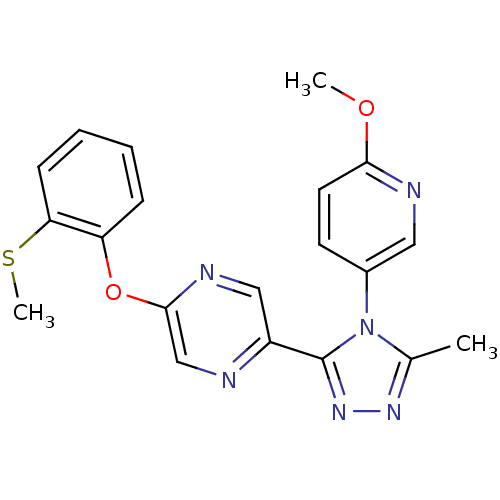
(2-(4-(6-methoxypyridin-3-yl)-5-methyl-4H-1,2,4-tri...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccccc2SC)cn1 Show InChI InChI=1S/C20H18N6O2S/c1-13-24-25-20(26(13)14-8-9-18(27-2)22-10-14)15-11-23-19(12-21-15)28-16-6-4-5-7-17(16)29-3/h4-12H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257683
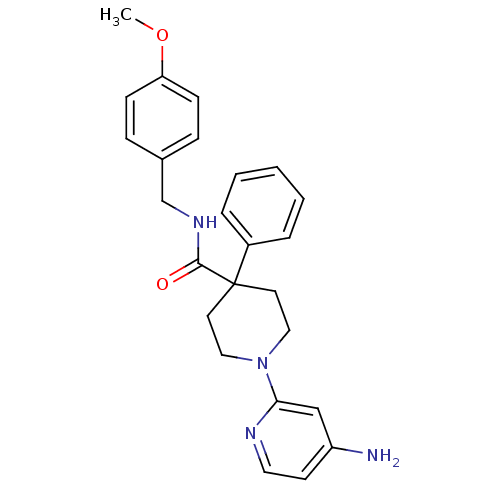
(1-(4-aminopyridin-2-yl)-N-(4-methoxybenzyl)-4-phen...)Show SMILES COc1ccc(CNC(=O)C2(CCN(CC2)c2cc(N)ccn2)c2ccccc2)cc1 Show InChI InChI=1S/C25H28N4O2/c1-31-22-9-7-19(8-10-22)18-28-24(30)25(20-5-3-2-4-6-20)12-15-29(16-13-25)23-17-21(26)11-14-27-23/h2-11,14,17H,12-13,15-16,18H2,1H3,(H2,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295774
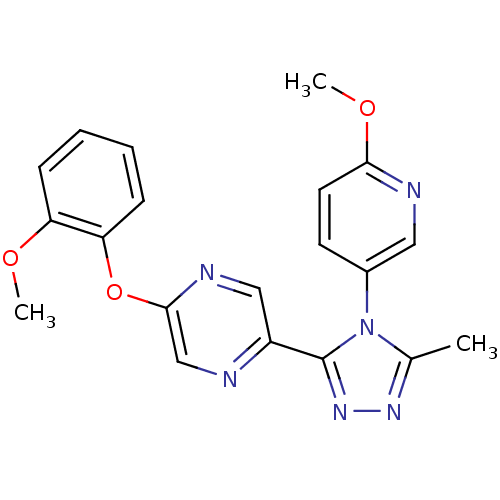
(2-(2-methoxyphenoxy)-5-(4-(6-methoxypyridin-3-yl)-...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccccc2OC)cn1 Show InChI InChI=1S/C20H18N6O3/c1-13-24-25-20(26(13)14-8-9-18(28-3)22-10-14)15-11-23-19(12-21-15)29-17-7-5-4-6-16(17)27-2/h4-12H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257685
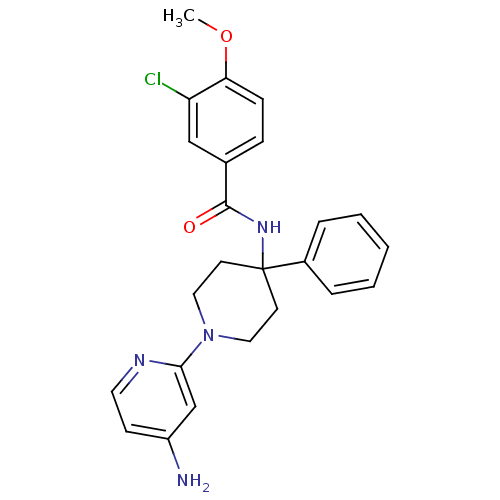
(CHEMBL492330 | N-(1-(4-aminopyridin-2-yl)-4-phenyl...)Show SMILES COc1ccc(cc1Cl)C(=O)NC1(CCN(CC1)c1cc(N)ccn1)c1ccccc1 Show InChI InChI=1S/C24H25ClN4O2/c1-31-21-8-7-17(15-20(21)25)23(30)28-24(18-5-3-2-4-6-18)10-13-29(14-11-24)22-16-19(26)9-12-27-22/h2-9,12,15-16H,10-11,13-14H2,1H3,(H2,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50295770

(2-(4-(6-methoxypyridin-3-yl)-5-methyl-4H-1,2,4-tri...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccccc2)cn1 Show InChI InChI=1S/C19H16N6O2/c1-13-23-24-19(25(13)14-8-9-17(26-2)21-10-14)16-11-22-18(12-20-16)27-15-6-4-3-5-7-15/h3-12H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50262270

(2-(4-fluoro-2-methylphenyl)-5-(4-(6-methoxypyridin...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(cn1)-c1ccc(F)cc1C Show InChI InChI=1S/C20H17FN6O/c1-12-8-14(21)4-6-16(12)17-10-23-18(11-22-17)20-26-25-13(2)27(20)15-5-7-19(28-3)24-9-15/h4-11H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned vasopressin V1A receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257833

(3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-benzyl...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)n1c2ncccc2n(Cc2ccccc2)c1=O Show InChI InChI=1S/C23H24N6O/c24-18-8-12-25-21(15-18)27-13-9-19(10-14-27)29-22-20(7-4-11-26-22)28(23(29)30)16-17-5-2-1-3-6-17/h1-8,11-12,15,19H,9-10,13-14,16H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257686
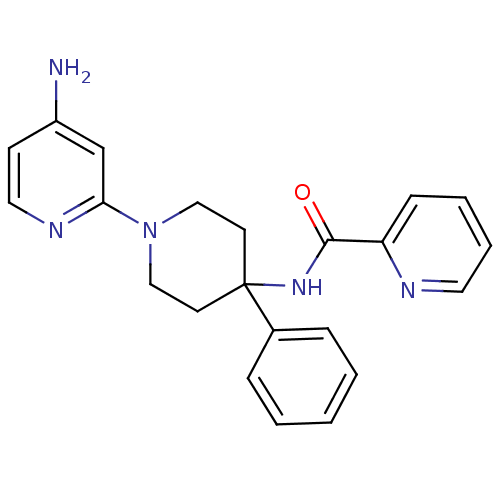
(CHEMBL522317 | N-(1-(4-aminopyridin-2-yl)-4-phenyl...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)(NC(=O)c1ccccn1)c1ccccc1 Show InChI InChI=1S/C22H23N5O/c23-18-9-13-25-20(16-18)27-14-10-22(11-15-27,17-6-2-1-3-7-17)26-21(28)19-8-4-5-12-24-19/h1-9,12-13,16H,10-11,14-15H2,(H2,23,25)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257834
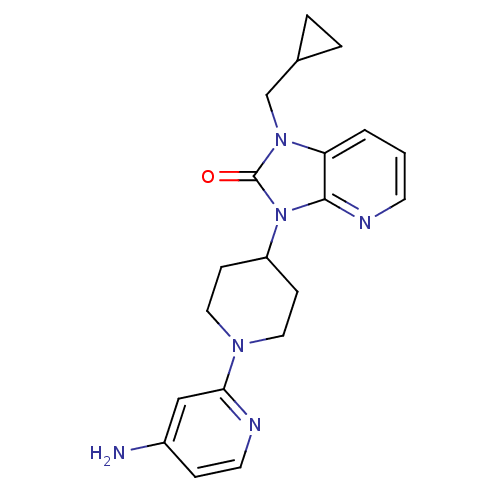
(3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-(cyclo...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)n1c2ncccc2n(CC2CC2)c1=O Show InChI InChI=1S/C20H24N6O/c21-15-5-9-22-18(12-15)24-10-6-16(7-11-24)26-19-17(2-1-8-23-19)25(20(26)27)13-14-3-4-14/h1-2,5,8-9,12,14,16H,3-4,6-7,10-11,13H2,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 746 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257831
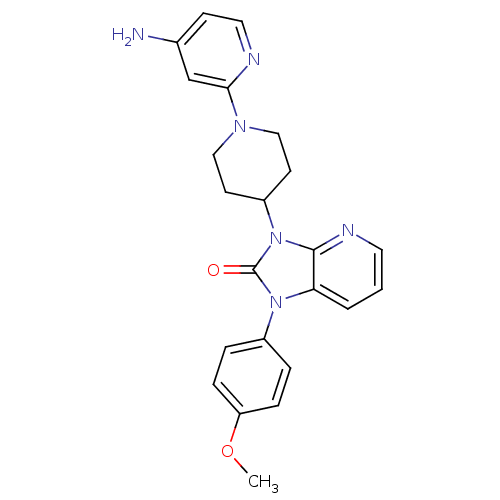
(3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-(4-met...)Show SMILES COc1ccc(cc1)-n1c2cccnc2n(C2CCN(CC2)c2cc(N)ccn2)c1=O Show InChI InChI=1S/C23H24N6O2/c1-31-19-6-4-17(5-7-19)28-20-3-2-11-26-22(20)29(23(28)30)18-9-13-27(14-10-18)21-15-16(24)8-12-25-21/h2-8,11-12,15,18H,9-10,13-14H2,1H3,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257832
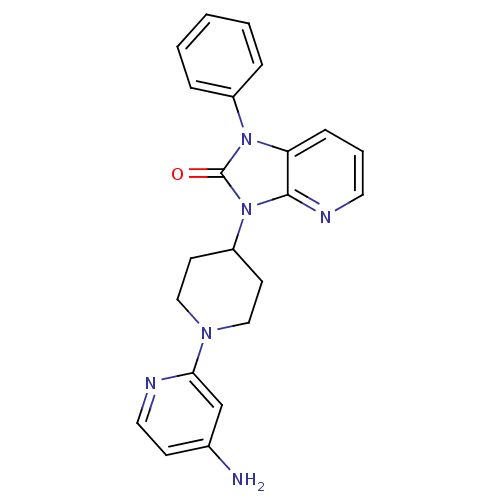
(3-(1-(4-aminopyridin-2-yl)piperidin-4-yl)-1-phenyl...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)n1c2ncccc2n(-c2ccccc2)c1=O Show InChI InChI=1S/C22H22N6O/c23-16-8-12-24-20(15-16)26-13-9-18(10-14-26)28-21-19(7-4-11-25-21)27(22(28)29)17-5-2-1-3-6-17/h1-8,11-12,15,18H,9-10,13-14H2,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 903 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257781
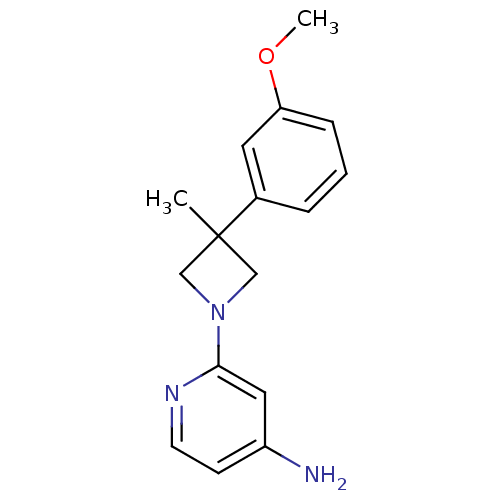
(2-(3-(3-methoxyphenyl)-3-methylazetidin-1-yl)pyrid...)Show InChI InChI=1S/C16H19N3O/c1-16(12-4-3-5-14(8-12)20-2)10-19(11-16)15-9-13(17)6-7-18-15/h3-9H,10-11H2,1-2H3,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50295770

(2-(4-(6-methoxypyridin-3-yl)-5-methyl-4H-1,2,4-tri...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccccc2)cn1 Show InChI InChI=1S/C19H16N6O2/c1-13-23-24-19(25(13)14-8-9-17(26-2)21-10-14)16-11-22-18(12-20-16)27-15-6-4-3-5-7-15/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned vasopressin V1A receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50295770

(2-(4-(6-methoxypyridin-3-yl)-5-methyl-4H-1,2,4-tri...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(Oc2ccccc2)cn1 Show InChI InChI=1S/C19H16N6O2/c1-13-23-24-19(25(13)14-8-9-17(26-2)21-10-14)16-11-22-18(12-20-16)27-15-6-4-3-5-7-15/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned vasopressin V1A receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257780
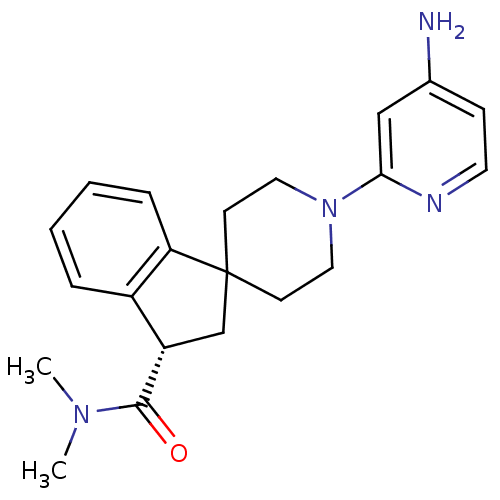
((S)-1'-(4-aminopyridin-2-yl)-N,N-dimethyl-2,3-dihy...)Show SMILES CN(C)C(=O)[C@H]1CC2(CCN(CC2)c2cc(N)ccn2)c2ccccc12 |r| Show InChI InChI=1S/C21H26N4O/c1-24(2)20(26)17-14-21(18-6-4-3-5-16(17)18)8-11-25(12-9-21)19-13-15(22)7-10-23-19/h3-7,10,13,17H,8-9,11-12,14H2,1-2H3,(H2,22,23)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257782

(CHEMBL493515 | N-(1-(4-aminopyridin-2-yl)piperidin...)Show SMILES COc1ccc(cc1)C(=O)N(C1CCN(CC1)c1cc(N)ccn1)c1cc(C)ccn1 Show InChI InChI=1S/C24H27N5O2/c1-17-7-11-27-23(15-17)29(24(30)18-3-5-21(31-2)6-4-18)20-9-13-28(14-10-20)22-16-19(25)8-12-26-22/h3-8,11-12,15-16,20H,9-10,13-14H2,1-2H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257784
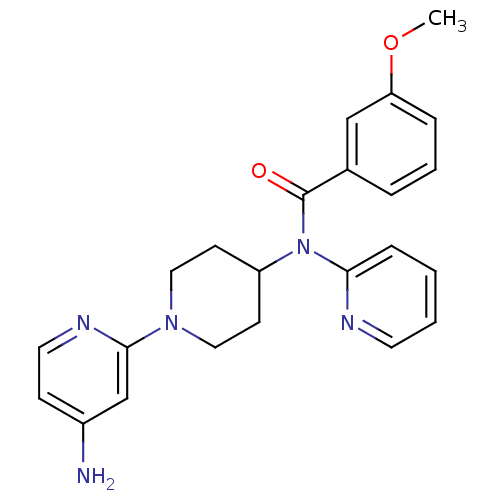
(CHEMBL493517 | N-(1-(4-aminopyridin-2-yl)piperidin...)Show SMILES COc1cccc(c1)C(=O)N(C1CCN(CC1)c1cc(N)ccn1)c1ccccn1 Show InChI InChI=1S/C23H25N5O2/c1-30-20-6-4-5-17(15-20)23(29)28(21-7-2-3-11-25-21)19-9-13-27(14-10-19)22-16-18(24)8-12-26-22/h2-8,11-12,15-16,19H,9-10,13-14H2,1H3,(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50262270

(2-(4-fluoro-2-methylphenyl)-5-(4-(6-methoxypyridin...)Show SMILES COc1ccc(cn1)-n1c(C)nnc1-c1cnc(cn1)-c1ccc(F)cc1C Show InChI InChI=1S/C20H17FN6O/c1-12-8-14(21)4-6-16(12)17-10-23-18(11-22-17)20-26-25-13(2)27(20)15-5-7-19(28-3)24-9-15/h4-11H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned vasopressin V2 receptor by cell based beta-lactamase assay |
Bioorg Med Chem Lett 19: 2634-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.160
BindingDB Entry DOI: 10.7270/Q2736QXW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257719

((S)-N-(1-(4-aminopyridin-2-yl)-4-phenylpiperidin-4...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)(NC(=O)[C@@H]1CCCO1)c1ccccc1 |r| Show InChI InChI=1S/C21H26N4O2/c22-17-8-11-23-19(15-17)25-12-9-21(10-13-25,16-5-2-1-3-6-16)24-20(26)18-7-4-14-27-18/h1-3,5-6,8,11,15,18H,4,7,9-10,12-14H2,(H2,22,23)(H,24,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257830
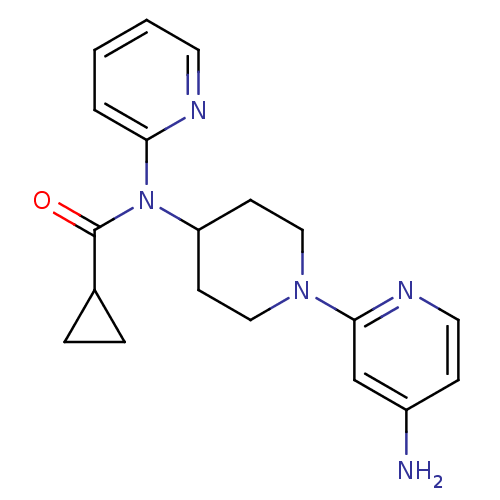
(CHEMBL493781 | N-(1-(4-aminopyridin-2-yl)piperidin...)Show InChI InChI=1S/C19H23N5O/c20-15-6-10-22-18(13-15)23-11-7-16(8-12-23)24(19(25)14-4-5-14)17-3-1-2-9-21-17/h1-3,6,9-10,13-14,16H,4-5,7-8,11-12H2,(H2,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257783
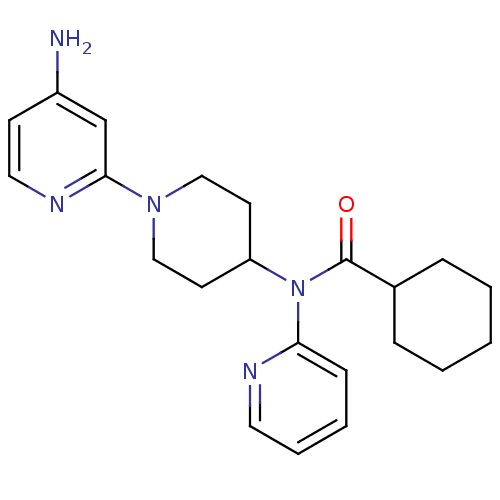
(CHEMBL493516 | N-(1-(4-aminopyridin-2-yl)piperidin...)Show SMILES Nc1ccnc(c1)N1CCC(CC1)N(C(=O)C1CCCCC1)c1ccccn1 Show InChI InChI=1S/C22H29N5O/c23-18-9-13-25-21(16-18)26-14-10-19(11-15-26)27(20-8-4-5-12-24-20)22(28)17-6-2-1-3-7-17/h4-5,8-9,12-13,16-17,19H,1-3,6-7,10-11,14-15H2,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50257722
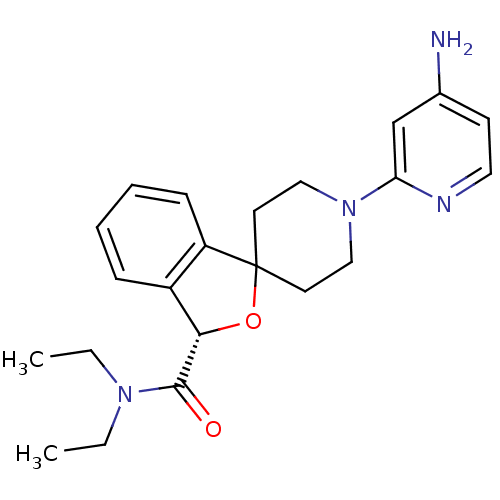
((S)-1'-(4-aminopyridin-2-yl)-N,N-diethyl-3H-spiro[...)Show SMILES CCN(CC)C(=O)[C@H]1OC2(CCN(CC2)c2cc(N)ccn2)c2ccccc12 |r| Show InChI InChI=1S/C22H28N4O2/c1-3-25(4-2)21(27)20-17-7-5-6-8-18(17)22(28-20)10-13-26(14-11-22)19-15-16(23)9-12-24-19/h5-9,12,15,20H,3-4,10-11,13-14H2,1-2H3,(H2,23,24)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 1702-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.106
BindingDB Entry DOI: 10.7270/Q21G0M40 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ataxin-1
(Homo sapiens (Human)) | BDBM321944

((E)-1-(4-((1-Methyl-1H-pyrazol-4-yl)methyl)piperaz...)Show SMILES Cc1nnn(Cc2cc(ccc2\C=C\C(=O)N2CCN(Cc3cnn(C)c3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C22H25F3N8O/c1-16-27-29-33(28-16)15-19-11-20(22(23,24)25)5-3-18(19)4-6-21(34)32-9-7-31(8-10-32)14-17-12-26-30(2)13-17/h3-6,11-13H,7-10,14-15H2,1-2H3/b6-4+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM321962
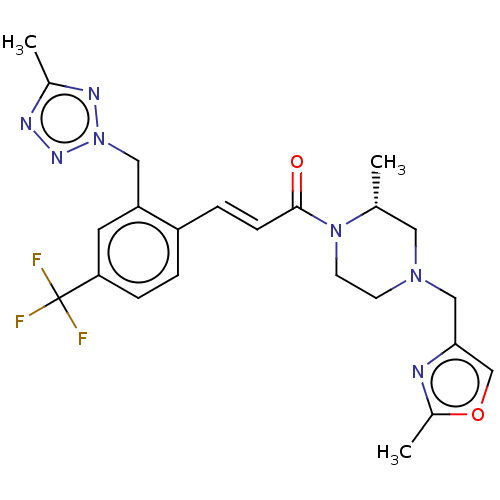
((R,E)-3-(2-((5-Methyl-2H-tetrazol-2-yl)methyl)-4-(...)Show SMILES C[C@@H]1CN(Cc2coc(C)n2)CCN1C(=O)\C=C\c1ccc(cc1Cn1nnc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C23H26F3N7O2/c1-15-11-31(13-21-14-35-17(3)27-21)8-9-32(15)22(34)7-5-18-4-6-20(23(24,25)26)10-19(18)12-33-29-16(2)28-30-33/h4-7,10,14-15H,8-9,11-13H2,1-3H3/b7-5+/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM321944

((E)-1-(4-((1-Methyl-1H-pyrazol-4-yl)methyl)piperaz...)Show SMILES Cc1nnn(Cc2cc(ccc2\C=C\C(=O)N2CCN(Cc3cnn(C)c3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C22H25F3N8O/c1-16-27-29-33(28-16)15-19-11-20(22(23,24)25)5-3-18(19)4-6-21(34)32-9-7-31(8-10-32)14-17-12-26-30(2)13-17/h3-6,11-13H,7-10,14-15H2,1-2H3/b6-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM322065
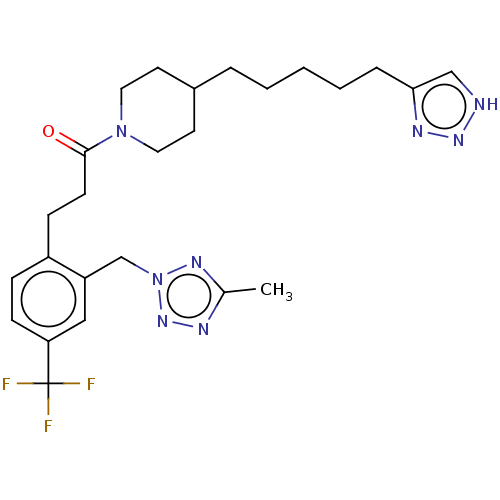
(1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...)Show SMILES Cc1nnn(Cc2cc(ccc2CCC(=O)N2CCC(CCCCCc3c[nH]nn3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C25H33F3N8O/c1-18-30-34-36(32-18)17-21-15-22(25(26,27)28)9-7-20(21)8-10-24(37)35-13-11-19(12-14-35)5-3-2-4-6-23-16-29-33-31-23/h7,9,15-16,19H,2-6,8,10-14,17H2,1H3,(H,29,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM321962

((R,E)-3-(2-((5-Methyl-2H-tetrazol-2-yl)methyl)-4-(...)Show SMILES C[C@@H]1CN(Cc2coc(C)n2)CCN1C(=O)\C=C\c1ccc(cc1Cn1nnc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C23H26F3N7O2/c1-15-11-31(13-21-14-35-17(3)27-21)8-9-32(15)22(34)7-5-18-4-6-20(23(24,25)26)10-19(18)12-33-29-16(2)28-30-33/h4-7,10,14-15H,8-9,11-13H2,1-3H3/b7-5+/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM322065

(1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...)Show SMILES Cc1nnn(Cc2cc(ccc2CCC(=O)N2CCC(CCCCCc3c[nH]nn3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C25H33F3N8O/c1-18-30-34-36(32-18)17-21-15-22(25(26,27)28)9-7-20(21)8-10-24(37)35-13-11-19(12-14-35)5-3-2-4-6-23-16-29-33-31-23/h7,9,15-16,19H,2-6,8,10-14,17H2,1H3,(H,29,31,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM322066
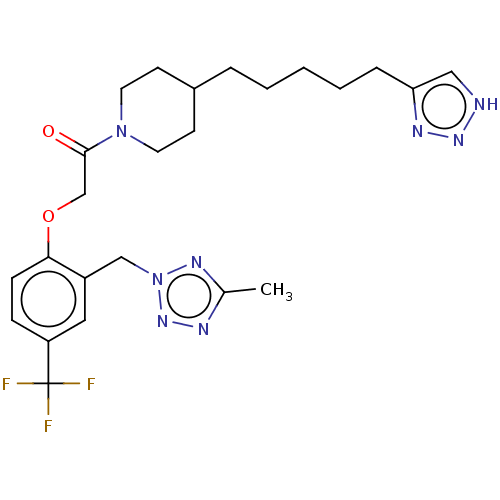
(1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...)Show SMILES Cc1nnn(Cc2cc(ccc2OCC(=O)N2CCC(CCCCCc3c[nH]nn3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C24H31F3N8O2/c1-17-29-33-35(31-17)15-19-13-20(24(25,26)27)7-8-22(19)37-16-23(36)34-11-9-18(10-12-34)5-3-2-4-6-21-14-28-32-30-21/h7-8,13-14,18H,2-6,9-12,15-16H2,1H3,(H,28,30,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM322066

(1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...)Show SMILES Cc1nnn(Cc2cc(ccc2OCC(=O)N2CCC(CCCCCc3c[nH]nn3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C24H31F3N8O2/c1-17-29-33-35(31-17)15-19-13-20(24(25,26)27)7-8-22(19)37-16-23(36)34-11-9-18(10-12-34)5-3-2-4-6-21-14-28-32-30-21/h7-8,13-14,18H,2-6,9-12,15-16H2,1H3,(H,28,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM241106
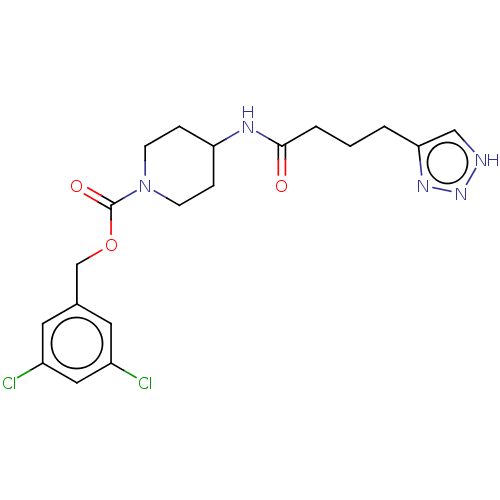
(US9409895, 17 | US9630945, 17)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)c1 Show InChI InChI=1S/C19H23Cl2N5O3/c20-14-8-13(9-15(21)10-14)12-29-19(28)26-6-4-16(5-7-26)23-18(27)3-1-2-17-11-22-25-24-17/h8-11,16H,1-7,12H2,(H,23,27)(H,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) assessed as decrease in choline release |
Bioorg Med Chem Lett 28: 2279-2284 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.030
BindingDB Entry DOI: 10.7270/Q2DB84DV |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM322063

((E)-N-(1-(3-(4-Chloro-2-((5-methyl-2H-tetrazol-2-y...)Show SMILES Cc1nnn(Cc2cc(Cl)ccc2\C=C\C(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)n1 Show InChI InChI=1S/C23H28ClN9O2/c1-16-27-31-33(29-16)15-18-13-19(24)7-5-17(18)6-8-23(35)32-11-9-20(10-12-32)26-22(34)4-2-3-21-14-25-30-28-21/h5-8,13-14,20H,2-4,9-12,15H2,1H3,(H,26,34)(H,25,28,30)/b8-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM322063

((E)-N-(1-(3-(4-Chloro-2-((5-methyl-2H-tetrazol-2-y...)Show SMILES Cc1nnn(Cc2cc(Cl)ccc2\C=C\C(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)n1 Show InChI InChI=1S/C23H28ClN9O2/c1-16-27-31-33(29-16)15-18-13-19(24)7-5-17(18)6-8-23(35)32-11-9-20(10-12-32)26-22(34)4-2-3-21-14-25-30-28-21/h5-8,13-14,20H,2-4,9-12,15H2,1H3,(H,26,34)(H,25,28,30)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM321899
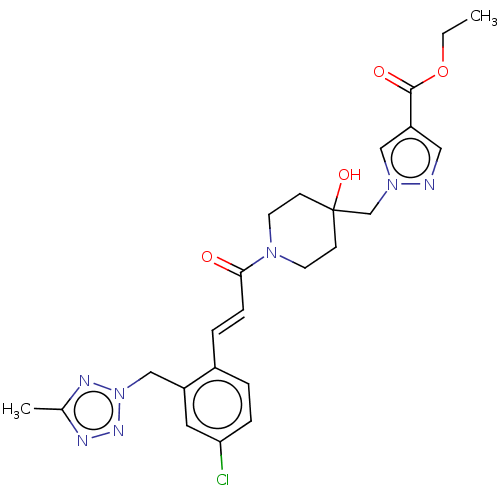
((E)-Ethyl 1-((1-(3-(4-chloro-2-((5-methyl-2H-tetra...)Show SMILES CCOC(=O)c1cnn(CC2(O)CCN(CC2)C(=O)\C=C\c2ccc(Cl)cc2Cn2nnc(C)n2)c1 Show InChI InChI=1S/C24H28ClN7O4/c1-3-36-23(34)20-13-26-31(14-20)16-24(35)8-10-30(11-9-24)22(33)7-5-18-4-6-21(25)12-19(18)15-32-28-17(2)27-29-32/h4-7,12-14,35H,3,8-11,15-16H2,1-2H3/b7-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM321868
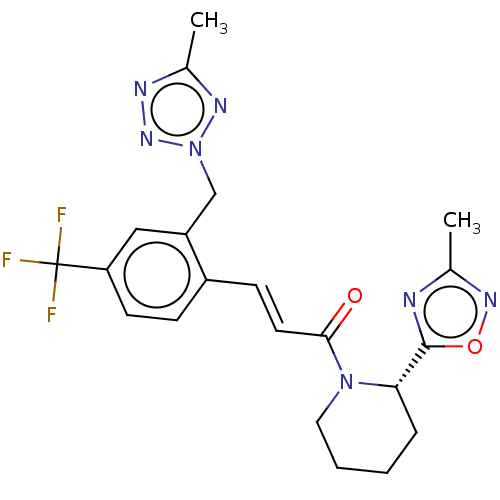
(US10183025, Example 5a | US9763957, Example 5b)Show SMILES Cc1noc(n1)[C@@H]1CCCCN1C(=O)\C=C\c1ccc(cc1Cn1nnc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N7O2/c1-13-25-20(33-28-13)18-5-3-4-10-30(18)19(32)9-7-15-6-8-17(21(22,23)24)11-16(15)12-31-27-14(2)26-29-31/h6-9,11,18H,3-5,10,12H2,1-2H3/b9-7+/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data