

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50228471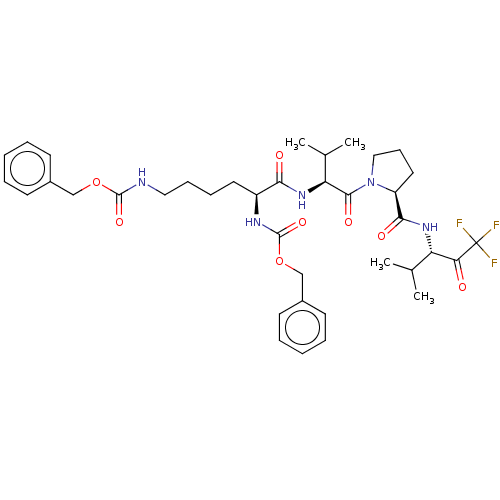 (CHEMBL131548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against human leukocyte Elastase | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM81925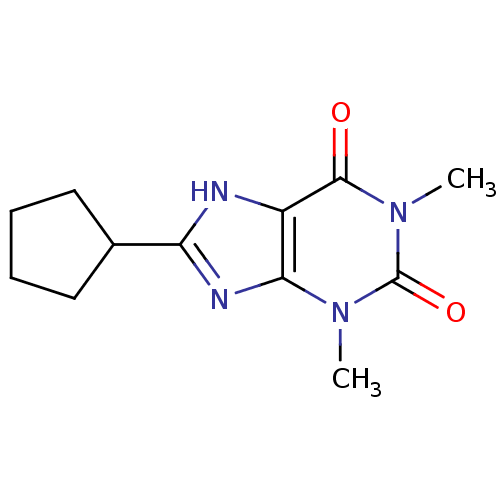 (8-Cyclopentyl-1,3-dimethyl-3,7-dihydro-purine-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1 (Homo sapiens (Human)) | BDBM50014738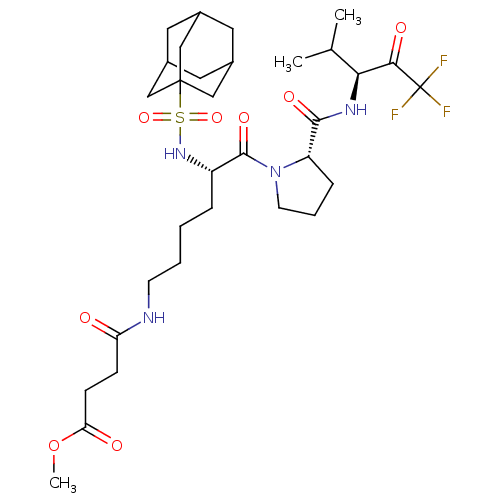 (CHEMBL130253 | N-(Adamantyl-sulfonyl)-N-(methoxy s...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against human Elastase | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50009552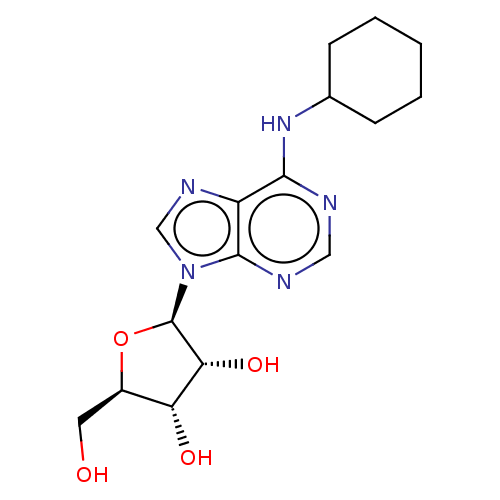 (2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1 (Homo sapiens (Human)) | BDBM50014735 ((1-{1-Methyl-2-oxo-2-[2-(3,3,3-trifluoro-1-isoprop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against human Elastase | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50014735 ((1-{1-Methyl-2-oxo-2-[2-(3,3,3-trifluoro-1-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated In vitro for inhibition of human neutrophil elastase | Bioorg Med Chem Lett 2: 1235-1238 (1992) Article DOI: 10.1016/S0960-894X(00)80220-6 BindingDB Entry DOI: 10.7270/Q2DB81SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding of Adenosine A2 receptor in whole rat brain membrane using [3H]CHA as a Radioligand | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50069667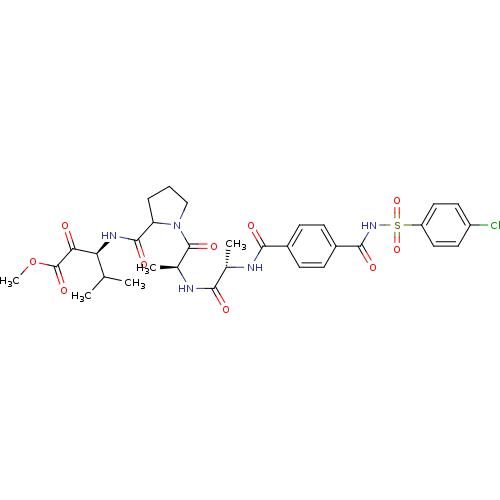 ((S)-3-{[1-((S)-2-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cinc Curated by ChEMBL | Assay Description Tested for rate of substrate hydrolysis in the presence of human neutrophil elastase | Bioorg Med Chem Lett 8: 63-4 (1999) BindingDB Entry DOI: 10.7270/Q2K936PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50421993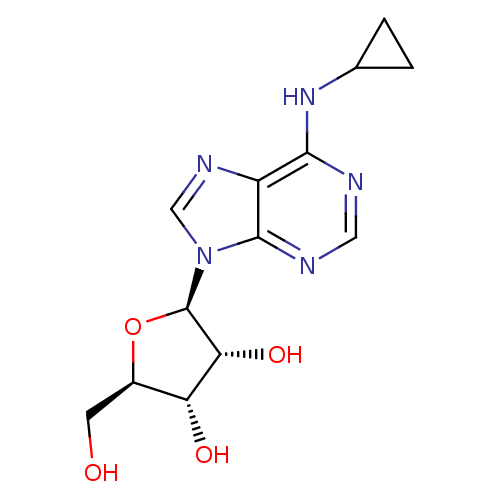 (CHEMBL2113423) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50009552 (2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding of Adenosine A2 receptor in whole rat brain membrane using [3H]CHA as a Radioligand | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM42467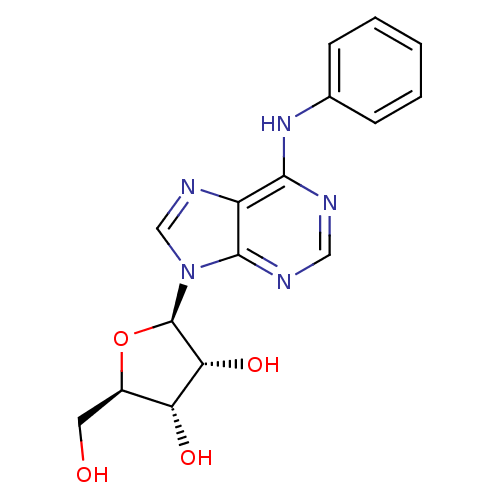 ((2R,3R,4S,5R)-2-(6-anilino-9-purinyl)-5-(hydroxyme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM81925 (8-Cyclopentyl-1,3-dimethyl-3,7-dihydro-purine-2,6-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding of Adenosine A2 receptor in whole rat brain membrane using [3H]CHA as a Radioligand | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281590 (CHEMBL150384 | N-(1-Benzyl-3,3,3-trifluoro-2-oxo-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its potency to inhibit human neutrophil elastase activity | Bioorg Med Chem Lett 3: 525-530 (1993) Article DOI: 10.1016/S0960-894X(01)81220-8 BindingDB Entry DOI: 10.7270/Q23778NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50228562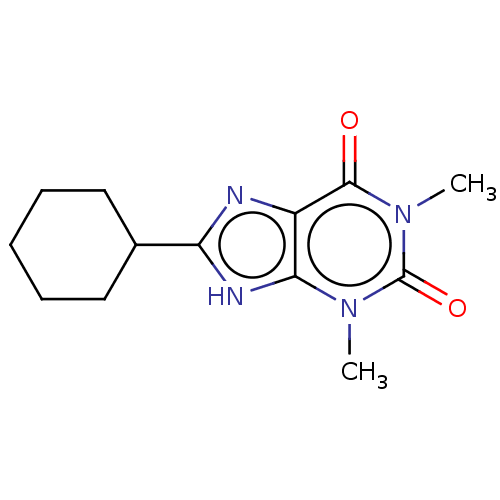 (CHEMBL321505) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding of Adenosine A2 receptor in whole rat brain membrane using [3H]CHA as a Radioligand | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50014732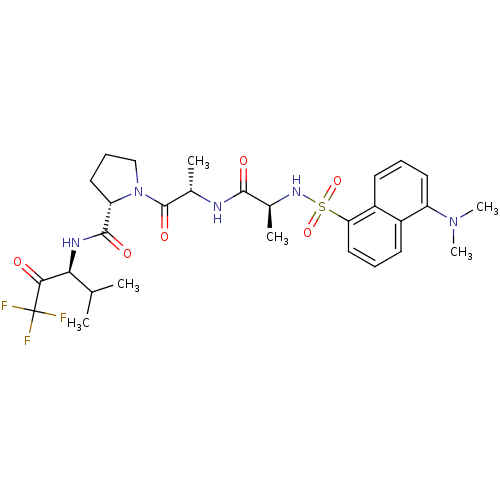 (1-{2-[2-(5-Dimethylamino-naphthalene-1-sulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against human Elastase for more active diasteriomer | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50008413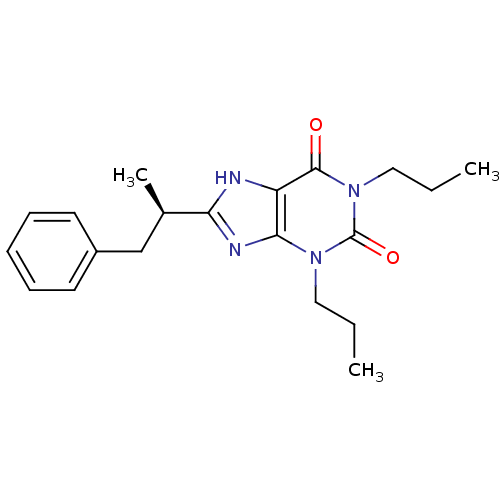 (8-(1-Methyl-2-phenyl-ethyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor was determined using radioligand [3H]-CHA in whole rat brain membranes at 25 degree C | J Med Chem 36: 4015-20 (1994) BindingDB Entry DOI: 10.7270/Q23T9G92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50008413 (8-(1-Methyl-2-phenyl-ethyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50014730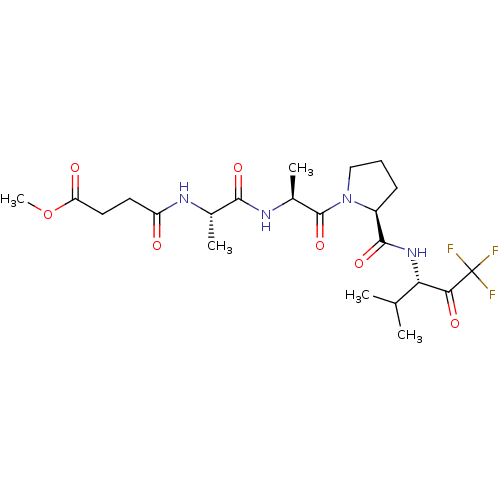 (CHEMBL130271 | N-(1-{1-Methyl-2-oxo-2-[2-(3,3,3-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against human Elastase for more active diasteriomer | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50073850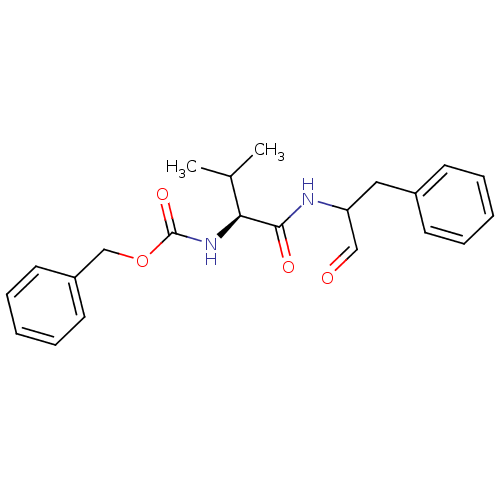 ((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc. Curated by ChEMBL | Assay Description Tested for inhibitory activity against calpain. | Bioorg Med Chem Lett 9: 2365-70 (1999) BindingDB Entry DOI: 10.7270/Q2F1907J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50014732 (1-{2-[2-(5-Dimethylamino-naphthalene-1-sulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated In vitro for inhibition of human neutrophil elastase | Bioorg Med Chem Lett 2: 1235-1238 (1992) Article DOI: 10.1016/S0960-894X(00)80220-6 BindingDB Entry DOI: 10.7270/Q2DB81SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Gallus gallus) | BDBM50073850 ((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against chicken gizzard smooth muscle calpain | Bioorg Med Chem Lett 9: 139-40 (1999) BindingDB Entry DOI: 10.7270/Q2DN4476 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50012199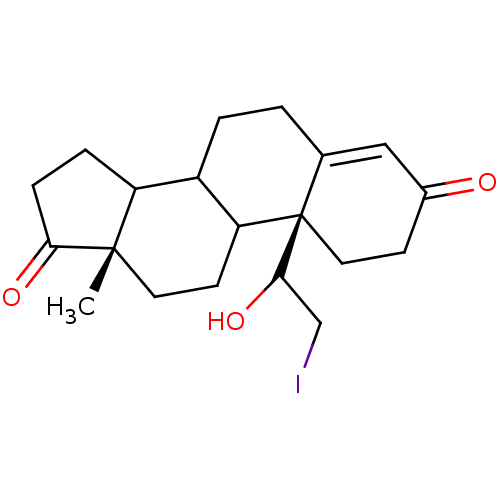 ((R isomer)10-(1-Hydroxy-2-iodo-ethyl)-13-methyl-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of binding to human Placental Cytochrome P450 19A1 | J Med Chem 34: 1748-50 (1991) BindingDB Entry DOI: 10.7270/Q2F76BH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82015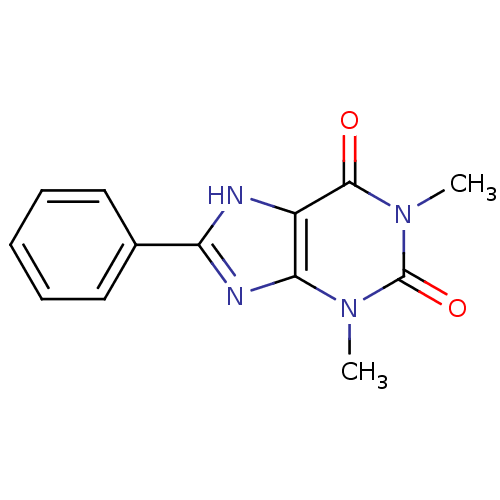 (1,3-Dimethyl-8-phenyl-3,9-dihydro-purine-2,6-dione...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-formylglutathione hydrolase (Sus scrofa) | BDBM50069665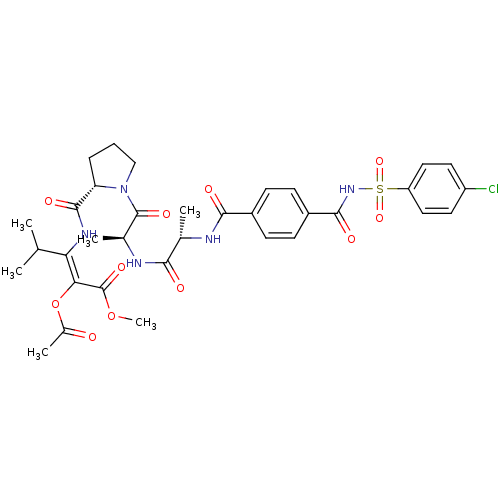 ((E)-2-Acetoxy-3-{[(S)-1-((S)-2-{(S)-2-[4-(4-chloro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cinc Curated by ChEMBL | Assay Description Tested for inhibition of substrate hydrolysis in the presence of porcine kidney esterase at a concentration of 10 nM | Bioorg Med Chem Lett 8: 63-4 (1999) BindingDB Entry DOI: 10.7270/Q2K936PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-formylglutathione hydrolase (Sus scrofa) | BDBM50069665 ((E)-2-Acetoxy-3-{[(S)-1-((S)-2-{(S)-2-[4-(4-chloro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cinc Curated by ChEMBL | Assay Description pA2 value towards endothelin receptor A was determined as functional ETA antagonism | Bioorg Med Chem Lett 8: 63-4 (1999) BindingDB Entry DOI: 10.7270/Q2K936PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50014730 (CHEMBL130271 | N-(1-{1-Methyl-2-oxo-2-[2-(3,3,3-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated In vitro for inhibition of human neutrophil elastase | Bioorg Med Chem Lett 2: 1235-1238 (1992) Article DOI: 10.1016/S0960-894X(00)80220-6 BindingDB Entry DOI: 10.7270/Q2DB81SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50014738 (CHEMBL130253 | N-(Adamantyl-sulfonyl)-N-(methoxy s...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity for porcine Elastase | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281589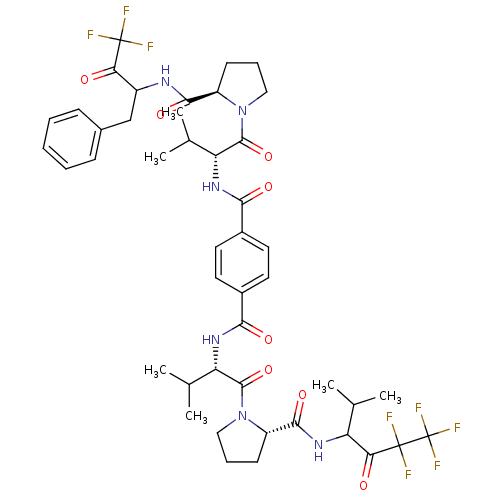 (5N-(1-benzyl-3,3,3-trifluoro-2-oxopropyl)-1-[3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its potency to inhibit human neutrophil elastase activity | Bioorg Med Chem Lett 3: 525-530 (1993) Article DOI: 10.1016/S0960-894X(01)81220-8 BindingDB Entry DOI: 10.7270/Q23778NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50014743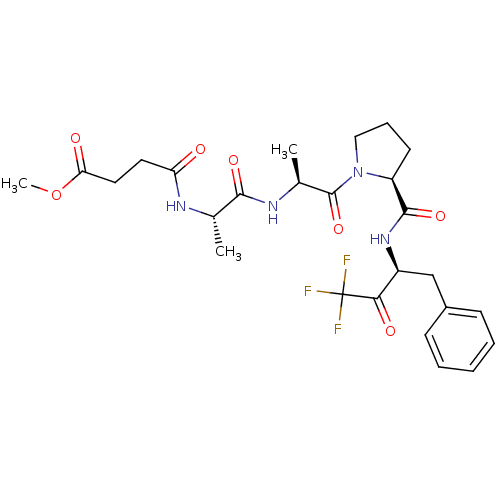 (CHEMBL132171 | N-(1-{2-[2-(1-Benzyl-3,3,3-trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-chymotrypsin | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065147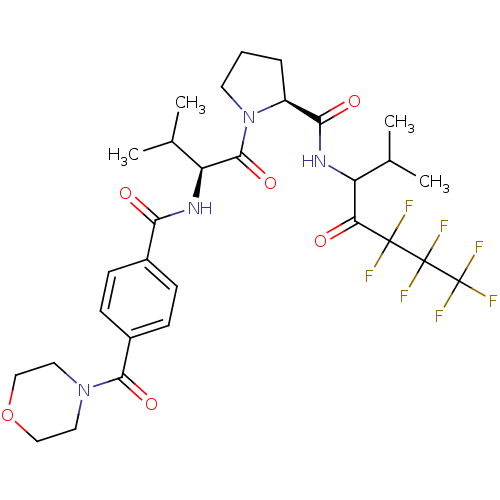 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035495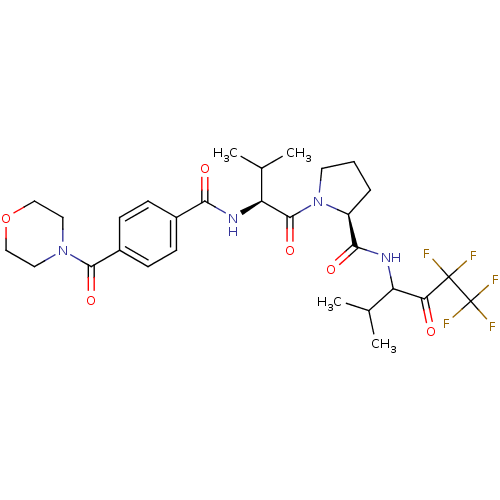 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065158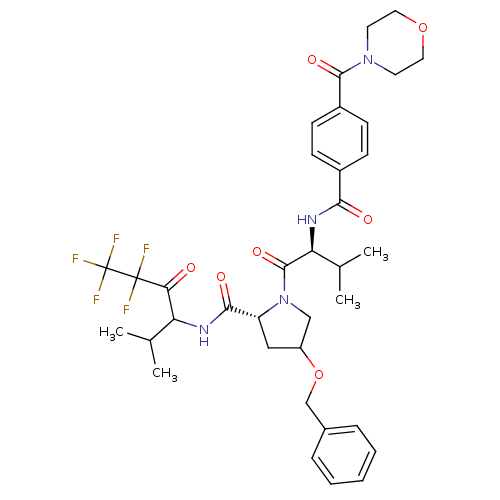 ((R)-4-Benzyloxy-1-{(S)-3-methyl-2-[4-(morpholine-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM82015 (1,3-Dimethyl-8-phenyl-3,9-dihydro-purine-2,6-dione...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding of Adenosine A2 receptor in whole rat brain membrane using [3H]CHA as a Radioligand | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50043209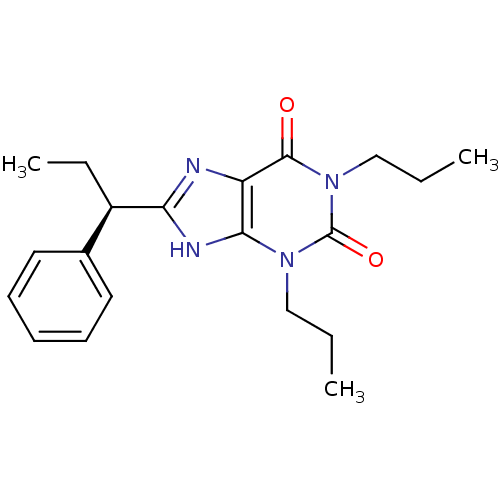 (8-(1-Phenyl-propyl)-1,3-dipropyl-3,9-dihydro-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 23.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor was determined using radioligand [3H]-CHA in whole rat brain membranes at 25 degree C | J Med Chem 36: 4015-20 (1994) BindingDB Entry DOI: 10.7270/Q23T9G92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8503 ((2R)-2-[(4-fluoro-3-methylbenzene)sulfonamido]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of Bacillus anthracis recombinant lethal factor expressed in Escherichia coli by linear double-reciprocal plot analysis | Bioorg Med Chem 22: 419-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.009 BindingDB Entry DOI: 10.7270/Q2QV3NZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50043214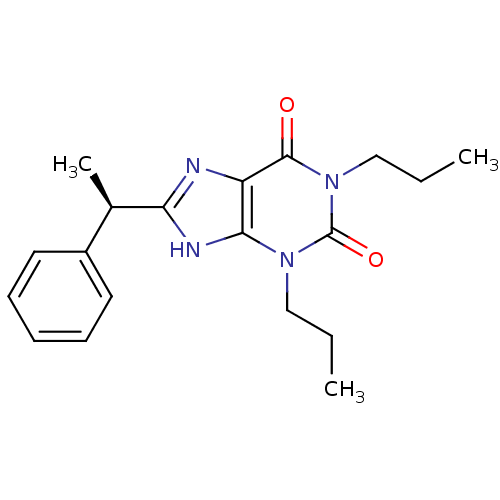 (8-(1-Phenyl-ethyl)-1,3-dipropyl-3,9-dihydro-purine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor was determined using radioligand [3H]-CHA in whole rat brain membranes at 25 degree C | J Med Chem 36: 4015-20 (1994) BindingDB Entry DOI: 10.7270/Q23T9G92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50014747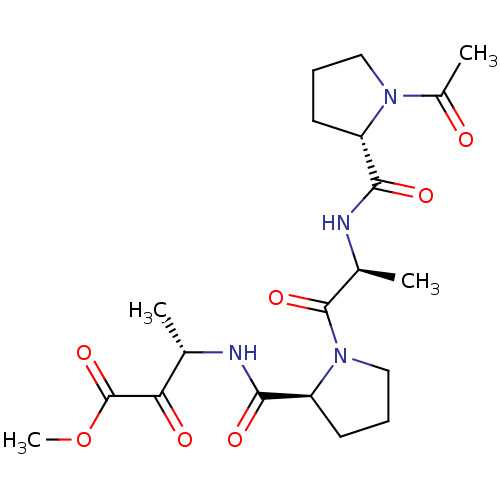 (3-[(1-{2-[(1-Acetyl-pyrrolidine-2-carbonyl)-amino]...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against rat Cathepsin G | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50014736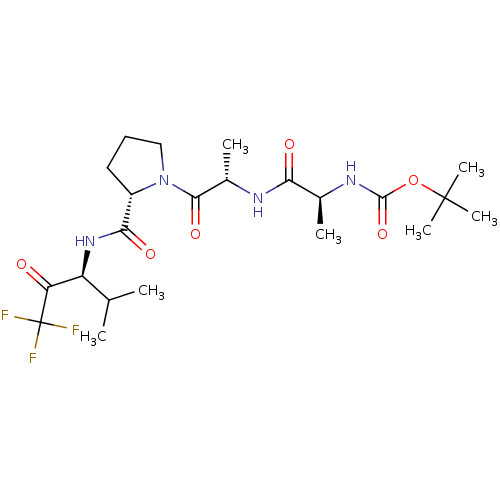 ((1-{1-Methyl-2-oxo-2-[2-(3,3,3-trifluoro-1-isoprop...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity for porcine Elastase | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011216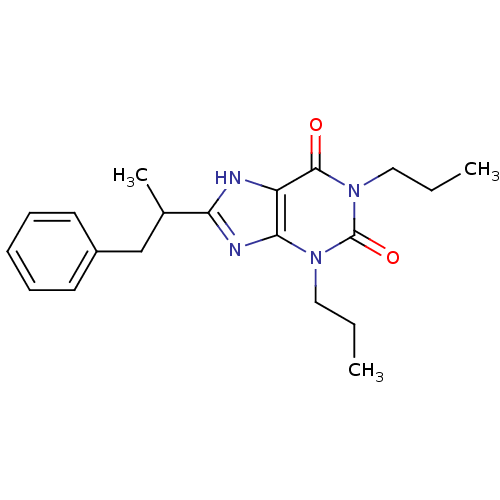 (1-Methyl-8-(1-methyl-2-phenyl-ethyl)-1,3-dipropyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor was determined using radioligand [3H]-CHA in whole rat brain membranes at 25 degree C | J Med Chem 36: 4015-20 (1994) BindingDB Entry DOI: 10.7270/Q23T9G92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50014730 (CHEMBL130271 | N-(1-{1-Methyl-2-oxo-2-[2-(3,3,3-tr...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity for porcine Elastase | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50080206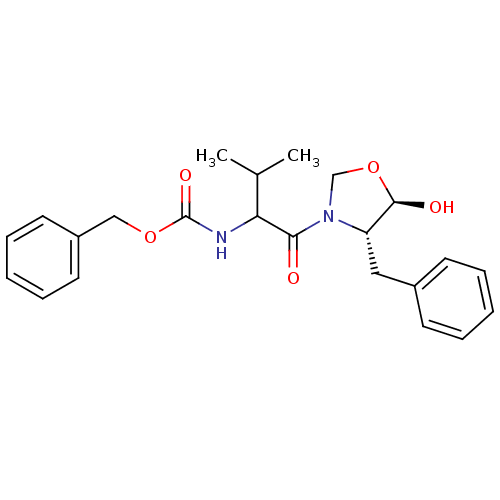 (CHEMBL311735 | [1-((4S,5R)-4-Benzyl-5-hydroxy-oxaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc. Curated by ChEMBL | Assay Description Tested for inhibitory activity against calpain. | Bioorg Med Chem Lett 9: 2365-70 (1999) BindingDB Entry DOI: 10.7270/Q2F1907J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011216 (1-Methyl-8-(1-methyl-2-phenyl-ethyl)-1,3-dipropyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptor | J Med Chem 33: 3127-30 (1991) BindingDB Entry DOI: 10.7270/Q2ZC83GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011222 (8-(1-Phenyl-propyl)-1,3-dipropyl-3,7-dihydro-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor was determined using radioligand [3H]-CHA in whole rat brain membranes at 25 degree C | J Med Chem 36: 4015-20 (1994) BindingDB Entry DOI: 10.7270/Q23T9G92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065157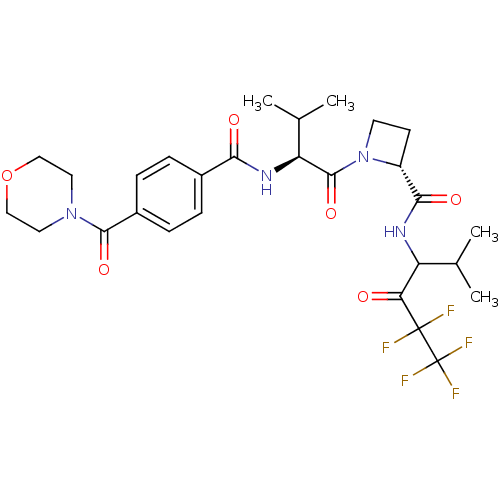 ((R)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065161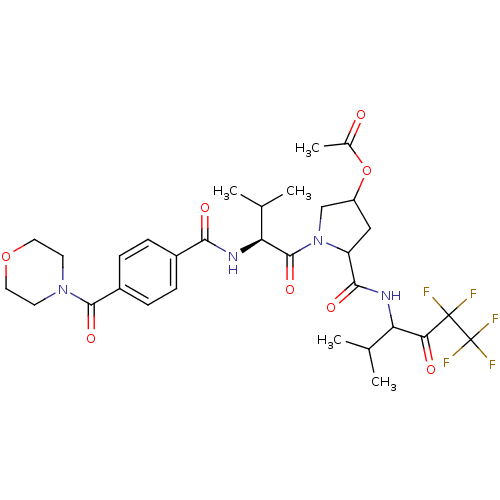 (Acetic acid 1-{(S)-3-methyl-2-[4-(morpholine-4-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280125 ((1-{1-Methyl-2-oxo-2-[2-(3,3,3-trifluoro-1-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated In vitro for inhibition of human neutrophil elastase | Bioorg Med Chem Lett 2: 1235-1238 (1992) Article DOI: 10.1016/S0960-894X(00)80220-6 BindingDB Entry DOI: 10.7270/Q2DB81SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1 (Homo sapiens (Human)) | BDBM50014736 ((1-{1-Methyl-2-oxo-2-[2-(3,3,3-trifluoro-1-isoprop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity against human Elastase | J Med Chem 33: 394-407 (1990) BindingDB Entry DOI: 10.7270/Q26D5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50043212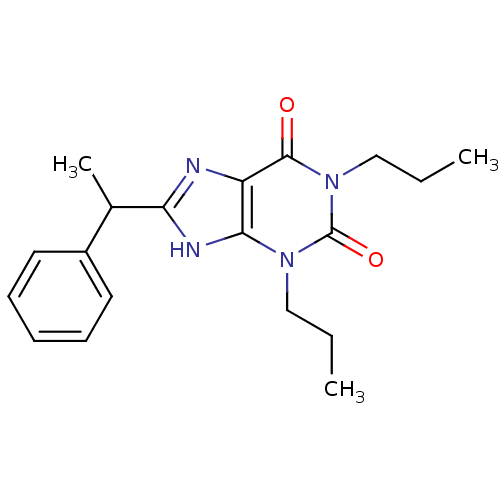 (8-(1-Phenyl-ethyl)-1,3-dipropyl-3,9-dihydro-purine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 49.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor was determined using radioligand [3H]-CHA in whole rat brain membranes at 25 degree C | J Med Chem 36: 4015-20 (1994) BindingDB Entry DOI: 10.7270/Q23T9G92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1334 total ) | Next | Last >> |