

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pirin (Homo sapiens (Human)) | BDBM50234078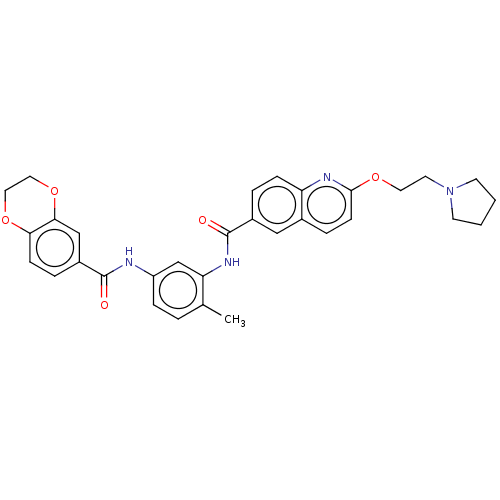 (CHEMBL4087666 | US9701664, Example 63) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of bisamide probe binding to pirin in human SKOV3 cells by SILAC-based quantitative mass spectrometry pull down assay | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pirin (Homo sapiens (Human)) | BDBM50234079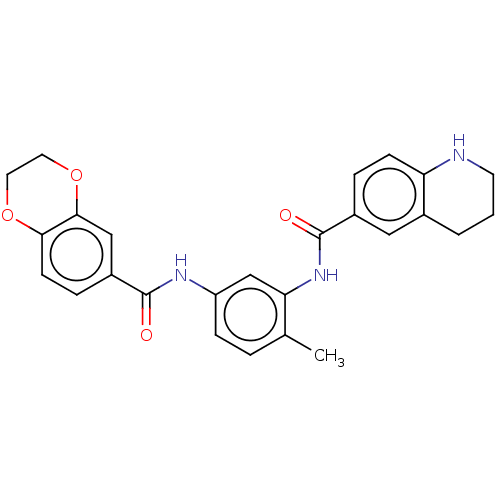 (CHEMBL4096048 | US9701664, Example 80) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of bisamide probe binding to pirin in human SKOV3 cells by SILAC-based quantitative mass spectrometry pull down assay | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234074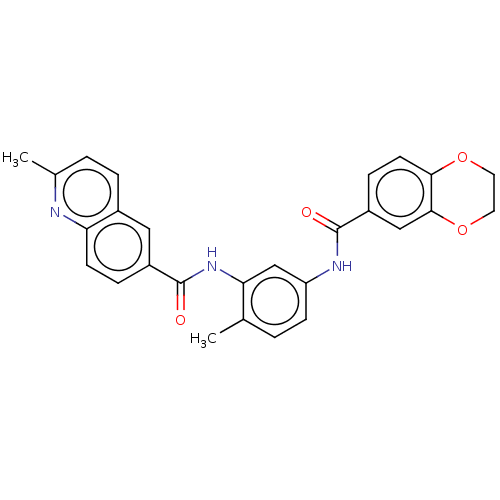 (CHEMBL4070633 | US9701664, Example 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human U20S cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addit... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234074 (CHEMBL4070633 | US9701664, Example 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human U20S cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addit... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234073 (CHEMBL4063330 | US9701664, Example 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234073 (CHEMBL4063330 | US9701664, Example 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234078 (CHEMBL4087666 | US9701664, Example 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234078 (CHEMBL4087666 | US9701664, Example 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234085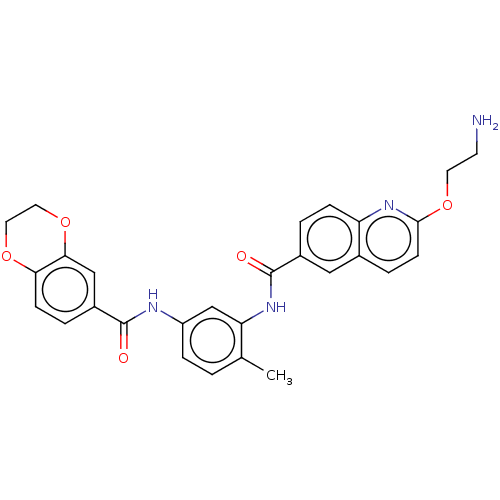 (CHEMBL4102297 | US9701664, Example 89) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234085 (CHEMBL4102297 | US9701664, Example 89) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071664 (5-Amino-2-[1-(3,5-dibromo-4-hydroxy-phenyl)-meth-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234078 (CHEMBL4087666 | US9701664, Example 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSPA1A mRNA level preincubated for 1 hr followed by 17-AAG ad... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234078 (CHEMBL4087666 | US9701664, Example 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSPA1A mRNA level preincubated for 1 hr followed by 17-AAG ad... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234090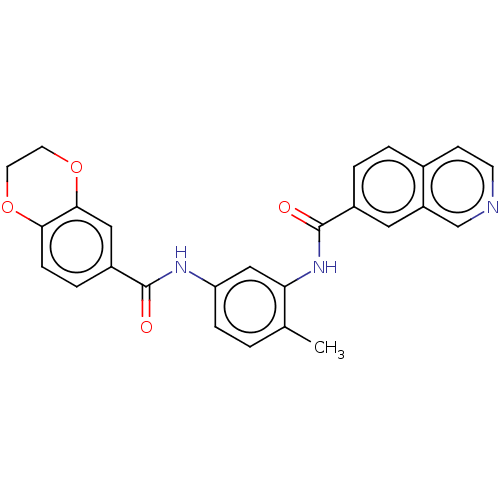 (CHEMBL4069552 | US9701664, Example 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234090 (CHEMBL4069552 | US9701664, Example 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234074 (CHEMBL4070633 | US9701664, Example 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234074 (CHEMBL4070633 | US9701664, Example 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071667 (5-Amino-2-[1-(3,5-dichloro-4-hydroxy-phenyl)-meth-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071666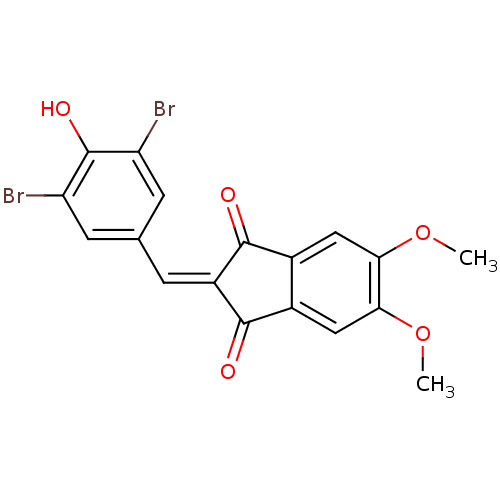 (2-(3,5-Dibromo-4-hydroxy-benzylidene)-5,6-dimethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234083 (CHEMBL4070302 | US9701664, Example 81) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234083 (CHEMBL4070302 | US9701664, Example 81) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071677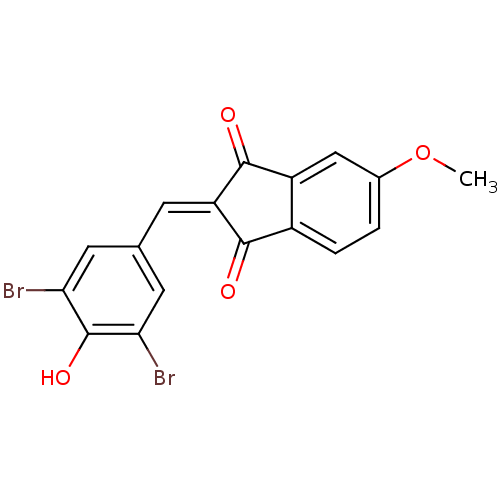 (2-[1-(3,5-Dibromo-4-hydroxy-phenyl)-meth-(E)-ylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234079 (CHEMBL4096048 | US9701664, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234079 (CHEMBL4096048 | US9701664, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071668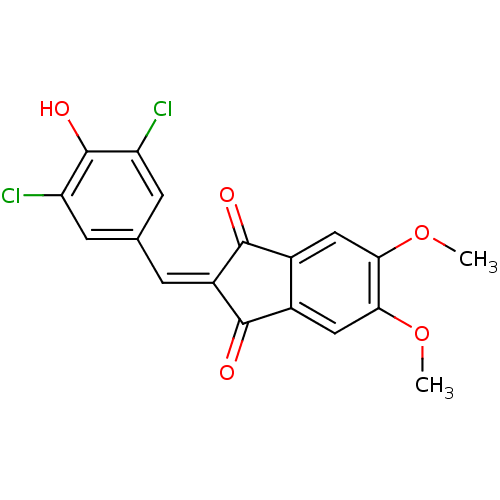 (2-(3,5-Dichloro-4-hydroxy-benzylidene)-5,6-dimetho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50234074 (CHEMBL4070633 | US9701664, Example 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 417 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human recombinant GST-tagged BRAF (S429 to E741 residues) expressed in baculovirus expression system using Ser/Thr 03 mixtu... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50234074 (CHEMBL4070633 | US9701664, Example 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human recombinant GST-tagged BRAF (S429 to E741 residues) expressed in baculovirus expression system using Ser/Thr 03 mixtu... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234076 (CHEMBL4077078) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 447 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234076 (CHEMBL4077078) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50071664 (5-Amino-2-[1-(3,5-dibromo-4-hydroxy-phenyl)-meth-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071676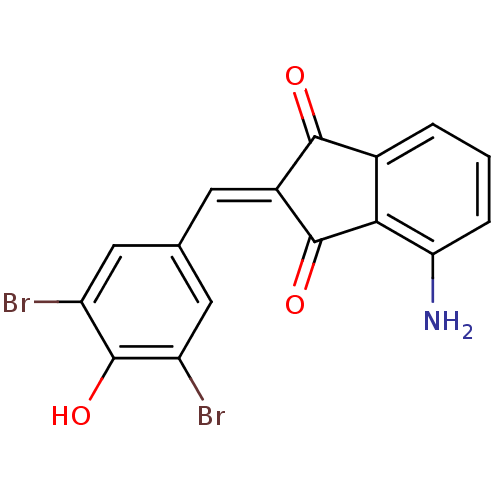 (4-Amino-2-[1-(3,5-dibromo-4-hydroxy-phenyl)-meth-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071665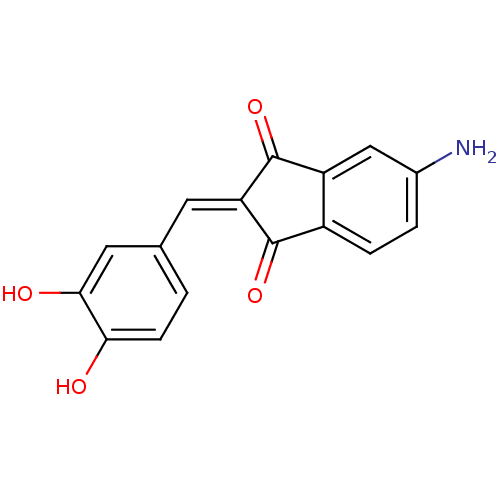 (5-Amino-2-[1-(3,4-dihydroxy-phenyl)-meth-(E)-ylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071670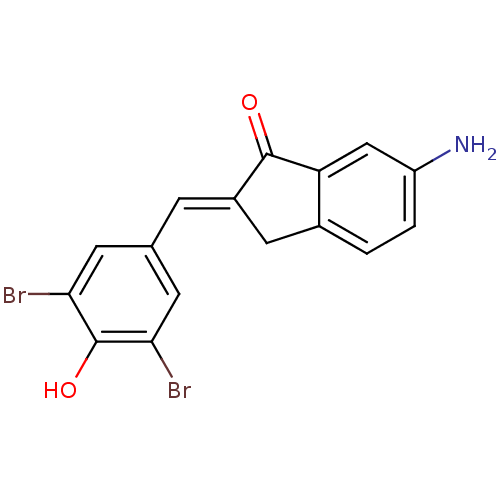 (6-Amino-2-[1-(3,5-dibromo-4-hydroxy-phenyl)-meth-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071669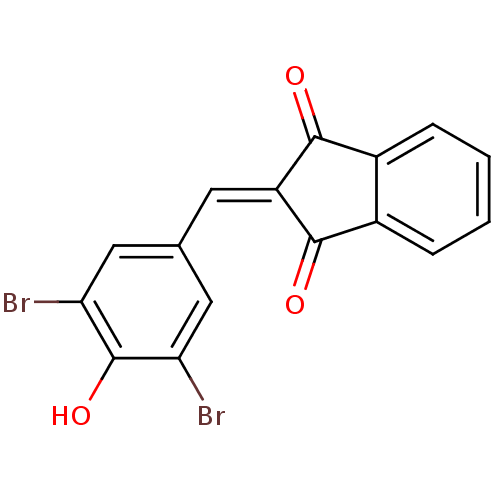 (2-(3,5-Dibromo-4-hydroxy-benzylidene)-indan-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071682 (2-[1-(3-Bromo-4-hydroxy-5-methoxy-phenyl)-meth-(E)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071678 (5-Bromo-2-[1-(3,5-dibromo-4-hydroxy-phenyl)-meth-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071675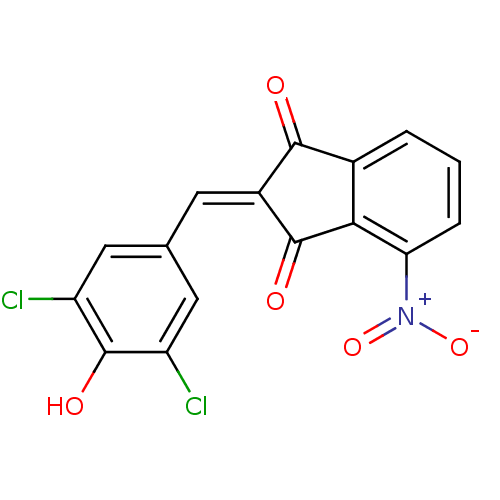 (2-[1-(3,5-Dichloro-4-hydroxy-phenyl)-meth-(Z)-ylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071681 (2-[1-(3,4-Dihydroxy-phenyl)-meth-(E)-ylidene]-5-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071673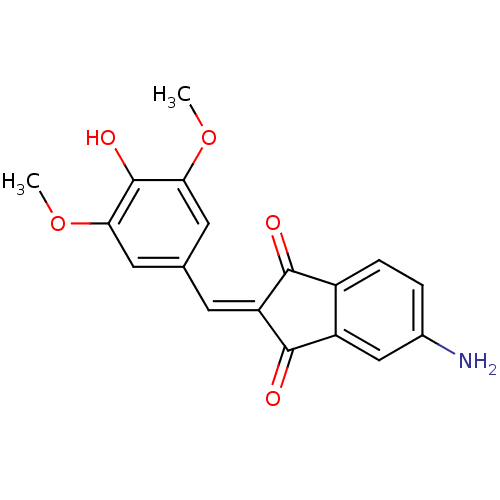 (5-Amino-2-[1-(4-hydroxy-3,5-dimethoxy-phenyl)-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071680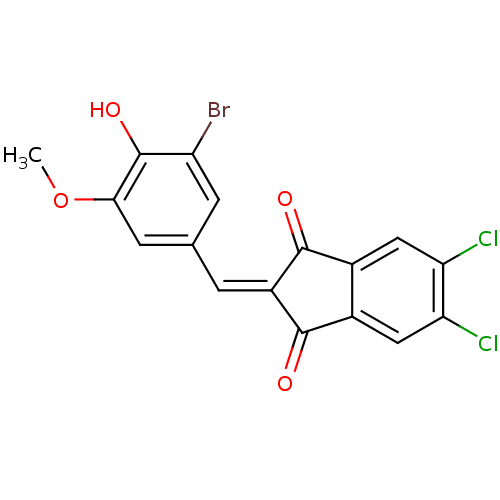 (2-(3-Bromo-4-hydroxy-5-methoxy-benzylidene)-5,6-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234072 (CHEMBL4099031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Homo sapiens (Human)) | BDBM50234072 (CHEMBL4099031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of 17-AAG-induced HSF1 pathway in human SKOV3 cells assessed as reduction in HSP72 induction preincubated for 1 hr followed by 17-AAG addi... | J Med Chem 60: 180-201 (2017) Article DOI: 10.1021/acs.jmedchem.6b01055 BindingDB Entry DOI: 10.7270/Q2KW5J9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50071667 (5-Amino-2-[1-(3,5-dichloro-4-hydroxy-phenyl)-meth-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma (Homo sapiens (Human)) | BDBM50071667 (5-Amino-2-[1-(3,5-dichloro-4-hydroxy-phenyl)-meth-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Protein Kinase A (PKA) | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071683 (2-[1-(3,4-Dihydroxy-phenyl)-meth-(Z)-ylidene]-4-ni...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50045936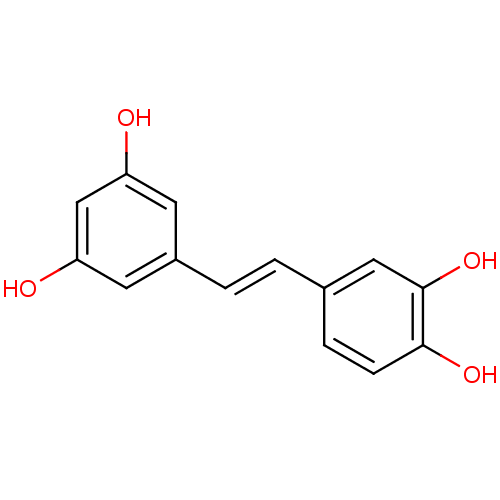 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071679 (5-Amino-2-[1-(3,5-dibromo-4-hydroxy-phenyl)-meth-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071674 (2-[1-(3,4-Dihydroxy-phenyl)-meth-(Z)-ylidene]-5-ni...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50067024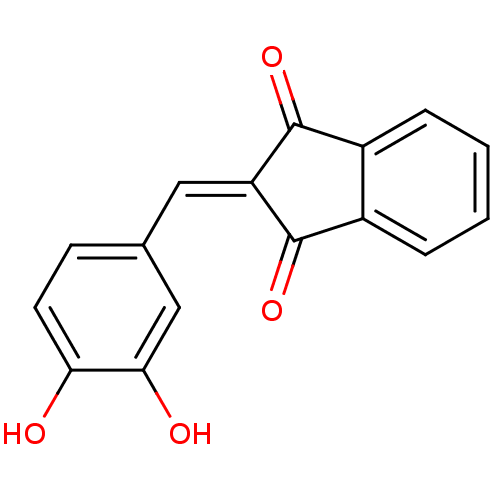 (2-(3,4-Dihydroxy-benzylidene)-indan-1,3-dione | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50071672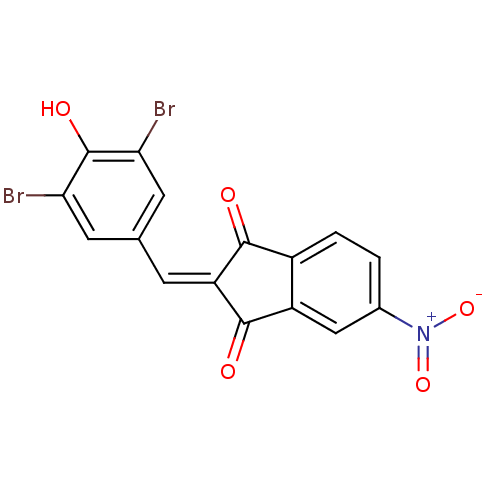 (2-[1-(3,5-Dibromo-4-hydroxy-phenyl)-meth-(E)-ylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 8: 2489-94 (1999) BindingDB Entry DOI: 10.7270/Q2D50M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 112 total ) | Next | Last >> |