

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118114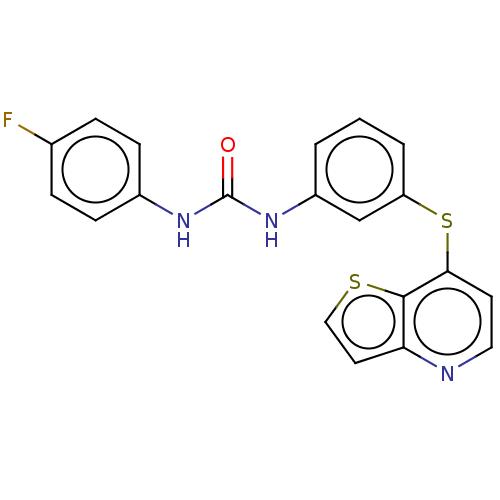 (CHEMBL3612230) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118116 (CHEMBL3612232) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118117 (CHEMBL3612233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118118 (CHEMBL3612234) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118115 (CHEMBL3612231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of human VEGFR2 after 1.5 to 2 hrs by TR-FRET assay | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118127 (CHEMBL3612227) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118128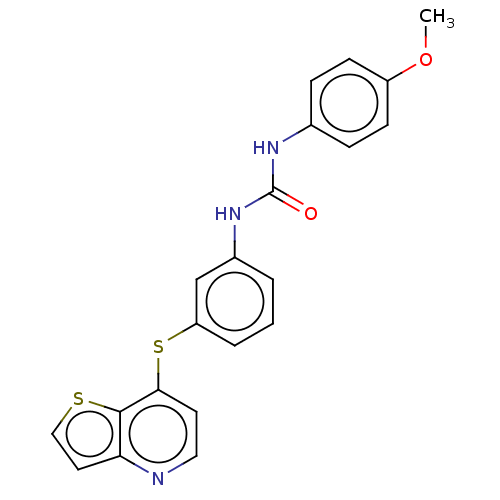 (CHEMBL3612228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118113 (CHEMBL3612229) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118122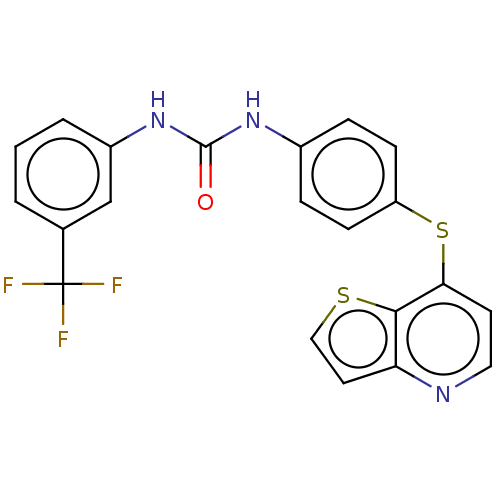 (CHEMBL3612325) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 988 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118119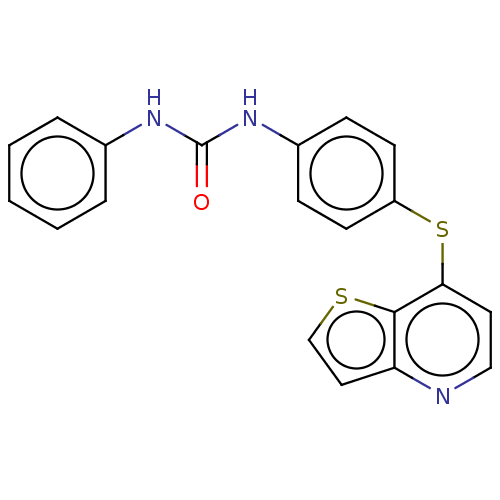 (CHEMBL3612235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118120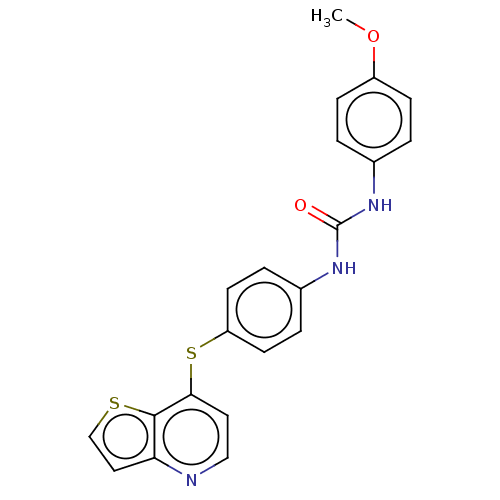 (CHEMBL3612323) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118123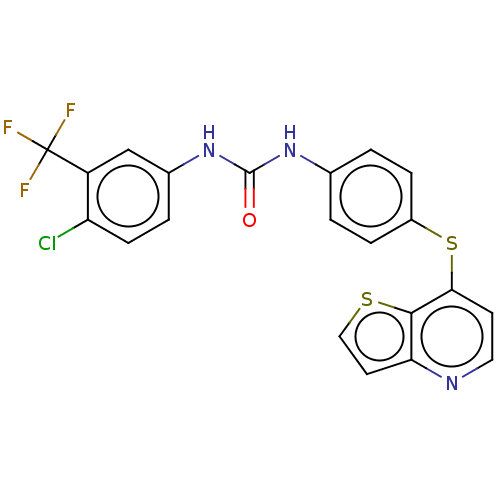 (CHEMBL3612326) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118121 (CHEMBL3612324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM192007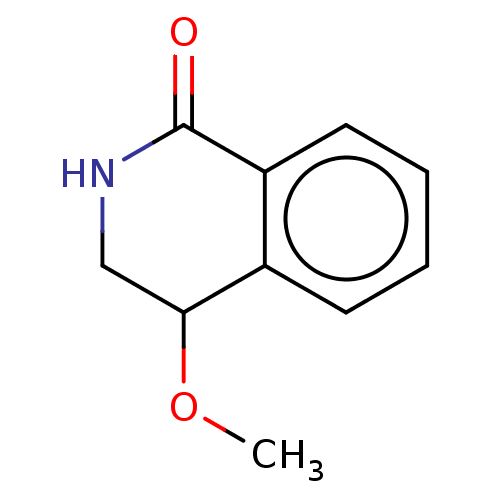 (Isoquinolin-1(2H)-ones, 1f) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118126 (CHEMBL3612226) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118124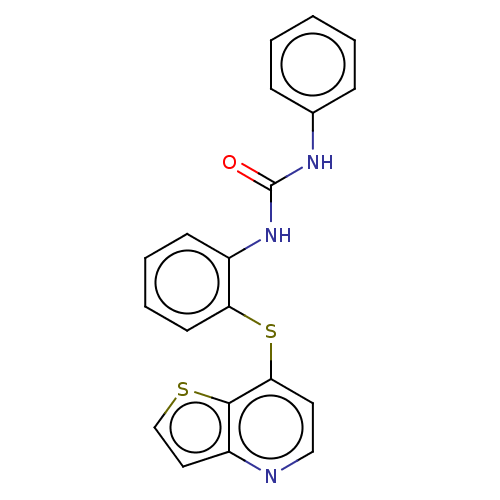 (CHEMBL3612224) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50118125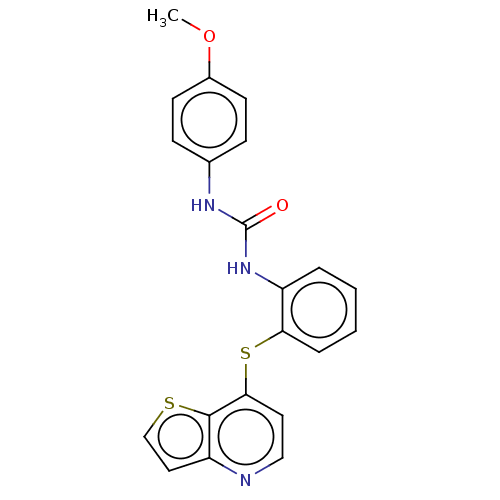 (CHEMBL3612225) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Minho Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using Tyr1 peptide as substrate after 1 hr by FRET-based assay in presence of 10 uM ATP | Bioorg Med Chem 23: 6497-509 (2015) Article DOI: 10.1016/j.bmc.2015.08.010 BindingDB Entry DOI: 10.7270/Q27946G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM191998 (Isoquinolin-1(2H)-ones, 2b) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM191997 (Isoquinolin-1(2H)-ones, 2a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM192000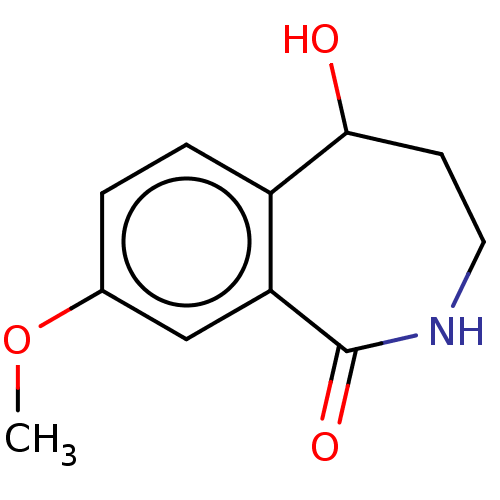 (Isoquinolin-1(2H)-ones, 2d) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM191992 (Isoquinolin-1(2H)-ones, 1a) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM192007 (Isoquinolin-1(2H)-ones, 1f) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM191995 (Isoquinolin-1(2H)-ones, 1d) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM191999 (Isoquinolin-1(2H)-ones, 2c) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM191997 (Isoquinolin-1(2H)-ones, 2a) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM191996 (Isoquinolin-1(2H)-ones, 1e) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM191993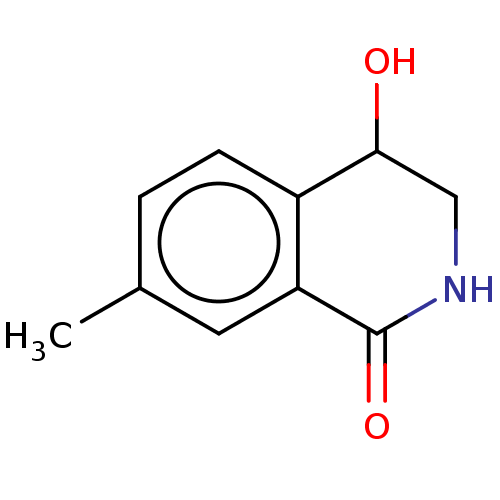 (Isoquinolin-1(2H)-ones, 1b) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM191998 (Isoquinolin-1(2H)-ones, 2b) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM192003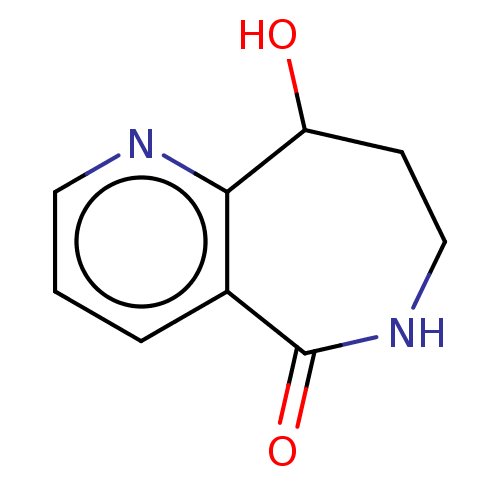 (Isoquinolin-1(2H)-ones, 4a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM191999 (Isoquinolin-1(2H)-ones, 2c) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM192000 (Isoquinolin-1(2H)-ones, 2d) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM192006 (Isoquinolin-1(2H)-ones, 6) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM191992 (Isoquinolin-1(2H)-ones, 1a) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM192006 (Isoquinolin-1(2H)-ones, 6) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM192002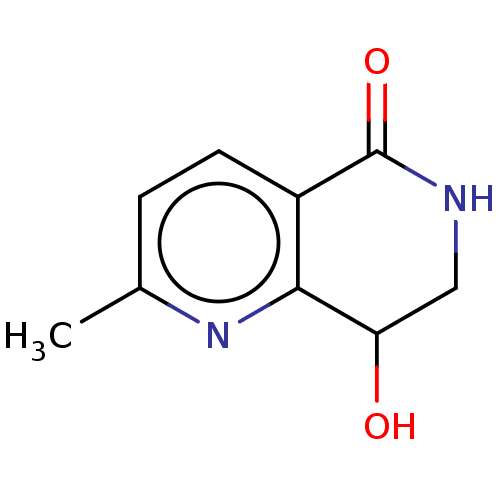 (Isoquinolin-1(2H)-ones, 3b) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM192001 (Isoquinolin-1(2H)-ones, 3a) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM192005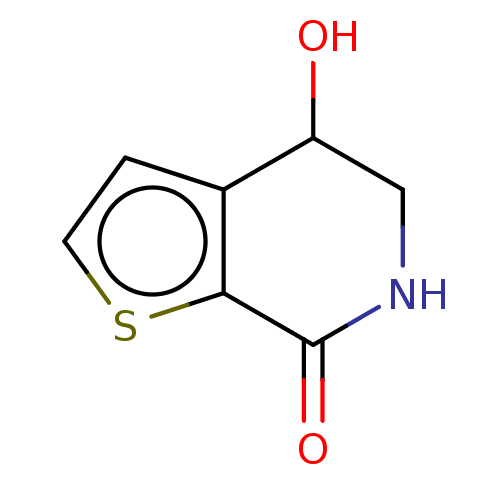 (Isoquinolin-1(2H)-ones, 5) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.37E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM191993 (Isoquinolin-1(2H)-ones, 1b) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM191994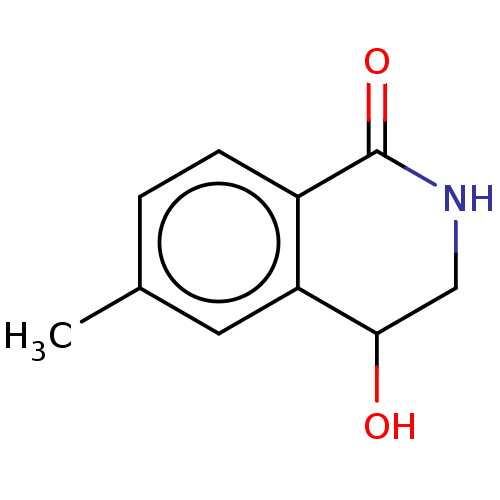 (Isoquinolin-1(2H)-ones, 1c) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM191995 (Isoquinolin-1(2H)-ones, 1d) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM192004 (Isoquinolin-1(2H)-ones, 4b) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The inhibition assay of these compounds for AChE (electrophorus electricus) and BuChE (equine serum) were determined by the spectroscopic method desc... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM191994 (Isoquinolin-1(2H)-ones, 1c) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM191996 (Isoquinolin-1(2H)-ones, 1e) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM192005 (Isoquinolin-1(2H)-ones, 5) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM192004 (Isoquinolin-1(2H)-ones, 4b) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM192001 (Isoquinolin-1(2H)-ones, 3a) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Évora | Assay Description The in vitro BuChE assay was performed similarly to the method described above. A standard enzyme activity curve was drawn for each enzyme using seve... | Bioorg Chem 67: 1-8 (2016) Article DOI: 10.1016/j.bioorg.2016.05.004 BindingDB Entry DOI: 10.7270/Q2XP73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |