 Found 33 hits with Last Name = 'penzien' and Initial = 'jb'
Found 33 hits with Last Name = 'penzien' and Initial = 'jb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395921
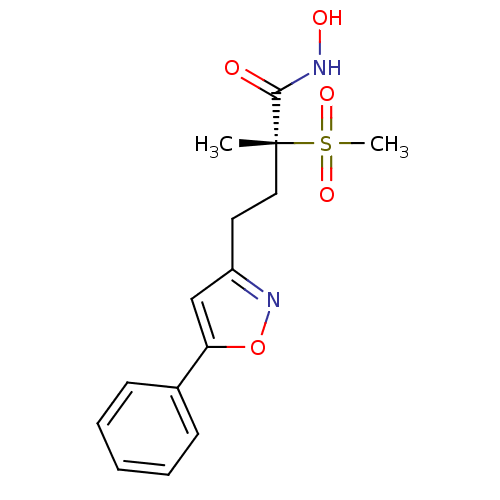
(CHEMBL2164511)Show SMILES C[C@@](CCc1cc(on1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C15H18N2O5S/c1-15(14(18)16-19,23(2,20)21)9-8-12-10-13(22-17-12)11-6-4-3-5-7-11/h3-7,10,19H,8-9H2,1-2H3,(H,16,18)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.511 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395911
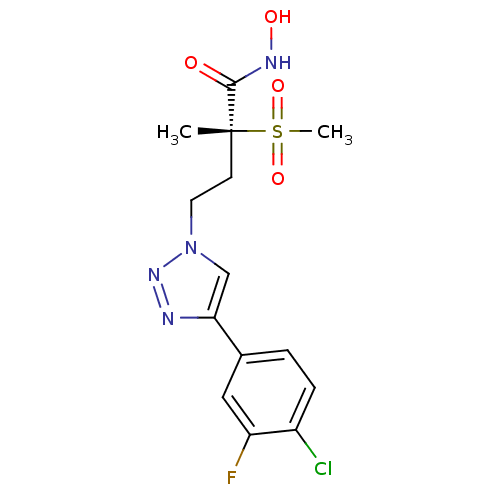
(CHEMBL2164521)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)10-4-3-9(15)7-11(10)16/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.657 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395920
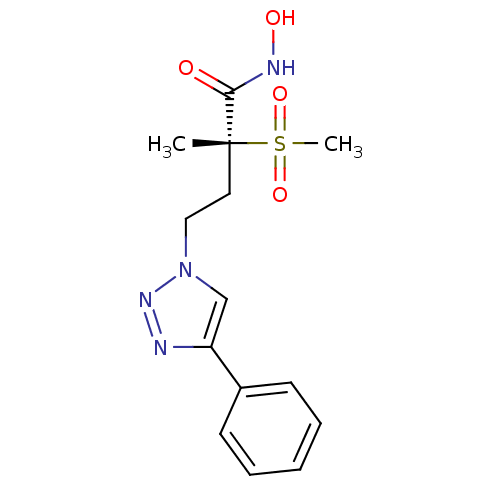
(CHEMBL2164512)Show SMILES C[C@@](CCn1cc(nn1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H18N4O4S/c1-14(13(19)16-20,23(2,21)22)8-9-18-10-12(15-17-18)11-6-4-3-5-7-11/h3-7,10,20H,8-9H2,1-2H3,(H,16,19)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395910
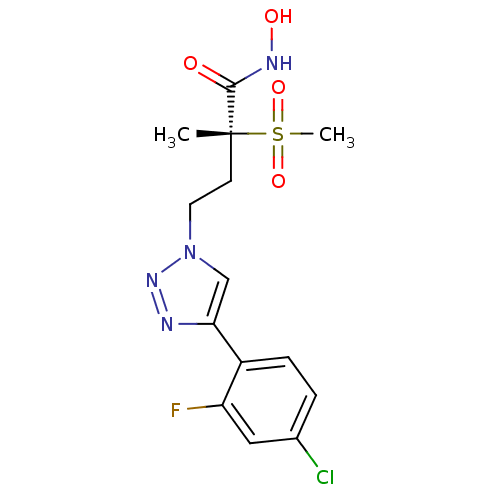
(CHEMBL2164522)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)c(F)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395909

(CHEMBL2164523)Show SMILES COc1ccc(-c2cn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)nn2)c(F)c1 |r| Show InChI InChI=1S/C15H19FN4O5S/c1-15(14(21)18-22,26(3,23)24)6-7-20-9-13(17-19-20)11-5-4-10(25-2)8-12(11)16/h4-5,8-9,22H,6-7H2,1-3H3,(H,18,21)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395908
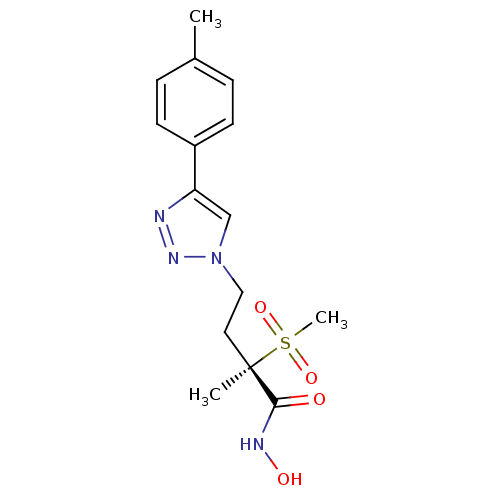
(CHEMBL2164524)Show SMILES Cc1ccc(cc1)-c1cn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)nn1 |r| Show InChI InChI=1S/C15H20N4O4S/c1-11-4-6-12(7-5-11)13-10-19(18-16-13)9-8-15(2,14(20)17-21)24(3,22)23/h4-7,10,21H,8-9H2,1-3H3,(H,17,20)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395919
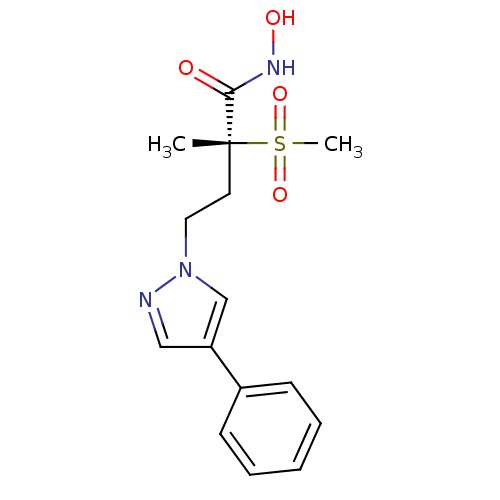
(CHEMBL2164513)Show SMILES C[C@@](CCn1cc(cn1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C15H19N3O4S/c1-15(14(19)17-20,23(2,21)22)8-9-18-11-13(10-16-18)12-6-4-3-5-7-12/h3-7,10-11,20H,8-9H2,1-2H3,(H,17,19)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395907
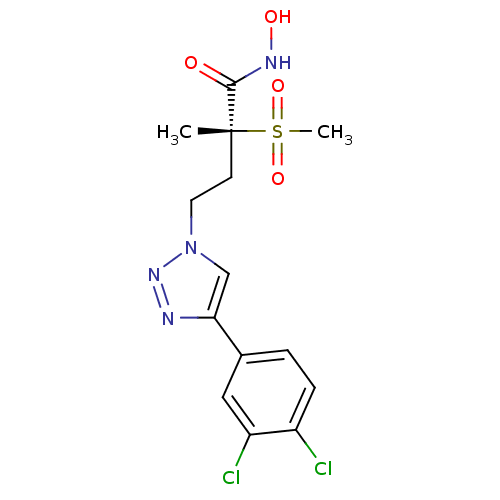
(CHEMBL2164525)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)c(Cl)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16Cl2N4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395906
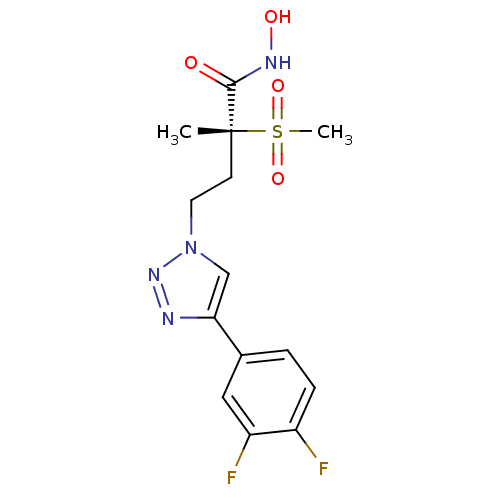
(CHEMBL2164526)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(F)c(F)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16F2N4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395905
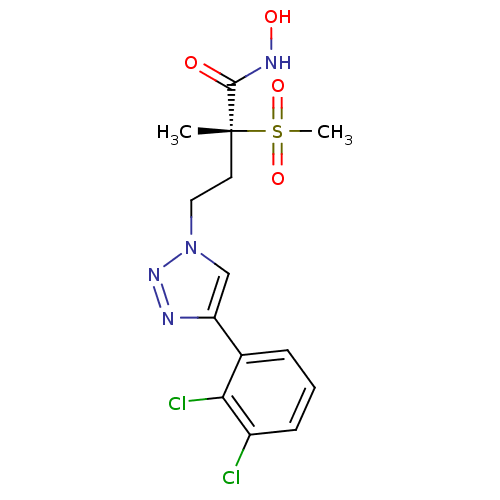
(CHEMBL2164184)Show SMILES C[C@@](CCn1cc(nn1)-c1cccc(Cl)c1Cl)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16Cl2N4O4S/c1-14(13(21)18-22,25(2,23)24)6-7-20-8-11(17-19-20)9-4-3-5-10(15)12(9)16/h3-5,8,22H,6-7H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395902
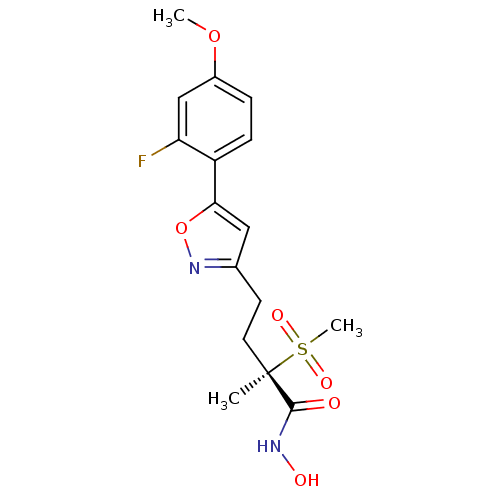
(CHEMBL2164187)Show SMILES COc1ccc(-c2cc(CC[C@](C)(C(=O)NO)S(C)(=O)=O)no2)c(F)c1 |r| Show InChI InChI=1S/C16H19FN2O6S/c1-16(15(20)18-21,26(3,22)23)7-6-10-8-14(25-19-10)12-5-4-11(24-2)9-13(12)17/h4-5,8-9,21H,6-7H2,1-3H3,(H,18,20)/t16-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395901
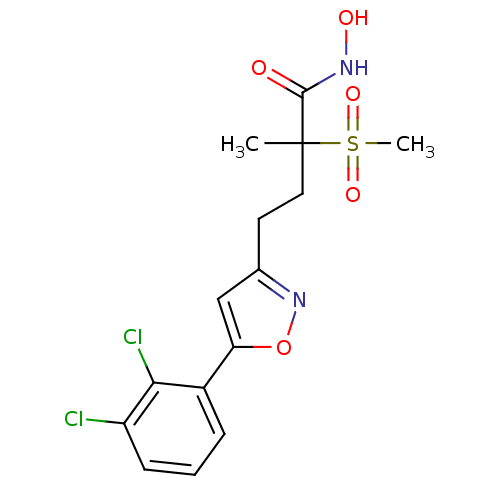
(CHEMBL2164188)Show SMILES CC(CCc1cc(on1)-c1cccc(Cl)c1Cl)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C15H16Cl2N2O5S/c1-15(14(20)18-21,25(2,22)23)7-6-9-8-12(24-19-9)10-4-3-5-11(16)13(10)17/h3-5,8,21H,6-7H2,1-2H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395898

(CHEMBL2164191)Show SMILES Cc1ccc(cc1)-c1cc(CCC(C)(C(=O)NO)S(C)(=O)=O)on1 Show InChI InChI=1S/C16H20N2O5S/c1-11-4-6-12(7-5-11)14-10-13(23-18-14)8-9-16(2,15(19)17-20)24(3,21)22/h4-7,10,20H,8-9H2,1-3H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395917
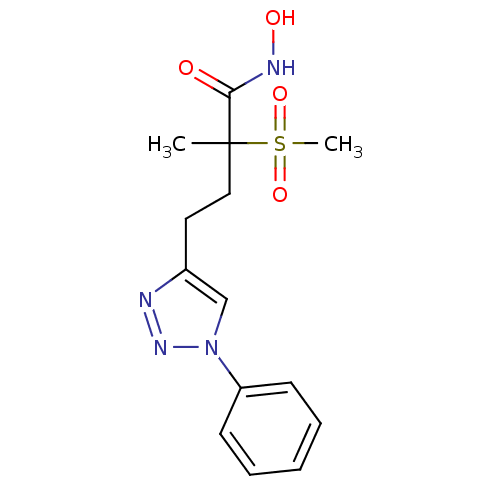
(CHEMBL2164515)Show SMILES CC(CCc1cn(nn1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C14H18N4O4S/c1-14(13(19)16-20,23(2,21)22)9-8-11-10-18(17-15-11)12-6-4-3-5-7-12/h3-7,10,20H,8-9H2,1-2H3,(H,16,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395918
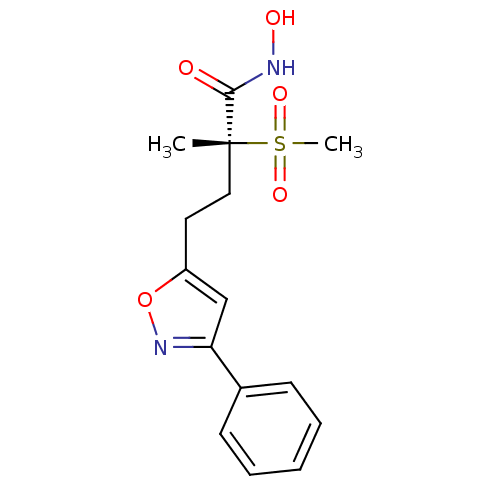
(CHEMBL2164514)Show SMILES C[C@@](CCc1cc(no1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C15H18N2O5S/c1-15(14(18)16-19,23(2,20)21)9-8-12-10-13(17-22-12)11-6-4-3-5-7-11/h3-7,10,19H,8-9H2,1-2H3,(H,16,18)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395897
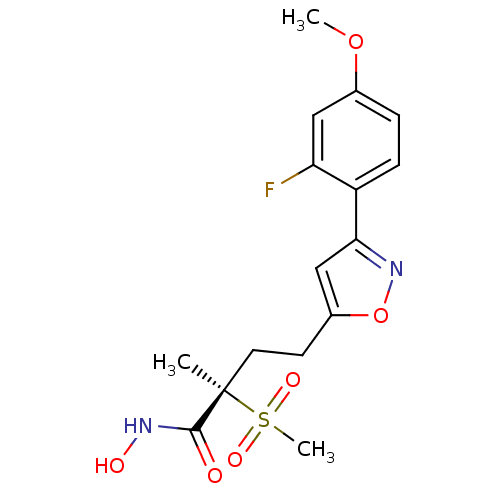
(CHEMBL2164192)Show SMILES COc1ccc(-c2cc(CC[C@](C)(C(=O)NO)S(C)(=O)=O)on2)c(F)c1 |r| Show InChI InChI=1S/C16H19FN2O6S/c1-16(15(20)18-21,26(3,22)23)7-6-11-9-14(19-25-11)12-5-4-10(24-2)8-13(12)17/h4-5,8-9,21H,6-7H2,1-3H3,(H,18,20)/t16-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395904
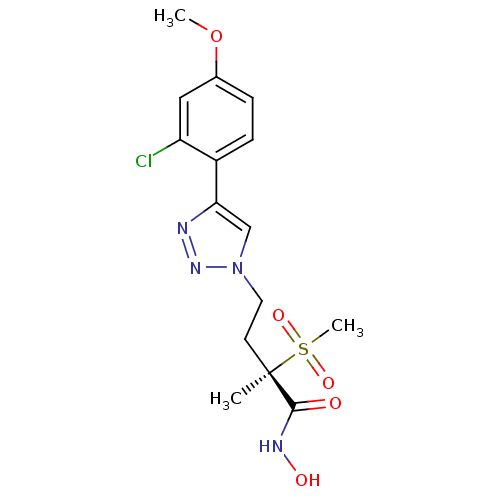
(CHEMBL2164185)Show SMILES COc1ccc(-c2cn(CC[C@](C)(C(=O)NO)S(C)(=O)=O)nn2)c(Cl)c1 |r| Show InChI InChI=1S/C15H19ClN4O5S/c1-15(14(21)18-22,26(3,23)24)6-7-20-9-13(17-19-20)11-5-4-10(25-2)8-12(11)16/h4-5,8-9,22H,6-7H2,1-3H3,(H,18,21)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395896
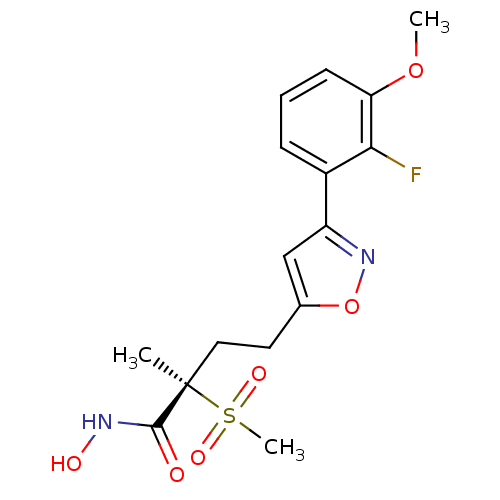
(CHEMBL2164193)Show SMILES COc1cccc(-c2cc(CC[C@](C)(C(=O)NO)S(C)(=O)=O)on2)c1F |r| Show InChI InChI=1S/C16H19FN2O6S/c1-16(15(20)18-21,26(3,22)23)8-7-10-9-12(19-25-10)11-5-4-6-13(24-2)14(11)17/h4-6,9,21H,7-8H2,1-3H3,(H,18,20)/t16-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395900
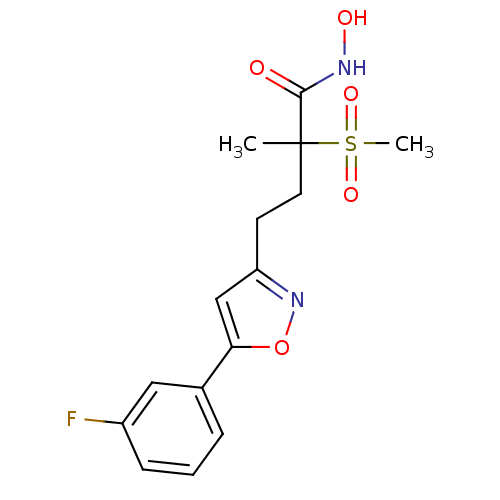
(CHEMBL2164189)Show SMILES CC(CCc1cc(on1)-c1cccc(F)c1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C15H17FN2O5S/c1-15(14(19)17-20,24(2,21)22)7-6-12-9-13(23-18-12)10-4-3-5-11(16)8-10/h3-5,8-9,20H,6-7H2,1-2H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395895
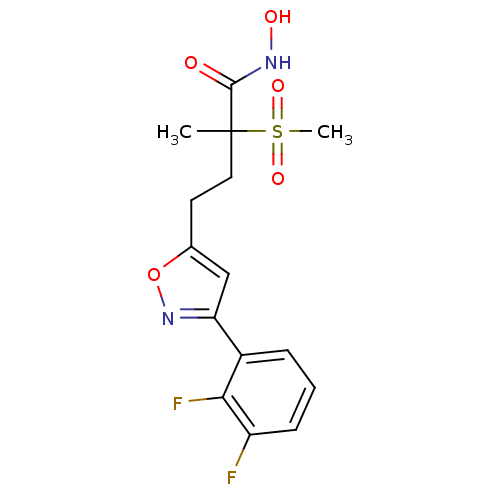
(CHEMBL2164194)Show SMILES CC(CCc1cc(no1)-c1cccc(F)c1F)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C15H16F2N2O5S/c1-15(14(20)18-21,25(2,22)23)7-6-9-8-12(19-24-9)10-4-3-5-11(16)13(10)17/h3-5,8,21H,6-7H2,1-2H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395903
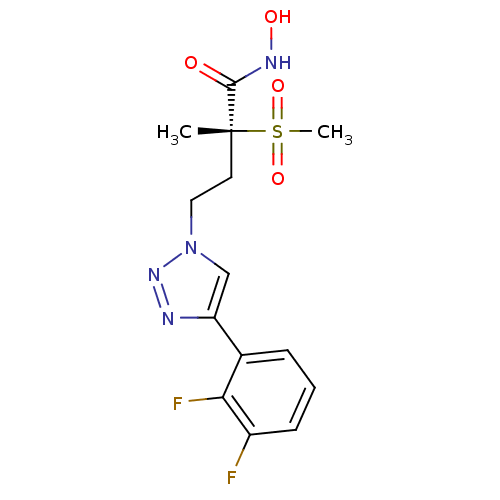
(CHEMBL2164186)Show SMILES C[C@@](CCn1cc(nn1)-c1cccc(F)c1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16F2N4O4S/c1-14(13(21)18-22,25(2,23)24)6-7-20-8-11(17-19-20)9-4-3-5-10(15)12(9)16/h3-5,8,22H,6-7H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395899
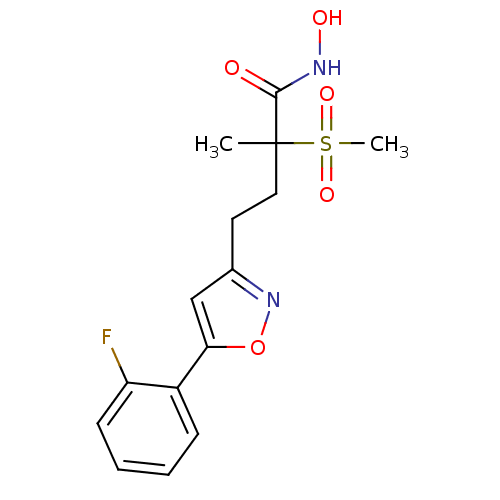
(CHEMBL2164190)Show SMILES CC(CCc1cc(on1)-c1ccccc1F)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C15H17FN2O5S/c1-15(14(19)17-20,24(2,21)22)8-7-10-9-13(23-18-10)11-5-3-4-6-12(11)16/h3-6,9,20H,7-8H2,1-2H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395916
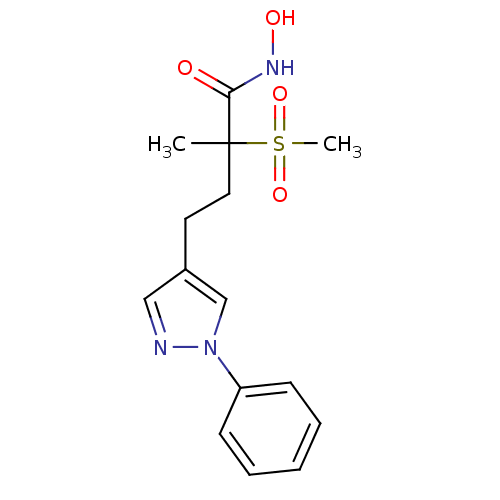
(CHEMBL2164516)Show SMILES CC(CCc1cnn(c1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C15H19N3O4S/c1-15(14(19)17-20,23(2,21)22)9-8-12-10-16-18(11-12)13-6-4-3-5-7-13/h3-7,10-11,20H,8-9H2,1-2H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395912
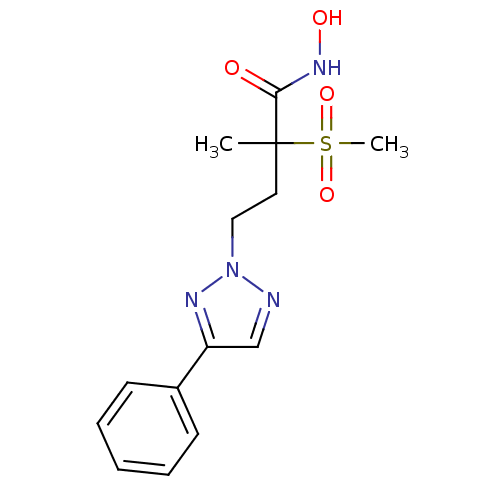
(CHEMBL2164520)Show SMILES CC(CCn1ncc(n1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C14H18N4O4S/c1-14(13(19)17-20,23(2,21)22)8-9-18-15-10-12(16-18)11-6-4-3-5-7-11/h3-7,10,20H,8-9H2,1-2H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395913
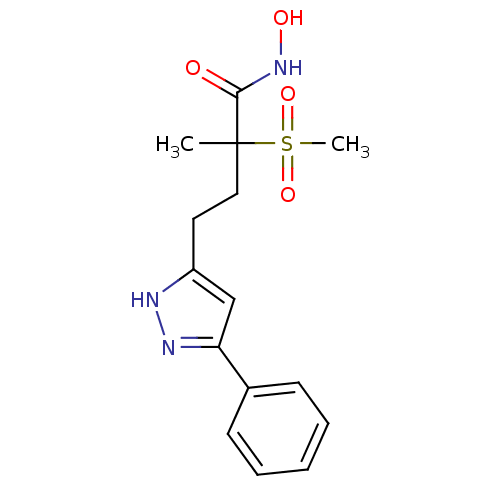
(CHEMBL2164519)Show SMILES CC(CCc1cc(n[nH]1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C15H19N3O4S/c1-15(14(19)18-20,23(2,21)22)9-8-12-10-13(17-16-12)11-6-4-3-5-7-11/h3-7,10,20H,8-9H2,1-2H3,(H,16,17)(H,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395914
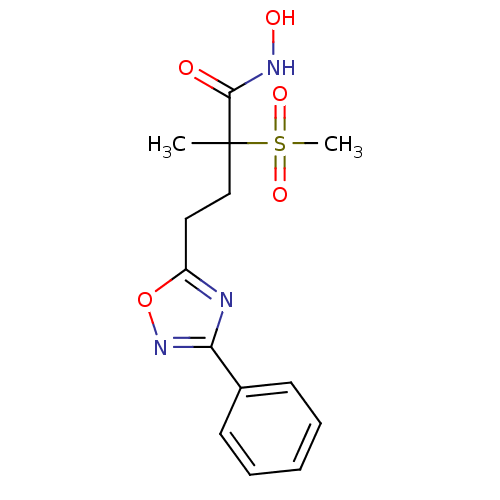
(CHEMBL2164518)Show SMILES CC(CCc1nc(no1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C14H17N3O5S/c1-14(13(18)16-19,23(2,20)21)9-8-11-15-12(17-22-11)10-6-4-3-5-7-10/h3-7,19H,8-9H2,1-2H3,(H,16,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395915
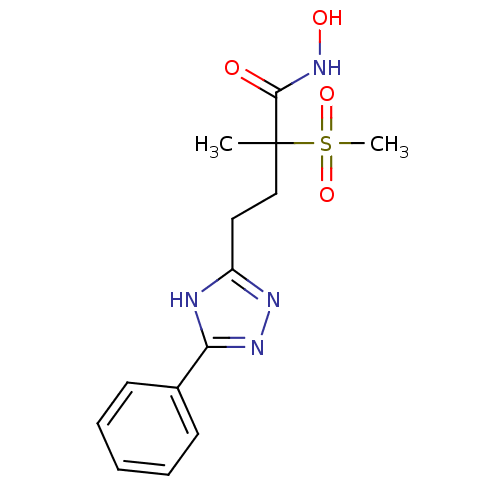
(CHEMBL2164517)Show SMILES CC(CCc1nnc([nH]1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C14H18N4O4S/c1-14(13(19)18-20,23(2,21)22)9-8-11-15-12(17-16-11)10-6-4-3-5-7-10/h3-7,20H,8-9H2,1-2H3,(H,18,19)(H,15,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378137
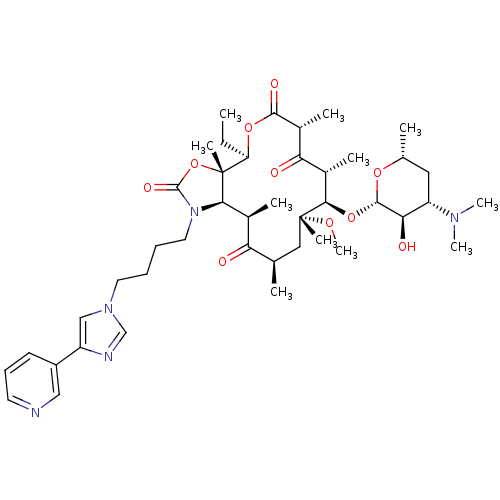
(Ketek | TELITHROMYCIN)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@@](C)(C[C@@H](C)C(=O)[C@H](C)[C@H]2N(CCCCn3cnc(c3)-c3cccnc3)C(=O)O[C@]12C)OC |r| Show InChI InChI=1S/C43H65N5O10/c1-12-33-43(8)37(48(41(53)58-43)19-14-13-18-47-23-31(45-24-47)30-16-15-17-44-22-30)27(4)34(49)25(2)21-42(7,54-11)38(28(5)35(50)29(6)39(52)56-33)57-40-36(51)32(46(9)10)20-26(3)55-40/h15-17,22-29,32-33,36-38,40,51H,12-14,18-21H2,1-11H3/t25-,26-,27+,28+,29-,32+,33-,36-,37-,38-,40+,42-,43-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch-clamp assay |
J Med Chem 52: 7446-57 (2009)
Article DOI: 10.1021/jm900729s
BindingDB Entry DOI: 10.7270/Q2ST7QSP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50299106

(3-Descladinosyl-11,12-dideoxy-6-Omethyl-3-oxo-12,1...)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@H]2N(C3CN(C3)[C@H](C)c3ccnc4ncccc34)C(=O)O[C@]12C |r| Show InChI InChI=1S/C43H63N5O10/c1-12-32-43(9)36(48(41(53)58-43)28-20-47(21-28)27(7)29-15-17-45-38-30(29)14-13-16-44-38)24(4)33(49)22(2)19-42(8,54)37(25(5)34(50)26(6)39(52)56-32)57-40-35(51)31(46(10)11)18-23(3)55-40/h13-17,22-28,31-32,35-37,40,51,54H,12,18-21H2,1-11H3/t22-,23-,24+,25+,26-,27-,31+,32-,35-,36-,37-,40+,42-,43-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch-clamp assay |
J Med Chem 52: 7446-57 (2009)
Article DOI: 10.1021/jm900729s
BindingDB Entry DOI: 10.7270/Q2ST7QSP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50299108
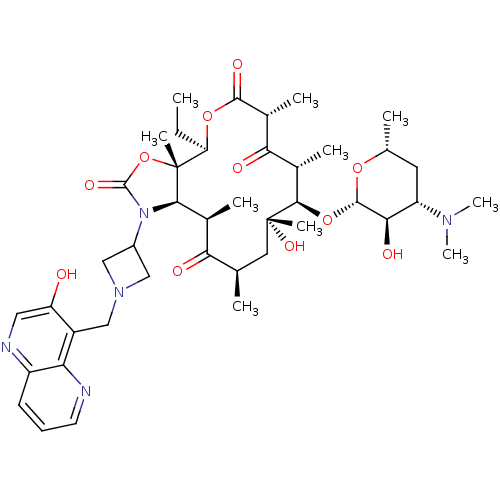
(3-Descladinosyl-11,12-dideoxy-6-O-methyl-3-oxo-12,...)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@H]2N(C3CN(Cc4c(O)cnc5cccnc45)C3)C(=O)O[C@]12C |r| Show InChI InChI=1S/C42H61N5O11/c1-11-31-42(8)36(47(40(53)58-42)26-18-46(19-26)20-27-30(48)17-44-28-13-12-14-43-32(27)28)23(4)33(49)21(2)16-41(7,54)37(24(5)34(50)25(6)38(52)56-31)57-39-35(51)29(45(9)10)15-22(3)55-39/h12-14,17,21-26,29,31,35-37,39,48,51,54H,11,15-16,18-20H2,1-10H3/t21-,22-,23+,24+,25-,29+,31-,35-,36-,37-,39+,41-,42-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch-clamp assay |
J Med Chem 52: 7446-57 (2009)
Article DOI: 10.1021/jm900729s
BindingDB Entry DOI: 10.7270/Q2ST7QSP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50299105

(3-Descladinosyl-11,12-dideoxy-6-O-methyl-3-oxo-12,...)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@H]2N(C3CN(Cc4ccnc5ncccc45)C3)C(=O)O[C@]12C |r| Show InChI InChI=1S/C42H61N5O10/c1-11-31-42(8)35(47(40(52)57-42)28-20-46(21-28)19-27-14-16-44-37-29(27)13-12-15-43-37)24(4)32(48)22(2)18-41(7,53)36(25(5)33(49)26(6)38(51)55-31)56-39-34(50)30(45(9)10)17-23(3)54-39/h12-16,22-26,28,30-31,34-36,39,50,53H,11,17-21H2,1-10H3/t22-,23-,24+,25+,26-,30+,31-,34-,35-,36-,39+,41-,42-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch-clamp assay |
J Med Chem 52: 7446-57 (2009)
Article DOI: 10.1021/jm900729s
BindingDB Entry DOI: 10.7270/Q2ST7QSP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50299107
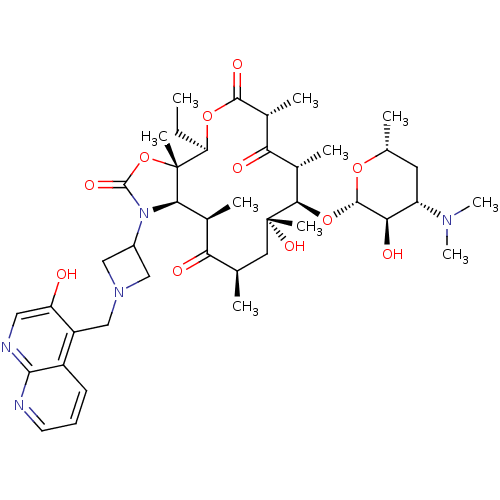
(3-Descladinosyl-11,12-dideoxy-6-O-methyl-3-oxo-12,...)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@H]2N(C3CN(Cc4c(O)cnc5ncccc45)C3)C(=O)O[C@]12C |r| Show InChI InChI=1S/C42H61N5O11/c1-11-31-42(8)35(47(40(53)58-42)26-18-46(19-26)20-28-27-13-12-14-43-37(27)44-17-30(28)48)23(4)32(49)21(2)16-41(7,54)36(24(5)33(50)25(6)38(52)56-31)57-39-34(51)29(45(9)10)15-22(3)55-39/h12-14,17,21-26,29,31,34-36,39,48,51,54H,11,15-16,18-20H2,1-10H3/t21-,22-,23+,24+,25-,29+,31-,34-,35-,36-,39+,41-,42-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch-clamp assay |
J Med Chem 52: 7446-57 (2009)
Article DOI: 10.1021/jm900729s
BindingDB Entry DOI: 10.7270/Q2ST7QSP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50299104

(3-Descladinosyl-11,12-dideoxy-6-O-methyl-3-oxo-12,...)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@H]2N(C3CN(Cc4ccnc5cccnc45)C3)C(=O)O[C@]12C |r| Show InChI InChI=1S/C42H61N5O10/c1-11-31-42(8)36(47(40(52)57-42)28-20-46(21-28)19-27-14-16-43-29-13-12-15-44-32(27)29)24(4)33(48)22(2)18-41(7,53)37(25(5)34(49)26(6)38(51)55-31)56-39-35(50)30(45(9)10)17-23(3)54-39/h12-16,22-26,28,30-31,35-37,39,50,53H,11,17-21H2,1-10H3/t22-,23-,24+,25+,26-,30+,31-,35-,36-,37-,39+,41-,42-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.96E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch-clamp assay |
J Med Chem 52: 7446-57 (2009)
Article DOI: 10.1021/jm900729s
BindingDB Entry DOI: 10.7270/Q2ST7QSP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data